The Floral Biology, Breeding System and Pollination Efficiency of Schima superba Gardn. et Champ. (Theaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Site
2.2. Floral Biology
2.3. Breeding System
2.4. Visitors
3. Results
3.1. Floral Biology
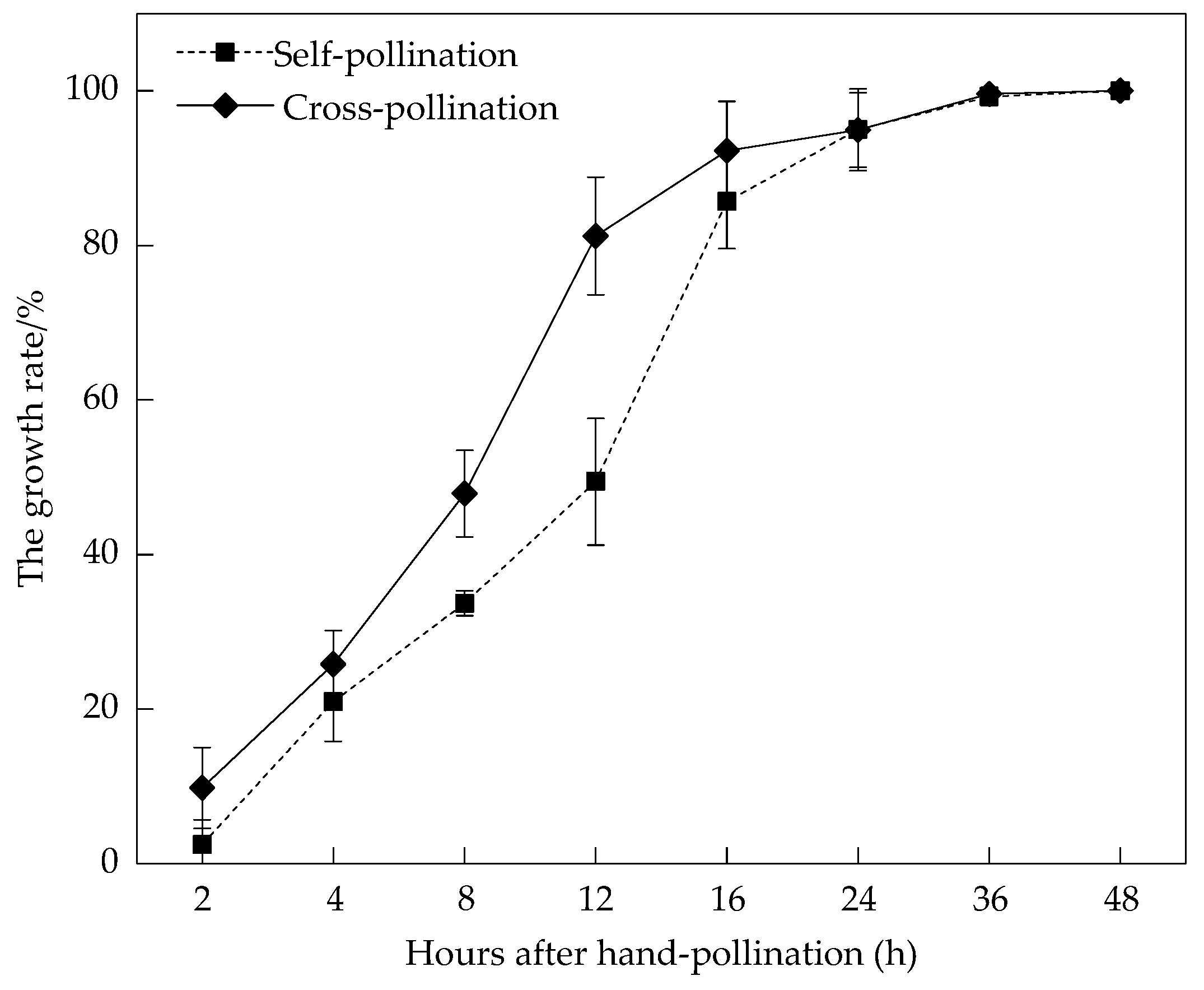
3.2. Pollen Viability and Stigma Receptivity
3.3. Breeding System
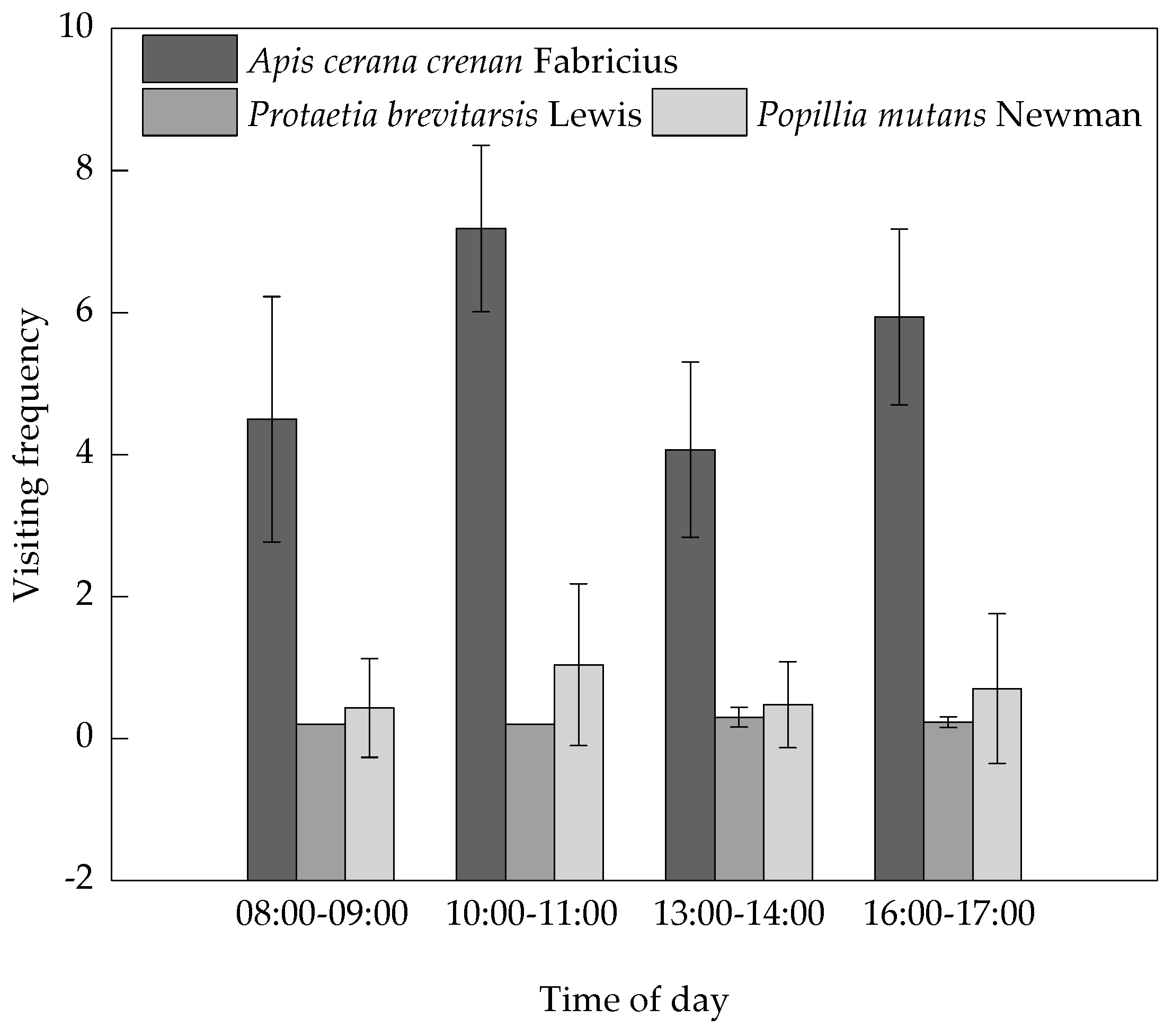
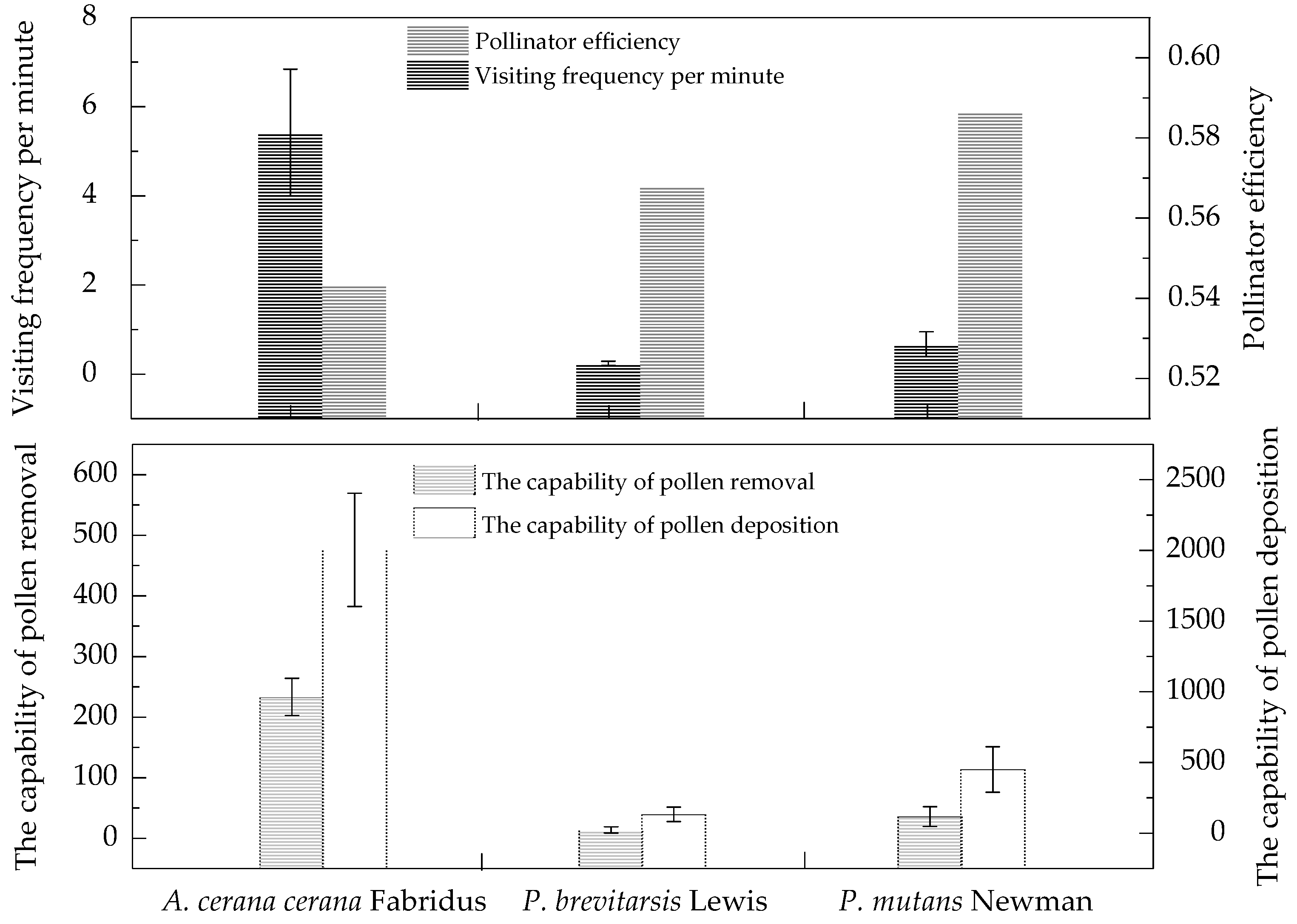
3.4. Pollinator Visitation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Charlesworth, D. Evolution of plant breeding systems. Curr. Biol. 2006, 16, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, D.; Oliveira, P. Reproductive ecology of buzz-pollinated Ouratea spectabilis trees (Ochnaceae) in Brazilian cerrados. Web Ecol. 2015, 14, 79–84. [Google Scholar] [CrossRef]
- Gong, X.; Guan, B.C.; Zhou, S.L.; Ge, G. Reproductive biology of the rare plant, Dysosma pleiantha (Berberidaceae): Breeding system, pollination and implications for conservation. Pak. J. Bot. 2015, 47, 951–957. [Google Scholar]
- Iwata, T.; Nagasaki, O.; Ishii, H.S.; Ushimaru, A. Inflorescence architecture affects pollinator behaviour and mating success in Spiranthes sinensis (Orchidaceae). New Phytol. 2012, 193, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Aronne, G.; Buonanno, M.; Micco, V.D. Reproducing under a warming climate: Long winter flowering and extended flower longevity in the only Mediterranean and maritime primula. Plant Biol. 2015, 17, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Khanduri, V.P.; Sharma, C.M.; Kumar, K.S.; Ghildiyal, S.K. Annual variation in flowering phenology, pollination, mating system, and pollen yield in two natural populations of Schima wallichii (DC.) Korth. Sci. World J. 2013, 2013, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Delmas, C.E.L.; Escaravage, N.; Cheptou, P.-O.; Charrier, O.; Ruzafa, S.; Winterton, P.; Pornon, A. Relative impact of mate versus pollinator availability on pollen limitation and outcrossing rates in a mass-flowering species. Plant Biol. 2014, 17, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, B.; Ge, R.; Fang, H. Pollination insects and their flower-visiting behaviors on endangered plant Amydalus pedunculata. Chin. J. Ecol. 2011, 30, 1370–1374. (In Chinese) [Google Scholar]
- Sigrist, M.R.; Sazima, M. Phenology, reproductive biology and diversity of buzzing bees of sympatric dichorisandra species (Commelinaceae): Breeding system and performance of pollinators. Plant Syst. Evol. 2014, 301, 1–11. [Google Scholar] [CrossRef]
- Barriault, I.; Gibernau, M.; Barabe, D. Flowering period, thermogenesis, and pattern of visiting insects in Arisaema triphyllum (Araceae) in Quebec. Botany 2009, 87, 324–329. [Google Scholar] [CrossRef]
- Young, H.J. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae). Am. J. Bot. 2002, 89, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.F.; Dressler, S.; Weitzman, A.L. Theaceae. In Flowering Plants Dicotyledons; Springer: Berlin, Germany, 2004; pp. 463–471. [Google Scholar]
- Bittrich, V.; Amaral, M.C.E.; Melo, G.A.R. Pollination biology of Ternstroemia laevigata and T. dentata (Theaceae). Plant Syst. Evol. 1993, 185, 1–6. [Google Scholar] [CrossRef]
- Caddell, G.M. Pollination, Mating System, and Distribution of Genetic Variation within Populations of Camellia japonica L. (Theaceae). Ph.D. Thesis, The University of North Carolina, Chapel Hill, NC, USA, 1989. [Google Scholar]
- Zhang, R.; Zhou, Z.C.; Luo, W.J.; Wang, Y.; Feng, Z.P. Effects of nitrogen deposition on growth and phosphate efficiency of Schima superba of different provenances grown in phosphorus-barren soil. Plant Soil 2013, 370, 435–445. [Google Scholar] [CrossRef]
- Ekeke, C.; Agbagwa, I.O. Breeding and pollination biology of Combretum constrictum (benth) laws. (Combretaceae). Trop. Plant Biol. 2015, 8, 51–59. [Google Scholar] [CrossRef]
- Chinese Academy of Sciences Editorial Board of Flora of China. Flora of China; Science Press: Beijing, China, 1998; Volume 49, p. 224. [Google Scholar]
- Dafni, A. Pollination Ecology: A Practical Approach; Oxford University Press: Oxford, UK, 1992; Volume 46, pp. 250–478. [Google Scholar]
- Zimmerman, M.; Pyke, G.H. Reproduction in polemonium: Assessing the factors limiting seed set. Am. Nat. 1988, 131, 723–738. [Google Scholar] [CrossRef]
- Bullock, S.T. Breeding systems in the flora of a tropical deciduous forest in Mexico. Biotropica 1985, 17, 287–301. [Google Scholar] [CrossRef]
- Zapata, T.R. Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 1978, 10, 221–230. [Google Scholar] [CrossRef]
- Han, Y.; Dai, C.; Yang, C.-F.; Wang, Q.-F.; Motley, T.J. Anther appendages of Incarvillea trigger a pollen-dispensing mechanism. Ann. Bot. 2008, 102, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Harder, L.D.; Wilson, W.G. Floral evolution and male reproductive success: Optimal dispensing schedule for pollen dispersal by animal-pollinated plants. Evol. Ecol. 1994, 8, 542–559. [Google Scholar] [CrossRef]
- Bernardello, G.; Crawford, D.J. A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernández islands (Chile). Bot. Rev. 2001, 67, 255–308. [Google Scholar] [CrossRef]
- Podolsky, R.D. Strange floral attractors: Pollinator attraction and the evolution of plant sexual systems. Science 1992, 258, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.S.; Sakai, S. Temporal variation in floral display size and individual floral sex allocation in racemes of Narthecium asiaticum (Liliaceae). Am. J. Bot. 2002, 89, 441. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Sweet, H.R. Impact of insect pollinator group and floral display size on outcrossing rate. Evolution 2006, 60, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Abe, T. Flowering phenology, display size, and fruit set in an understory dioecious shrub, Aucuba japonica (Cornaceae). Am. J. Bot. 2001, 88, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bawa, K.S.; Beach, J.H. Evolution of sexual systems in flowering plants. Ann. Missouri Bot. Gard. 1981, 68, S243. [Google Scholar] [CrossRef]
- Gottsberger, U.D.G. Some aspects of beetle pollination in the evolution of flowering plants. In Flowering Plants; Springer: Vienna, Austria, 1977; pp. 211–226. [Google Scholar]
- Amyc, D.; Rosanna, F. Floral development, stigma receptivity and pollen viability in eight nolana (Solanaceae) species. Euphytica 2010, 174, 105–117. [Google Scholar]
- Cruden, R.W. Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution 1977, 31, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.E. Late-acting self-incompatibility—The pariah breeding system in flowering plants. New Phytol. 2014, 203, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Yuan, D.Y.; Zou, F.; Gao, C.; Yang, Y.; Zhang, L.; Tan, X.F. Self-sterility in Camellia oleifera may be due to the prezygotic late-acting self-incompatibility. PLoS ONE 2014, 9, e99639. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. The Effects of Cross and Self-Fertilization in the Vegetable Kingdom; John Murray: London, UK, 1876. [Google Scholar]
- Fetscher, A.E. Resolution of male-female conflict in a hermaphroditic flower. Proc. Biol. Sci. 2001, 268, 525. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H. Sexual interference of the floral kind. Heredity 2002, 88, 154. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Cao, L.; Zhang, X.; Li, H. Floral biology, breeding system and pollination ecology of an endangered tree Tetracentron sinense oliv. (Trochodendraceae). Bot. Stud. 2013, 54, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ashman, T.L.; Morgan, M.T. Explaining phenotypic selection on plant attractive characters: Male function, gender balance or ecological context? Proc. R. Soc. B Biol. Sci. 2004, 271, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.J. The Pollination of Flowers by Insects; Academic Press for the Linnean Society of London: London, UK, 1978; pp. 119–128. [Google Scholar]
- Thomson, J.D.; Goodell, K. Pollen removal and deposition by honeybee and bumblebee visitors to apple and almond flowers. J. Appl. Ecol. 2001, 38, 1032–1044. [Google Scholar] [CrossRef]
- Thomson, J.D. Pollen transport and deposition by bumble bees in erythronium: Influences of floral nectar and bee grooming. J. Ecol. 1986, 74, 329–341. [Google Scholar] [CrossRef]
- Snow, A.A.; Roubik, D.W. Pollen deposition and removal by bees visiting two tree species in Panama. Biotropica 1987, 19, 57–63. [Google Scholar] [CrossRef]
- Suzuki, K.; Dohzono, I.; Hiei, K.; Fukuda, Y. Pollination effectiveness of three bumblebee species on flowers of Hosta sieboldiana (Liliaceae) and its relation to floral structure and pollinator sizes. Plant Species Biol. 2002, 17, 139–146. [Google Scholar] [CrossRef]


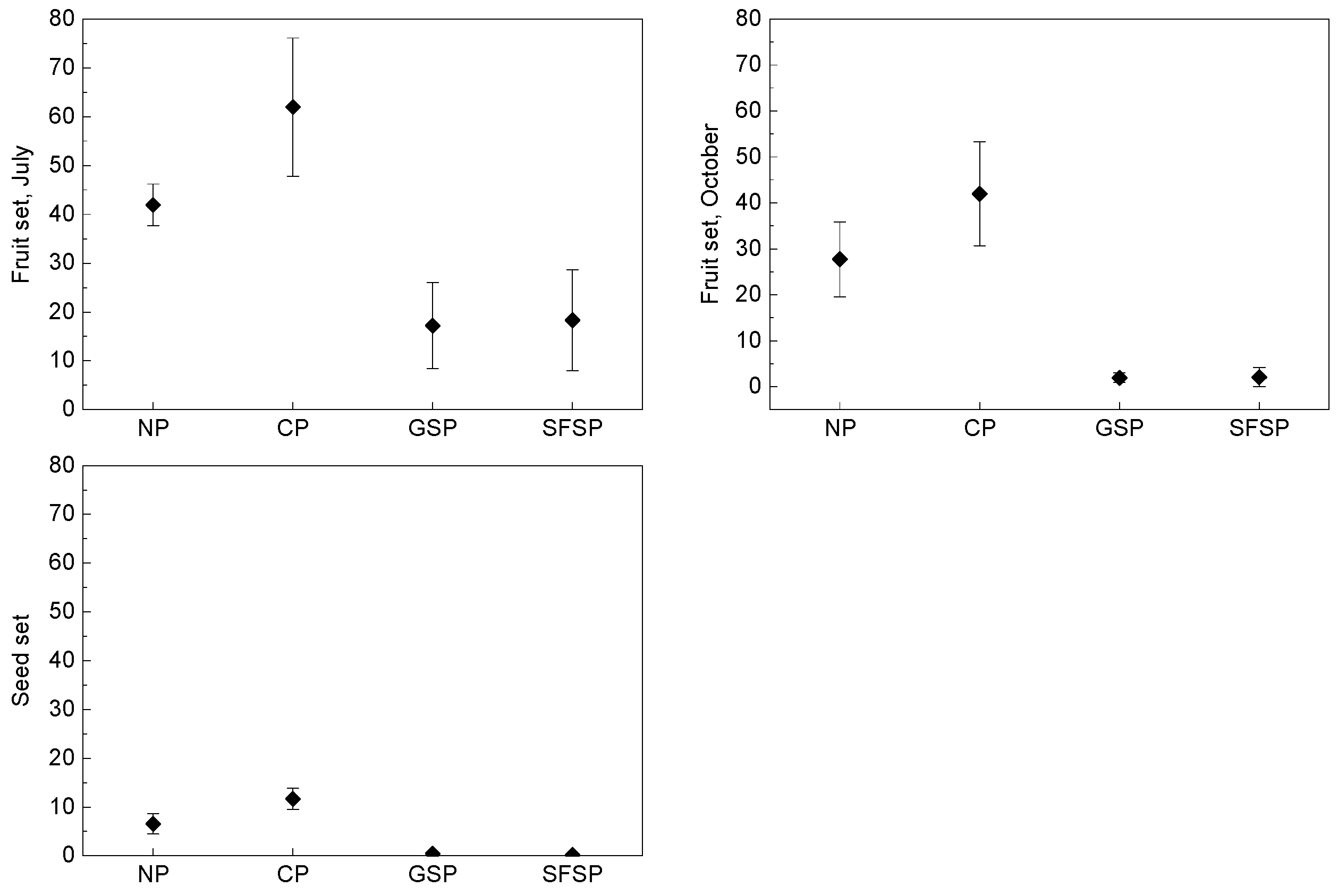
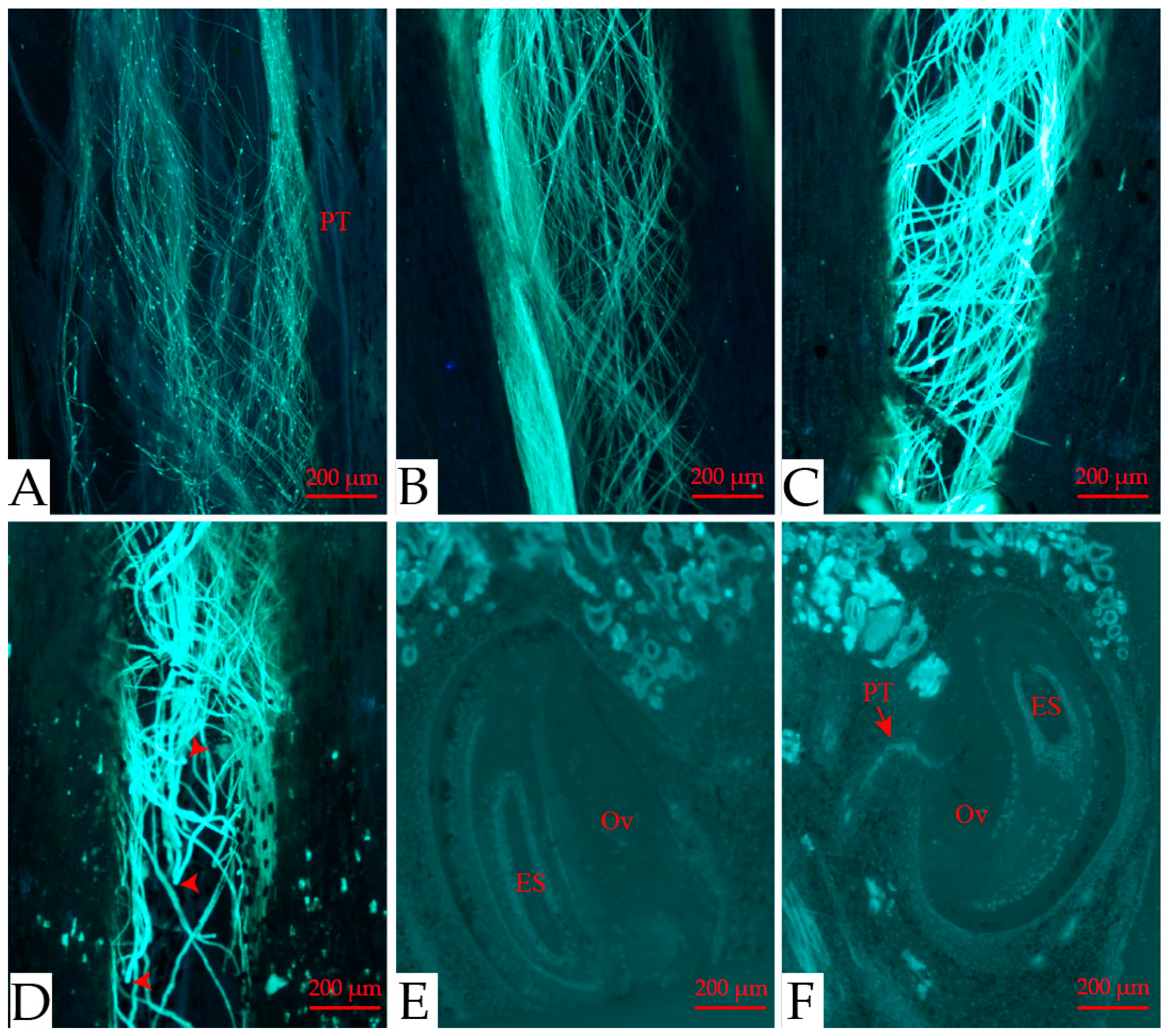
| Items | NIP | NFI | LC (mm) | LP (mm) | LS (mm) | LOS (mm) | Pollen grains | Ovules | Pollen-Ovule Ratio |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) | 86.68 (21.42) | 7 (2) | 31.57 (2.69) | 16.79 (2.18) | 8.30 (0.85) | 7.91 (2.42) | 100,300 (47,056) | 15.00 | 6686.67 |
| Range | 40.50–147.55 | 4–11 | 27.86–35.12 | 11.73–20.35 | 6.80–9.60 | 4.01–12.25 | 92,104–165,105 | - | - |
| N | 30 | 30 | 60 | 60 | 60 | 60 | 30 | 30 | 30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, R.; Song, P.; Zhou, Z. The Floral Biology, Breeding System and Pollination Efficiency of Schima superba Gardn. et Champ. (Theaceae). Forests 2017, 8, 404. https://doi.org/10.3390/f8100404
Yang H, Zhang R, Song P, Zhou Z. The Floral Biology, Breeding System and Pollination Efficiency of Schima superba Gardn. et Champ. (Theaceae). Forests. 2017; 8(10):404. https://doi.org/10.3390/f8100404
Chicago/Turabian StyleYang, Hanbo, Rui Zhang, Ping Song, and Zhichun Zhou. 2017. "The Floral Biology, Breeding System and Pollination Efficiency of Schima superba Gardn. et Champ. (Theaceae)" Forests 8, no. 10: 404. https://doi.org/10.3390/f8100404
APA StyleYang, H., Zhang, R., Song, P., & Zhou, Z. (2017). The Floral Biology, Breeding System and Pollination Efficiency of Schima superba Gardn. et Champ. (Theaceae). Forests, 8(10), 404. https://doi.org/10.3390/f8100404




