Sequential Management of Commercial Rosewood (Aniba rosaeodora Ducke) Plantations in Central Amazonia: Seeking Sustainable Models for Essential Oil Production
Abstract
:1. Introduction
2. Materials and Methods
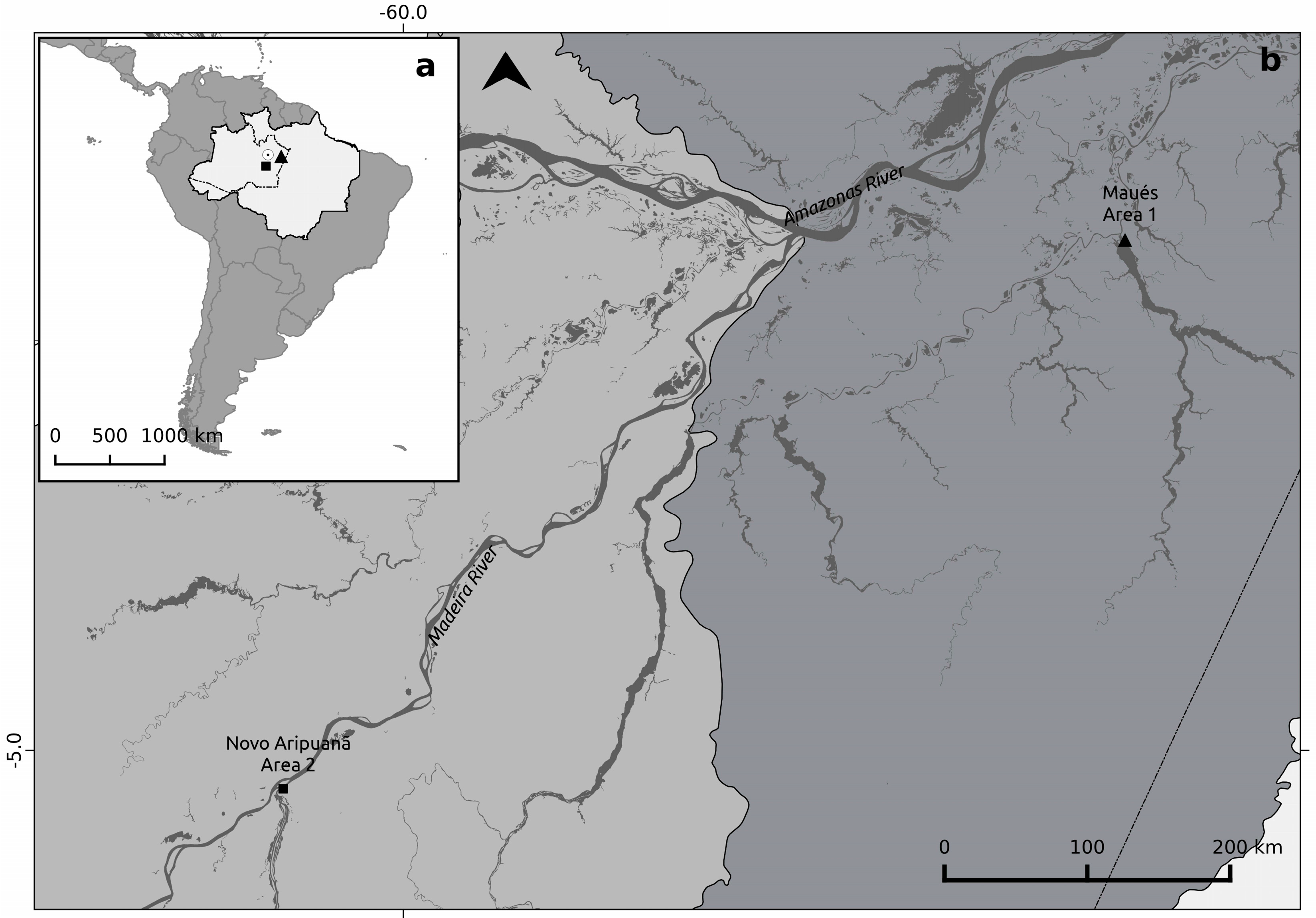
2.1. Study Sites Descripions
2.2. Field Sampling and Laboratory Methods
2.3. Management of Sprouted Above-Ground Biomass
2.4. Statistical Methods and Data Analysis
3. Results
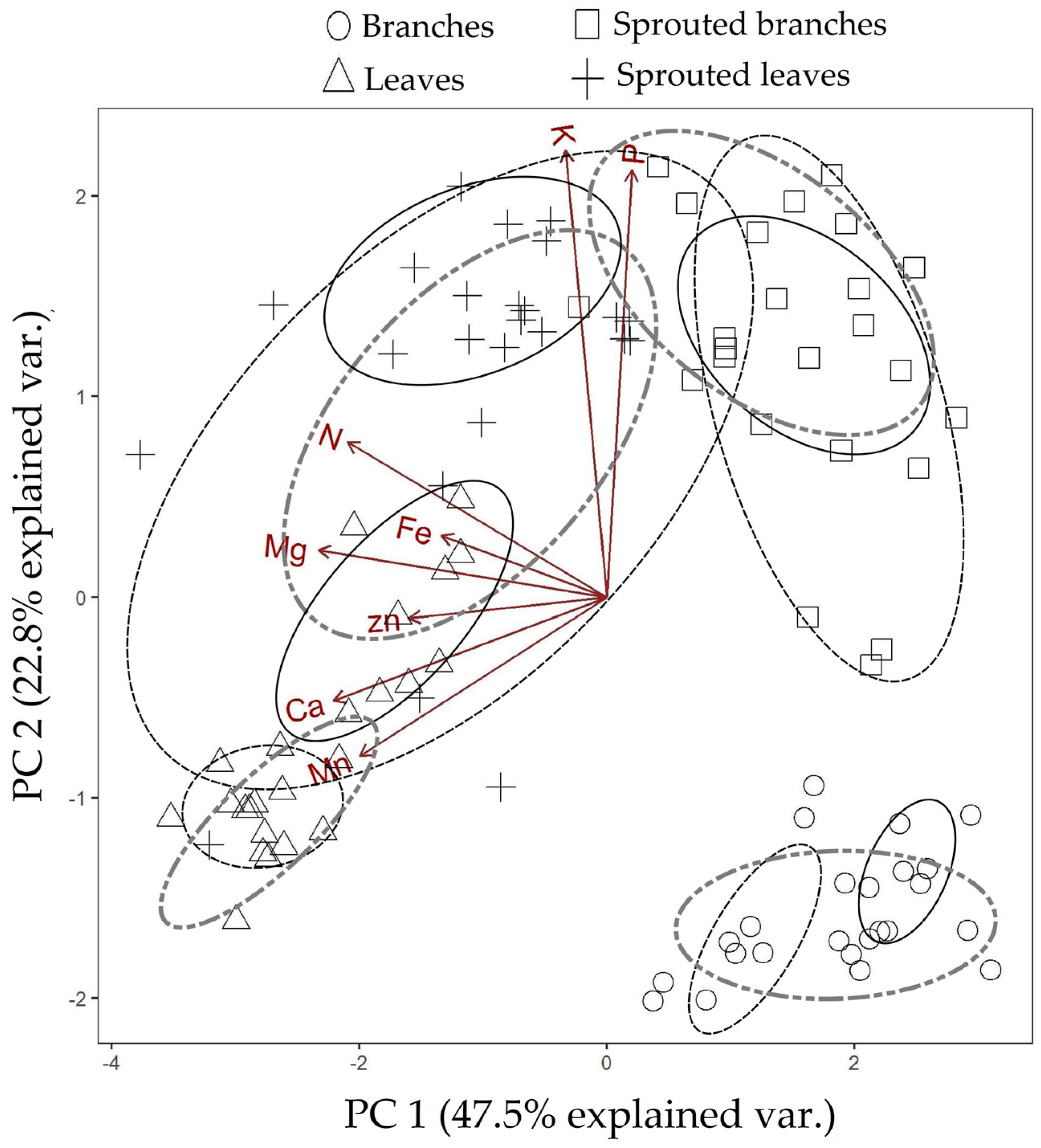
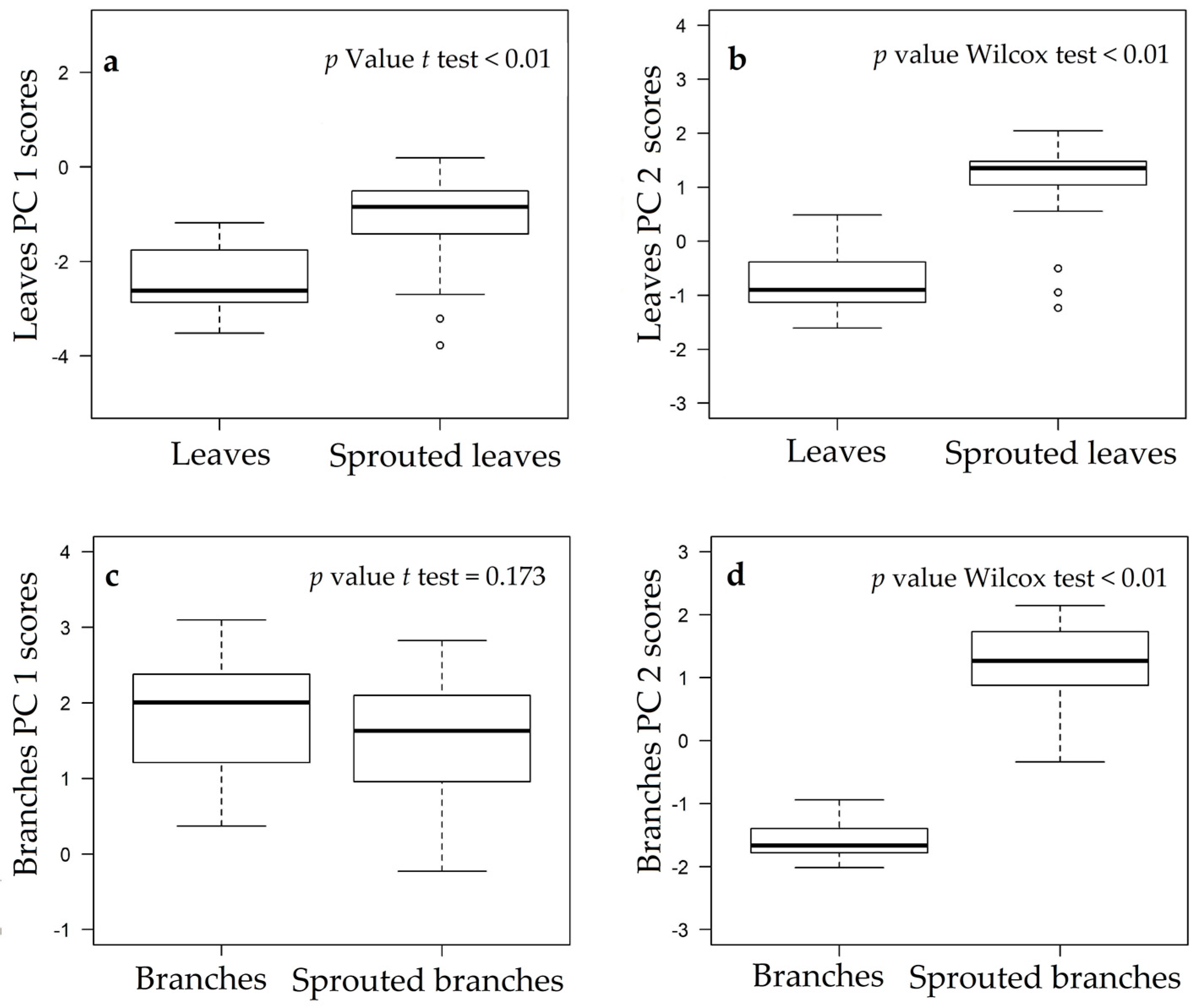
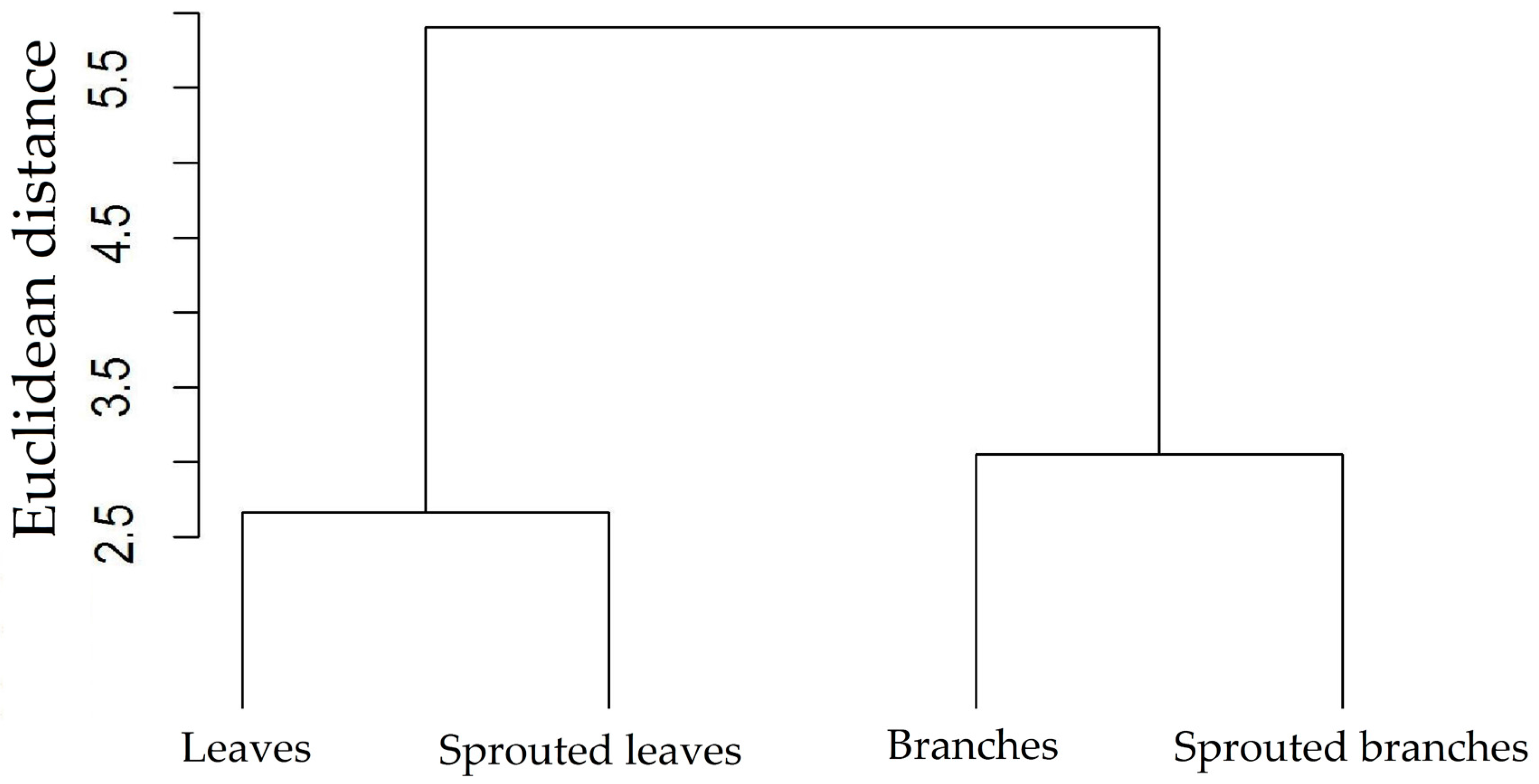
3.1. Changes in Nutritional Status Associated with the Management of Rosewood Plantations
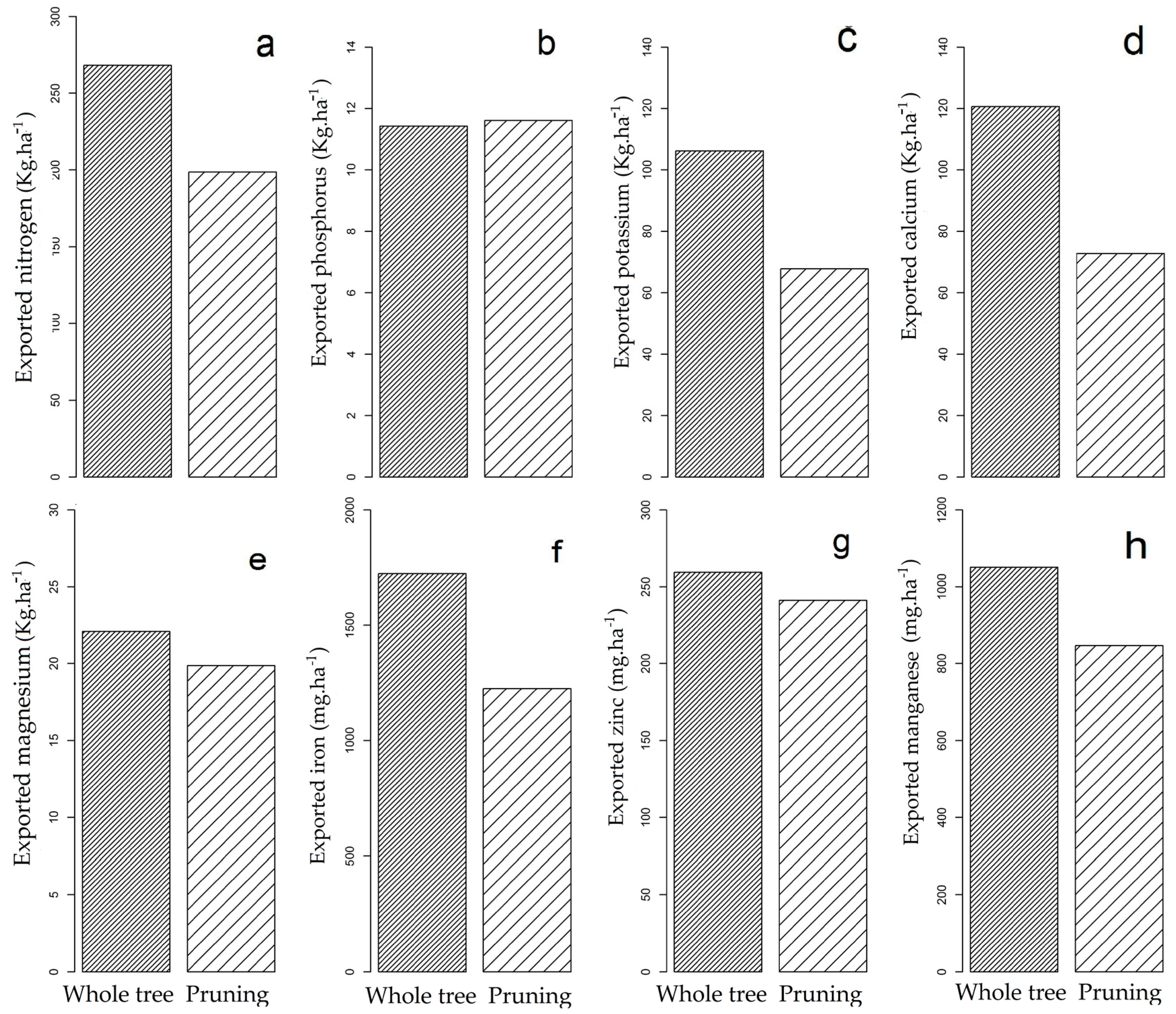
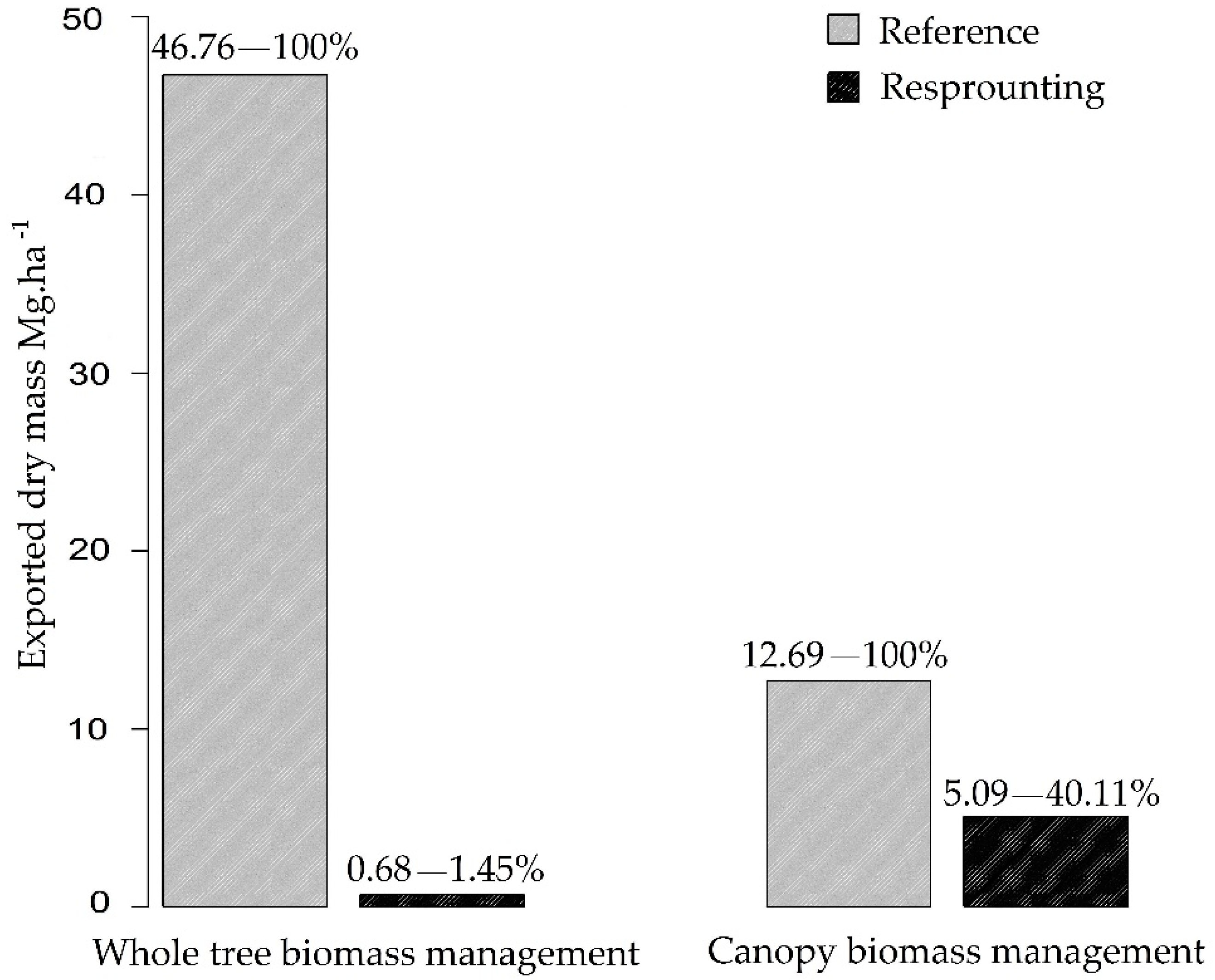
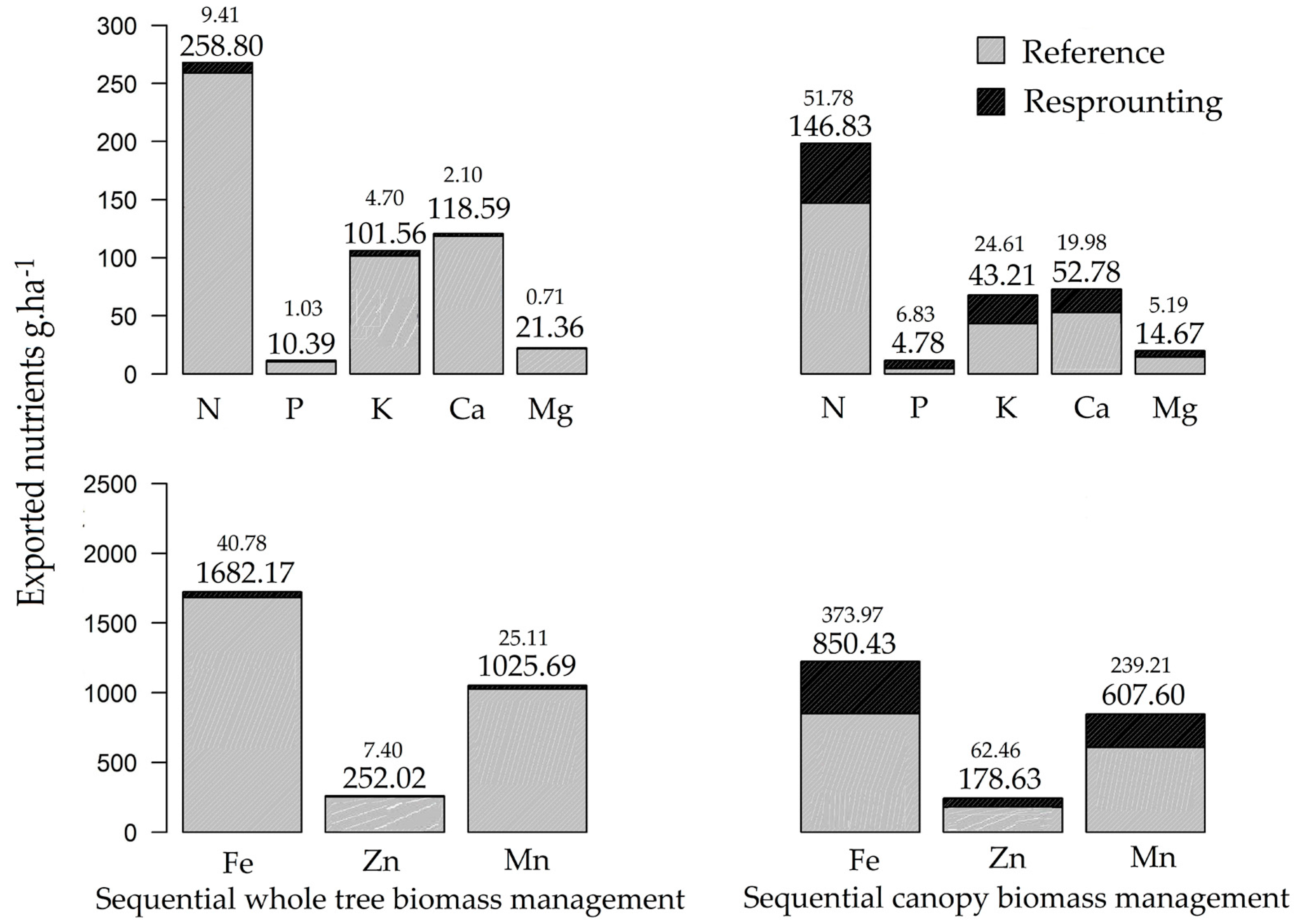
3.2. Nutrient Export during Sequential Management of Rosewood Plantations
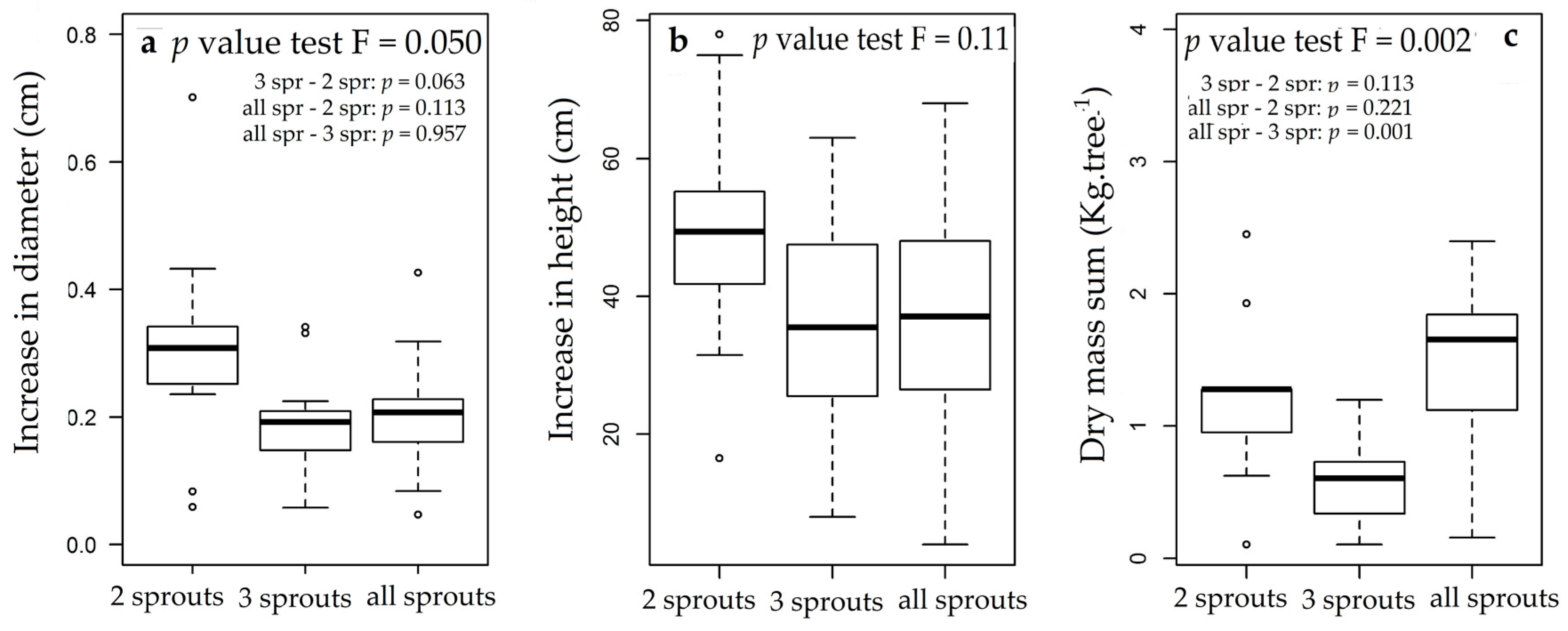
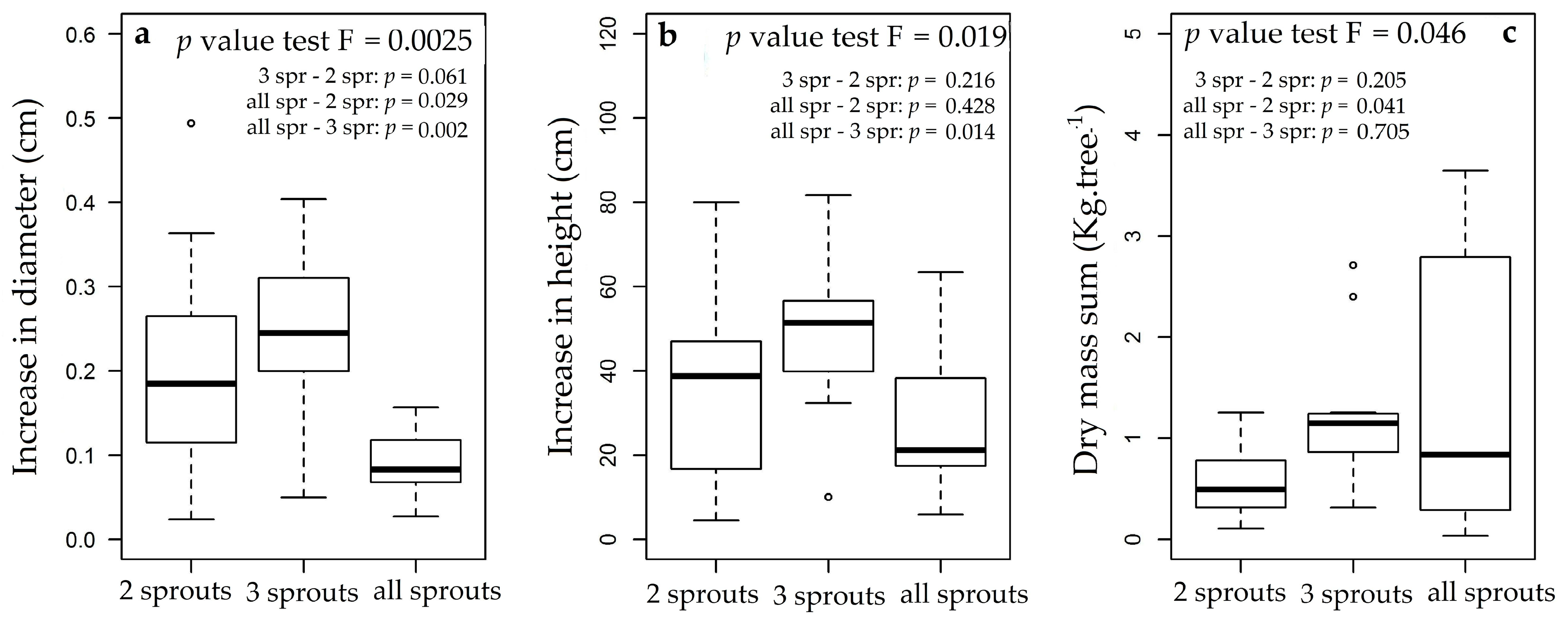
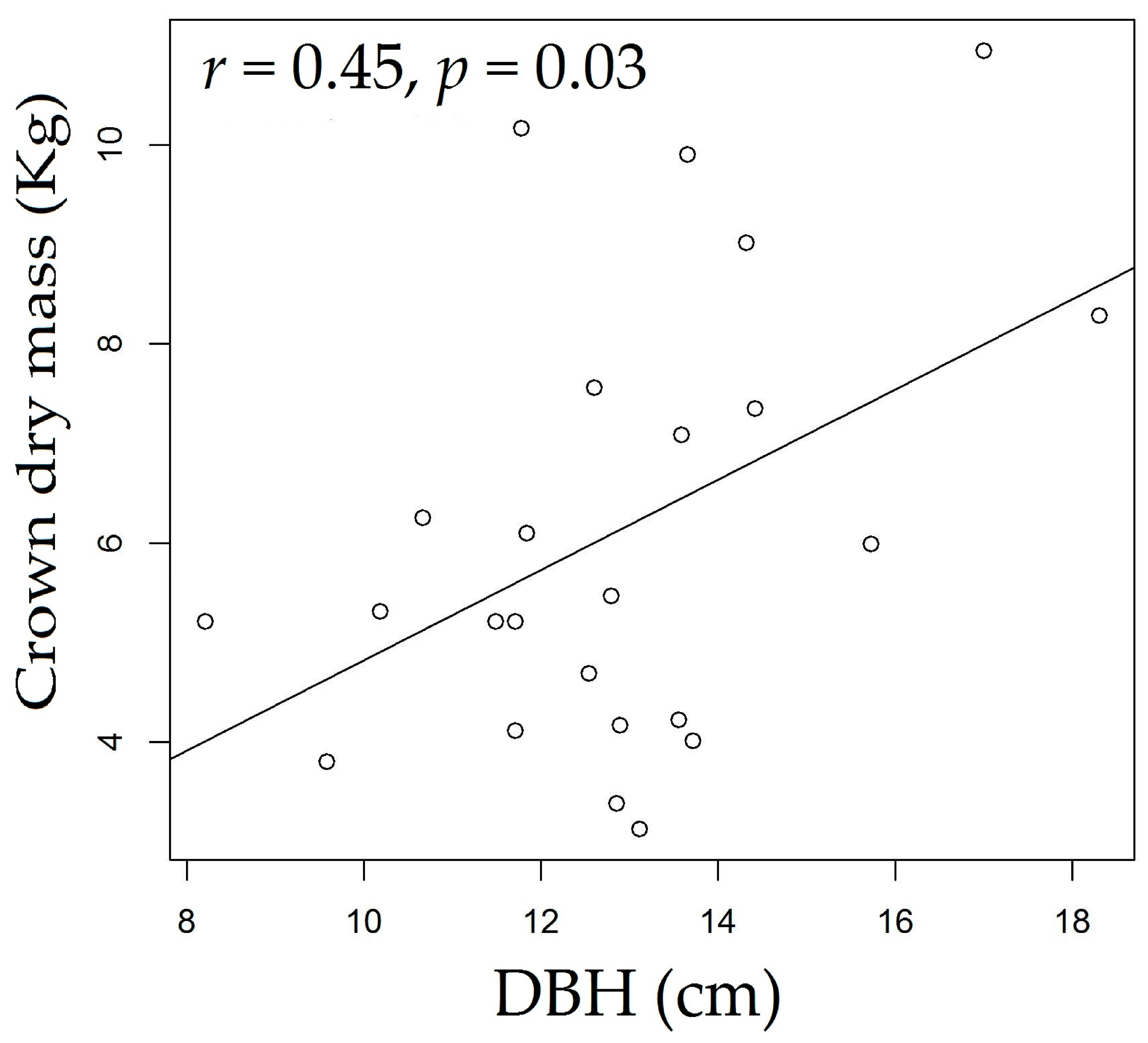
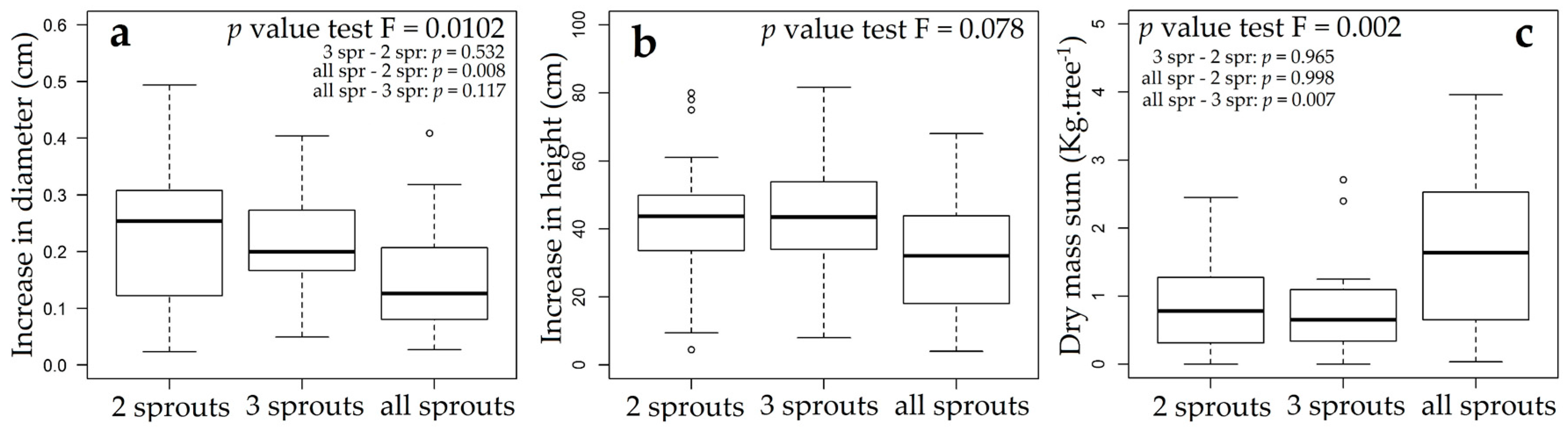
3.3. Rosewood Shoots Following Above-Ground Biomass Management
4. Discussion
4.1. Changes in Nutritional Status Associated with the Management of Rosewood Plantations
4.2. Nutrient Export during Sequential Management of Rosewood Plantations
4.3. Rosewood Shoots Following Above-Ground Biomass Management
4.4. Future Management of Rosewood Plantations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Soil Management System | Description: Cultivated Areas |
|---|---|
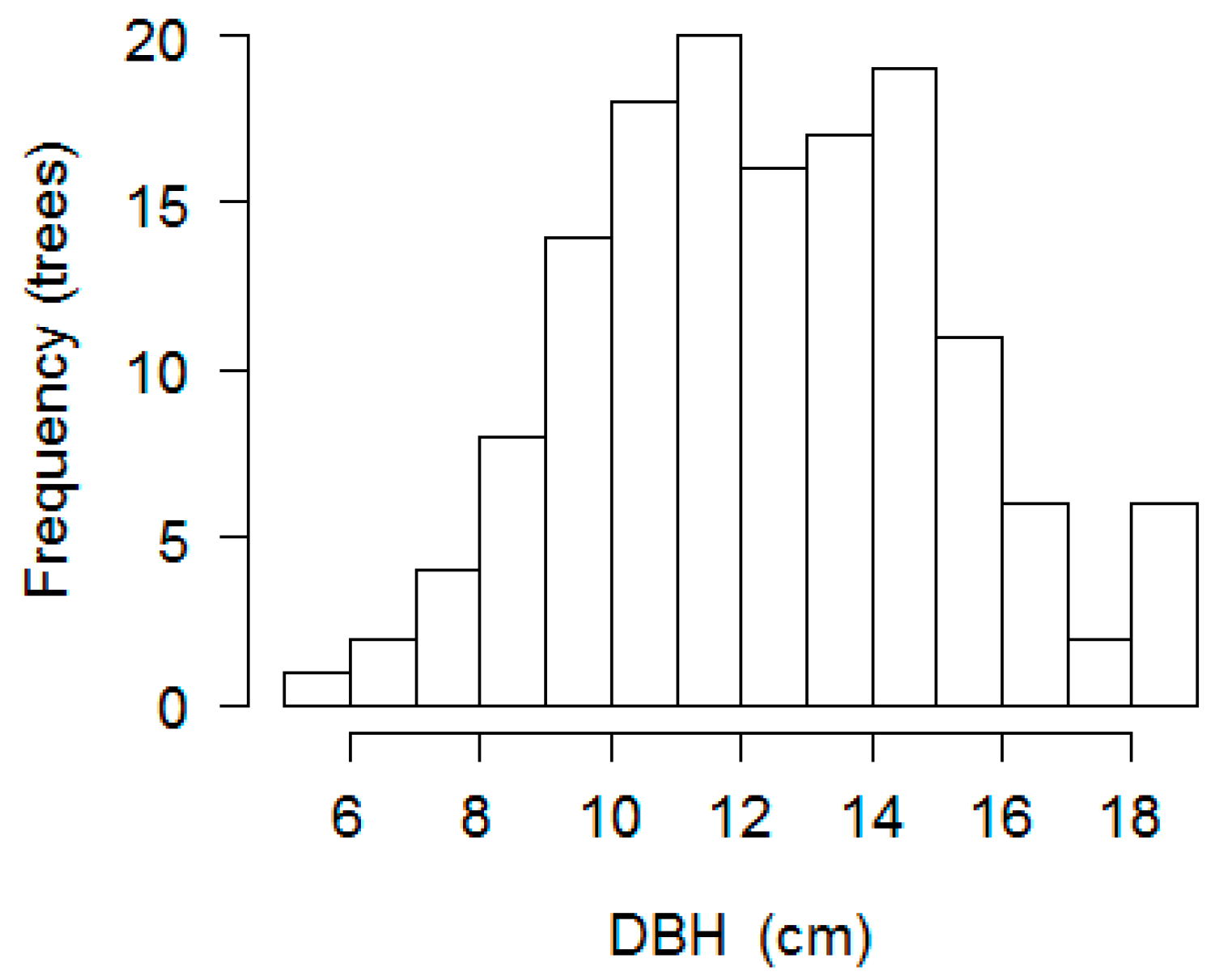
| Rosewood plantations (cultivated for ten years): C10. | Rosewood seedlings planted in 2005 with 3.0 × 4.0 m spacing, following cutting and burning of original vegetation. Individual rosewood plants had a mean AGFW of 102.47 ± 33.69 Kg, DBH of 13.88 ± 2.37 cm and H = 8.15± 1.39 m. (n = 36). Canopy percentage were 35.89 ± 6.91% and FF = 0.49 ± 0.061 |
| Rosewood plantations (cultivated for twelve years): C12. | Rosewood seedlings planted in 2003 with 1.5 × 2.0 m spacing, following cutting and burning of original vegetation. In 2010 following systematic thinning, spacing was 3.0 × 4.0 m. Individual rosewood plants had a mean AGFW of 89.89 ± 36.44 Kg, DBH of 12.80 ± 2.68 cm and H = 9.49 ± 1.13 m. (n = 36). Canopy percentage was 32.01 ± 8.06% and FF = 0.57 ± 0.053. |
| Rosewood plantations (cultivated for seventeen years): C17. | Rosewood seedlings planted in 1998 with 5.0 × 10 m spacing, following cutting and burning of original vegetation. Planting lines were maintained in the middle of naturally regenerating, occasionally cut, vegetation. Individual rosewood plants had AGFW of 96.02 ± 39.78 Kg, DBH of 12.7 ± 2.8 cm and H = 11.02 ± 1.97 m. (n = 36) Canopy percentage was 54.87 ± 17.99% and FF = 0.57 ± 0.106. Natural regeneration was uncontrolled and abundant. |
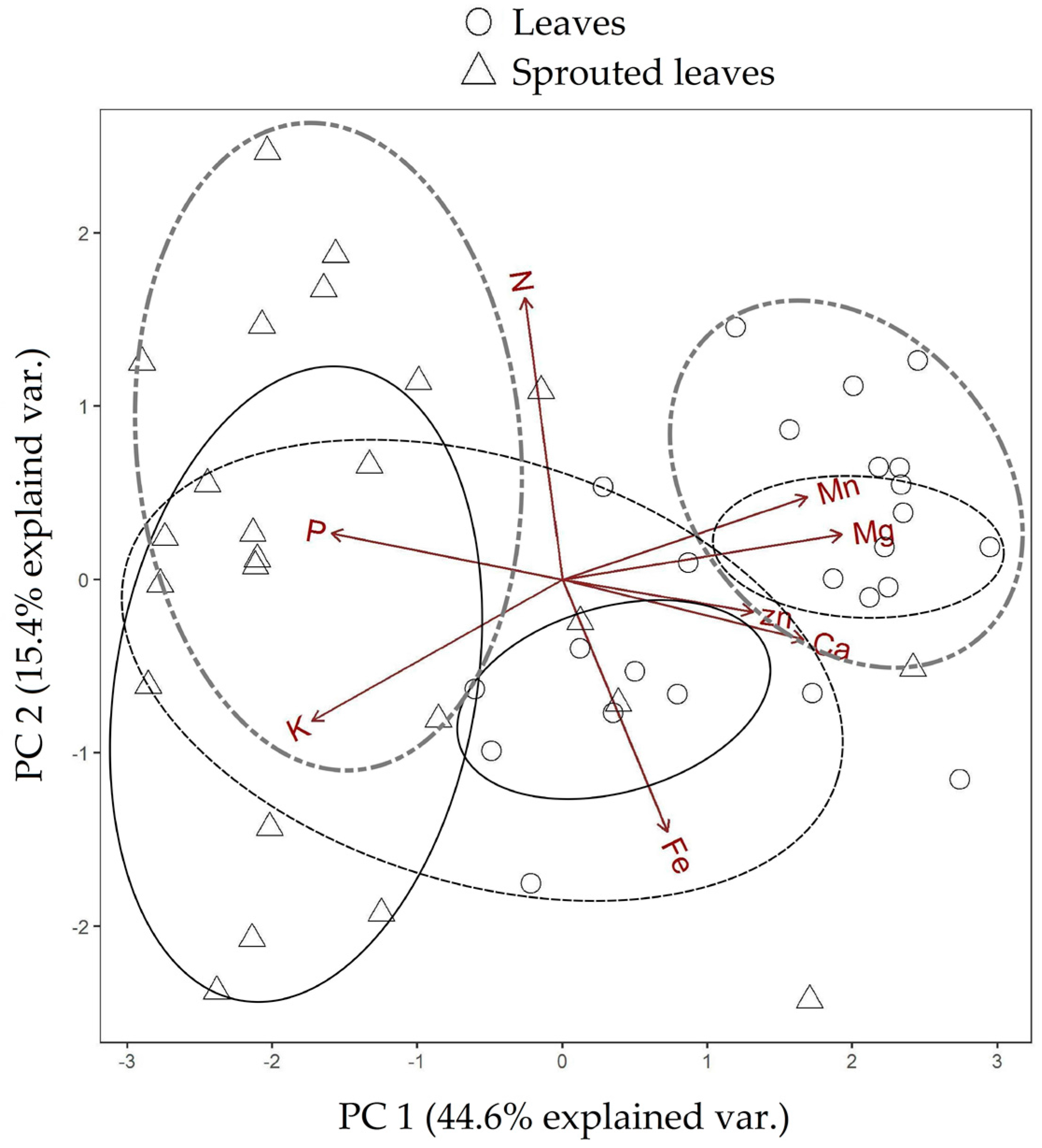
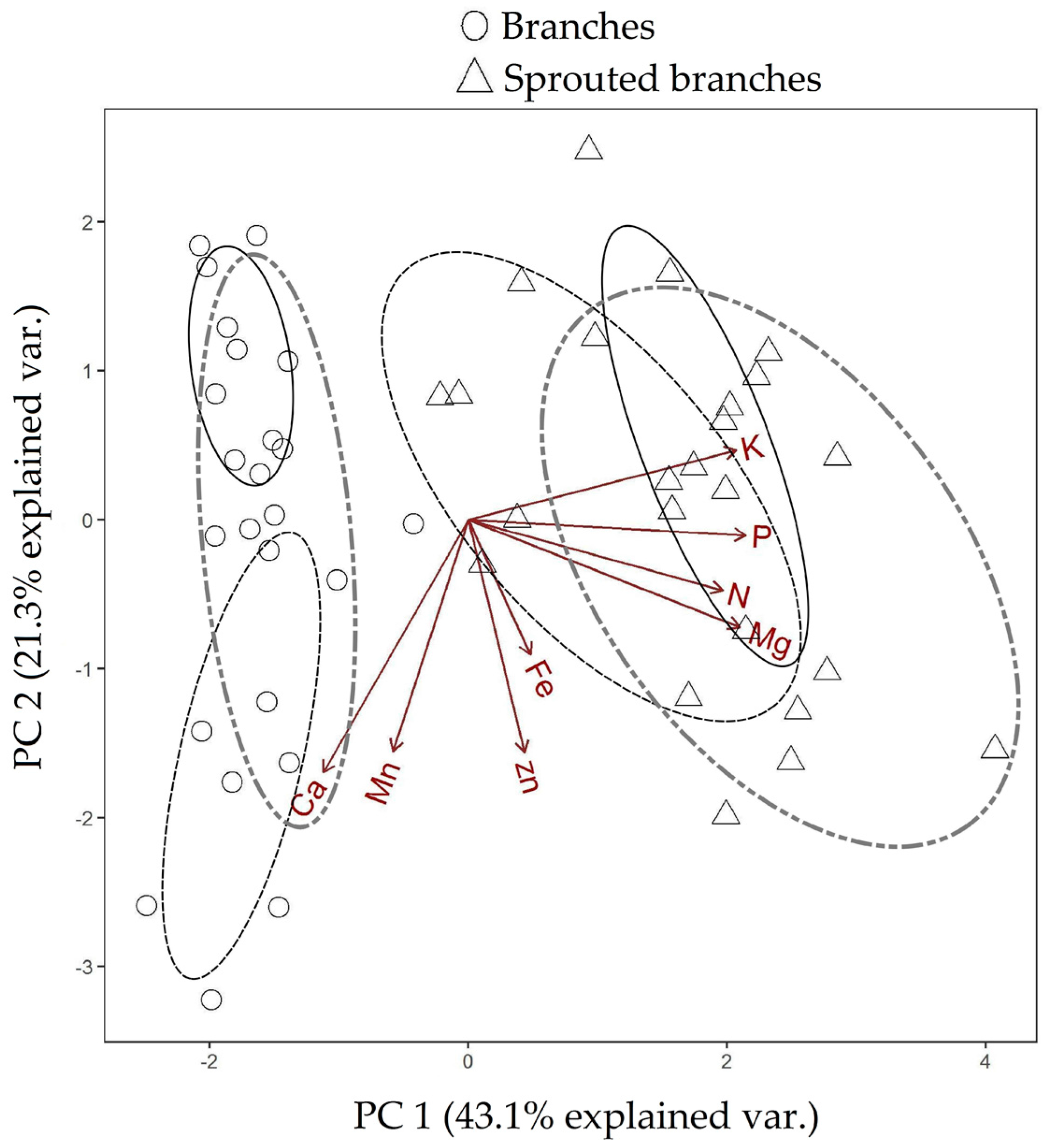
| Test Categories | p-Value PC 1 | p-Value PC 2 |
|---|---|---|
| Leaves 10-Sprouted leaves 10 | 0.043 | 0.006 |
| Leaves 12-Sprouted leaves 12 | 0.001 | 0.000 |
| Leaves 17-Sprouted leaves 17 | 0.064 | 0.000 |
| Branches 10-Sprouted branches 10 | 0.080 | 0.000 |
| Branches 12-Sprouted branches 12 | 0.054 | 0.000 |
| Branches 17-Sprouted branches 17 | 0.025 | 0.000 |


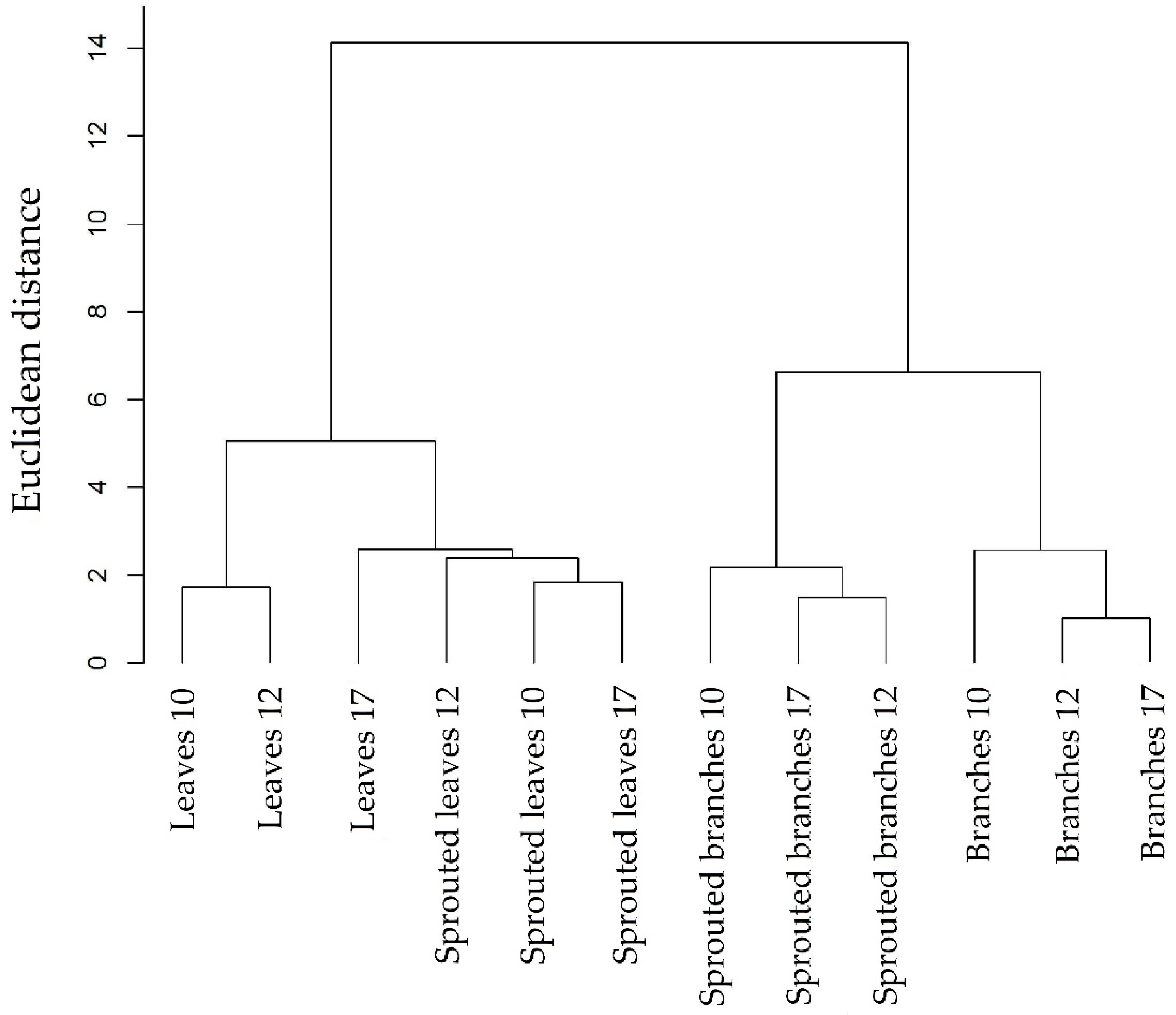

Appendix B







References
- Marques, C.A. A Importância Econômica Da Família Lauraceae Lindl. Floresta Ambient. 2001, 8, 195–206. [Google Scholar]
- Zanin, S.M.W.; Lordello, A.L.L. Alcalóides aporfinóides do gênero Ocotea (Lauraceae). Químic. Nova 2007, 30, 92–98. [Google Scholar] [CrossRef]
- Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). Portaria 06-N; Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis: Brasília, Brazil, 1992. [Google Scholar]
- The Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), Convention on International Trade in Endangered Species of Wild Fauna and Flora, Doha, Qatar, 18 March 2010. Available online: http://www.cites.org/esp/app/appendices.shtml (accessed on 15 November 2016).
- International Union for Conservation of Nature (IUCN). The IUCN Red List of Threatened Species, Version 2014.3 (IUCN, 2014). Available online: http://www.iucnredlist.org (accessed on 11 March 2015).
- Sampaio, P.T.B.; Barbosa, A.P.; Vieira, G.; Spironello, W.R.; Bruno, F.M.S. Biomassa da rebrota de copas de Rosewood (Aniba rosaeodora Ducke) em plantios sob sombra parcial em floresta primária. Acta Amazon. 2005, 35, 491–494. [Google Scholar] [CrossRef]
- Sampaio, P.T.B.; Santos, M.; Vieira, G.; Spironello, W.; Useche, F.L.; Bruno, F.M.S. Avaliação da rebrota da copa das árvores de pau-rosa (Aniba rosaeodora Ducke) em sistema de podas sucessivas. Acta Amazon. 2007, 37, 55–60. [Google Scholar] [CrossRef]
- McEwan, A.; Magagnotti, N.; Spinelli, R. The effects of number of stems per stool on cutting productivity in coppiced Eucalyptus plantations. Silva Fenn. 2016, 50. [Google Scholar] [CrossRef]
- Fredericksen, T.S.; Putz, F.E. Silvicultural intensification for tropical forest conservation. Biodivers. Conserv. 2003, 12, 1445–1453. [Google Scholar] [CrossRef]
- Secretaria de Estado do Meio Ambiente e Desenvolvimento Sustentável (SDS) 2006. Notícias: Semana do Meio Ambiente. Available online: http://www.sds.am.gov.br/noticia.php?xcod=2255 (accessed on 1 February 2015).
- Ministério do Meio Ambiente (MMA). Instrução Normativa MMA Nº 04; Ministério do Meio Ambiente: Brasília, Brazil, 2006.
- Ministério do Meio Ambiente (MMA). Instrução Normativa MMA Nº 05; Ministério do Meio Ambiente: Brasilia, Brazil, 2006.
- Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). Instrução Normativa IBAMA Nº 09; Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis: Brasilia, Brazil, 2011. [Google Scholar]
- May, P.H.; Barata, L.E. Rosewood exploitation in the Brazilian Amazon: Options for sustainable production. Econ. Bot. 2004, 58, 257–265. [Google Scholar] [CrossRef]
- Takeda, P.S. Avaliação de Biomassa e óleo da Rebrota de Galhos e Folhas de Rosewood (Aniba rosaeodora Ducke) em Plantios Comerciais Submetidos a Poda e Adubação. Master’s Dissertation, Instituto Nacional de Pesquisas da Amazônia/Universidade Federal Rural da Amazônia, Manaus, Brazil, 2008. (In Portuguese). [Google Scholar]
- Krainovic, P.M. Plantios de Pau-rosa (Aniba Rosaeodora Ducke) em Áreas com Histórico de Degradação por Atividades Agrícolas e Pecuárias. Master’s Dissertation, Instituto Nacional de Pesquisas da Amazônia, Manaus, Brazil, 2011. (In Portuguese). [Google Scholar]
- Krainovic, P.M.; Almeida, D.R.; Sampaio, P.T.B. New Allometric Equations to Support Sustainable Plantation Management of Rosewood (Aniba rosaeodora Ducke) in the Central Amazon. Forests 2017, 8, 327. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Couto, H.A.R.; Silva, A.C.M.D.; Marx, F.; Henke, C. Plant sources of Amazon rosewood oil. Químic. Nova 2007, 30, 1906–1910. [Google Scholar] [CrossRef]
- Chantraine, J.M.; Dhénin, J.M.; Moretti, C. Chemical variability of rosewood (Aniba rosaeodora Ducke) essential oil in French Guiana. J. Essent. Oil Res. 2009, 21, 486–495. [Google Scholar] [CrossRef]
- Fidelis, C.H.; Augusto, F.; Sampaio, P.T.; Krainovic, P.M.; Barata, L.E. Chemical characterization of rosewood (Aniba rosaeodora Ducke) leaf essential oil by comprehensive two-dimensional gas chromatography coupled with quadrupole mass spectrometry. J. Essent. Oil Res. 2012, 24, 245–251. [Google Scholar] [CrossRef]
- Fidelis, C.H.; Sampaio, P.T.; Krainovic, P.M.; Augusto, F.; Barata, L.E. Correlation between maturity of tree and GC × GC–qMS chemical profiles of essential oil from leaves of Aniba rosaeodora Ducke. Microchem. J. 2013, 109, 73–77. [Google Scholar] [CrossRef]
- Paul, M.; Catterall, C.P.; Pollard, P.C.; Kanowski, J. Recovery of soil properties and functions in different rainforest restoration pathways. For. Ecol. Manag. 2010, 259, 2083–2092. [Google Scholar] [CrossRef]
- Londero, E.K.; Schumacher, M.V.; Szymczak, D.A.; Viera, M. Exportação e reposição nutricional no primeiro desbaste de um povoamento de Pinus taeda L. em área de segunda rotação. Ciênc. Florest. 2011, 21. Available online: http://www.redalyc.org/articulo.oa?id=53420071009 (accessed on 15 January 2017). [CrossRef]
- Schumacher, M.V.; Witschoreck, R.; Calil, F.N.; Lopes, V.G. Biomassa e nutrientes no corte raso de um povoamento de Pinus taeda L. de 27 anos de idade em Cambará do Sul–RS. Ciênc. Florest. 2013, 23, 321–332. [Google Scholar] [CrossRef]
- Cram, S.; Sommer, I.; Fernández, P.; Galicia, L.; Ríos, C.; Barois, I. Soil natural capital modification through landuse and cover change in a tropical forest landscape: Implications for management. J. Trop. For. Sci. 2015, 27, 189–201. [Google Scholar]
- Barichello, R.; Schumacher, L.; Valdir, M.; Vogel, M.; Luiz, H. Quantificação da biomassa de um povoamento de Acacia mearnsii De Wild. NA região sul do Brasil. Ciênc. Florest. 2005, 15, 129–135. Available online: http://www.redalyc.org/articulo.oa?id=53415202 (accessed on 15 January 2017). [CrossRef]
- Lammel, D.R.; Nüsslein, K.; Tsai, S.M.; Cerri, C.C. Land use, soil and litter chemistry drive bacterial community structures in samples of the rainforest and Cerrado (Brazilian Savannah) biomes in Southern Amazonia. Eur. J. Soil Biol. 2015, 66, 32–39. [Google Scholar] [CrossRef]
- Nyland, R.D. Silviculture: Concepts and Applications; Waveland Press: New York, NY, USA, 2016; ISBN 1-7486-2714-X. [Google Scholar]
- Augusto, L.; Achat, D.L.; Bakker, M.R.; Bernier, F.; Bert, D.; Danjon, F.; Khlifa, R.; Meredieu, C.; Trichet, P. Biomass and nutrients in tree root systems-sustainable harvesting of an intensively managed Pinus pinaster (Ait.) planted forest. Glob. Chang. Biol. Bioenergy 2015, 7, 231–243. [Google Scholar] [CrossRef]
- Carneiro, M.A.C.; Souza, E.D.; Reis, E.F.; Pereira, H.S.; Azevedo, W.R. Atributos físicos, químicos e biológicos de solo de cerrado sob diferentes sistemas de uso e manejo. Rev. Brasil. Ciênc. Solo 2009, 33, 147–157. Available online: http://www.redalyc.org/articulo.oa?id=180214067016 (accessed on 5 February 2017). [CrossRef]
- Witschoreck, R.; Schumacher, M.V. Nutrient allocation in Eucalyptus saligna sm. Stands In The Region Of Guaíba-Rio Grande do Sul. Cerne 2015, 21, 625–632. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bradstock, R.A.; Keith, D.A.; Keeley, J.E. Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 2004, 85, 1085–1100. [Google Scholar] [CrossRef]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Davis, S.D. Towards understanding resprouting at the global scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, B.; Tormo, J.; Pausas, J.G. To resprout or not to resprout: Factors driving intraspecific variability in resprouting. Oikos 2012, 121, 1577–1584. [Google Scholar] [CrossRef]
- Vesk, P.A.; Westoby, M. Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 2004, 92, 310–320. [Google Scholar] [CrossRef]
- Ohashi, S.T.; dos Santos Rosa, L.; Mekdece, F.S. Influência do diâmetro e da altura de corte das cepas na brotação de Pau-rosa (Aniba rosaeodora Ducke). Rev. Ciênc. Agrár./Amazon. J. Agric. Environ. Sci. 2004, 41, 137–144. Available online: https://periodicos.ufra.edu.br/index.php/ajaes/article/view/2381 (accessed on 14 March 2017).
- Matula, R.; Svátek, M.; Kůrová, J.; Úradníček, L.; Kadavý, J.; Kneifl, M. The sprouting ability of the main tree species in Central European coppices: Implications for coppice restoration. Eur. J. For. Res. 2012, 131, 1501–1511. [Google Scholar] [CrossRef]
- Spinelli, R.; Pari, L.; Aminti, G.; Magagnotti, N.; Giovannelli, A. Mortality, re-sprouting vigor and physiology of coppice stumps after mechanized cutting. Ann. For. Sci. 2017, 74, 5. [Google Scholar] [CrossRef]
- Canullo, R.; Simonetti, E.; Cervellini, M.; Chelli, S.; Bartha, S.; Wellstein, C.; Campetella, G. Unravelling mechanisms of short-term vegetation dynamics in complex coppice forest systems. Folia Geobot. 2017, 1–11. [Google Scholar] [CrossRef]
- Moreira, B.; Pausas, J.G. Shedding light through the smoke on the germination of Mediterranean Basin flora. S. Afr. J. Bot. 2016. [Google Scholar] [CrossRef]
- Fleck, I.; Diaz, C.; Pascual, M.; Iniguez, J. Ecophysiological differences between first-year resprouts after wildfire and unburned vegetation of Arbutus unedo and Coriaria myrtifolia. Acta Oecol. 1995, 16, 55–69. [Google Scholar]
- Fang, X.; Li, J.; Xiong, Y.; Xu, D.; Fan, X.; Li, F. Responses of Caragana korshinskii Kom. to shoot removal: Mechanisms underlying regrowth. Ecol. Res. 2008, 23, 863–871. [Google Scholar] [CrossRef]
- Shibata, R.; Kurokawa, H.; Shibata, M.; Tanaka, H.; Iida, S.; Masaki, T.; Nakashizuka, T. Relationships between resprouting ability, species traits and resource allocation patterns in woody species in a temperate forest. Funct. Ecol. 2016, 30, 1205–1215. [Google Scholar] [CrossRef]
- Fittkau, E.J.; Irmler, U.; Junk, W.J.; Reiss, F.; Schmidt, G.W. Productivity, biomass, and population dynamics in Amazonian water bodies. In Tropical Ecological Systems; Springer: Berlin/Heidelberg, Germany, 1975; pp. 289–311. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- CLIMATE-DATA.ORG. Climate Data for World Cities. Electronic Publishing. 2016. Available online: http://en.climate-data.org/ (accessed on 2 December 2016).
- Tanaka, A.; Vieira, G. Autoecologia das espécies florestais em regime de plantio de enriquecimento em linha na floresta primária da Amazônia Central. Acta Amazon. 2006, 36, 193–204. [Google Scholar] [CrossRef]
- Lindell, L.; Åström, M.; Öberg, T. Land-use change versus natural controls on stream water chemistry in the Subandean Amazon, Peru. Appl. Geochem. 2010, 25, 485–495. [Google Scholar] [CrossRef]
- Silva, R.P. Alometria, Estoque e Dinâmica da Biomassa de Florestas Primárias e Secundárias na Região de Manaus (AM). Ph.D. Thesis, Universidade Federal do Amazonas, Manaus, Brazil, 2007; p. 152, (In Portuguese with English abstract). [Google Scholar]
- Embrapa. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Embrapa Informação Tecnológica; Embrapa Solos: Rio de Janeiro, Brazil, 2009. [Google Scholar]
- Malavolta, E. Manual de Calagem e Adubação das Principais Culturas; Agronômica Ceres: São Paulo, Brazil, 1987. [Google Scholar]
- Black, M.; Corbineau, F.; Grzesik, M.; Guyi, P.; Côme, D. Carbohydrate metabolism in the developing and maturing wheat embryo in relation to its desiccation tolerance. J. Exp. Bot. 1996, 47, 161–169. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smit, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Oliveira, I.A.D.; Campos, M.C.C.; Freitas, L.D.; Soares, M.D.R. Characterization of soils under different land uses in the southern region of the Amazonas. Acta Amazon. 2015, 45, 1–12. [Google Scholar] [CrossRef]
- Young, F.J.; Hammer, R.D. Defining geographic soil bodies by landscape position, soil taxonomy, and cluster analysis. Soil Sci. Soc. Am. J. 2000, 64, 989–998. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Pallardy, S.G. Physiology of Woody Plants; Academic Press: San Diego, CA, USA, 2010; ISBN 978-0-12-088765-1. [Google Scholar]
- Kabeya, D.; Sakai, S. The relative importance of carbohydrate and nitrogen for the resprouting ability of Quercus crispula seedlings. Ann. Bot. 2005, 96, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Moreira, B.; Tavsanoglu, Ç.; Pausas, J.G. Local versus regional intraspecific variability in regeneration traits. Oecologia 2012, 168, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zambrosi, F.C.B.; Ribeiro, R.V.; Machado, E.C.; Garcia, J.C. Phosphorus deficiency impairs shoot regrowth of sugarcane varieties. Exp. Agric. 2017, 53, 1–11. [Google Scholar] [CrossRef]
- Clemente, A.S.; Rego, F.C.; Correia, O.A. Growth, water relations and photosynthesis of seedlings and resprouts after fire. Acta Oecol. 2005, 27, 233–243. [Google Scholar] [CrossRef]
- Drake, P.L.; Mendham, D.S.; White, D.A.; Ogden, G.N. A comparison of growth, photosynthetic capacity and water stress in Eucalyptus globulus coppice regrowth and seedlings during early development. Tree Physiol. 2009, 29, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.F.D.C.; Barreto, D.C.D.S.; Santos Junior, U.M.D.; Fernandes, A.V.; Sampaio, P.D.T.B.; Buckeridge, M.S. Growth, photosynthesis and stress indicators in young rosewood plants (Aniba rosaeodora Ducke) under different light intensities. Braz. J. Plant Physiol. 2005, 17, 325–334. [Google Scholar] [CrossRef]
- DeSouza, J.; Silka, P.A.; Davis, S.D. Comparative physiology of burned and unburned Rhus laurina after chaparral wildfire. Oecologia 1986, 71, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Fageria, N.K. Soil chemical attributes of Amazonas state, Brazil. Commun. Soil Sci. Plant Anal. 2009, 40, 2912–2925. [Google Scholar] [CrossRef]
- Dechen, A.R.; Nachigall, G.R. Elementos requeridos à nutrição de plantas. In Fertilidade do Solo; Novais, R.F., Alvarez, V.V.H., Barros, N.F., Fonte, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; SBCS/UFV: Viçosa, Brazil, 2007; pp. 92–132. [Google Scholar]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 4th ed.; Artmed: Porto Alegre, Rio grande do Sul, Brazil, 2008; p. 820. [Google Scholar]
- Ferreira, S.J.F.; Mello-Ivo, W.; Biot, Y.; Luizão, F.J.; Ross, S.M. Propriedades físicas do solo após extração seletiva de madeira na Amazônia Central. Acta Amazon. 2002. [Google Scholar] [CrossRef]
- Van Raij, B. Fertilidade do Solo e Adubação; Associação Brasileira para Pesquisa da Potassa e do Fosfato: Piracicaba, Brasil, 1991. [Google Scholar]
- Vesk, P.A.; Westoby, M. Funding the bud bank: A review of the costs of buds. Oikos 2004, 106, 200–208. [Google Scholar] [CrossRef]
- Kauppi, A.; Kiviniitty, M.; Ferm, A. Growth habits and crown architecture of Betula pubescens Ehrh. of seed and sprout origin. Can. J. For. Res. 1988, 18, 1603–1613. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Oliveira, C.H.R.D. Produção de Eucalipto em alto Fuste e Talhadia e de Braquiária em Sistemas Silvipastoris com Diferentes Arranjos Espaciais. Ph.D. Thesis, Universidade Federal de Viçosa, Minas Gerais, Brazil, 2014. [Google Scholar]
- Kays, J.S.; Canham, C.D. Effects of time and frequency of cutting on hardwood root reserves and sprout growth. For. Sci. 1991, 37, 524–539. [Google Scholar]
- Borzak, C.L.; Potts, B.M.; O’Reilly-Wapstra, J.M. Survival and recovery of Eucalyptus globulus seedlings from severe defoliation. For. Ecol. Manag. 2016, 379, 243–251. [Google Scholar] [CrossRef]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, Ç.; Lloret, F.; Buhk, C.; Espelta, J.M. Fire-related traits for plant species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef]
- Loescher, W.H.; McCamant, T.; Keller, J.D. Carbohydrate reserves, translocation, and storage in woody plant roots. HortScience 1990, 25, 274–281. [Google Scholar]
- Vesk, P.A. Plant size and resprouting ability: Trading tolerance and avoidance of damage? J. Ecol. 2006, 94, 1027–1034. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, J.J. The evolutionary ecology of sprouting in woody plants. Int. J. Plant Sci. 2003, 164, S103–S114. [Google Scholar] [CrossRef]
- Sparrow, A.D.; Bellingham, P.J. More to resprouting than fire. Oikos 2001, 94, 195–198. [Google Scholar] [CrossRef]
- Ministério do Meio Ambiente (MMA). Portaria MMA Nº 443; Ministério do Meio Ambiente: Brasília, Brazil, 2014.
- Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). Lei Federal n° 12.651; de 25 de maio de 2012, alterada pela Lei 12.727, de 17 de outubro de 2012; Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis: Brasília, Brazil, 2012. [Google Scholar]
- Homma, A.K.O. Amazônia: Como aproveitar os benefícios da destruição? Estudos Avançados 2005, 19, 115–135. [Google Scholar] [CrossRef]
| Cultivated Areas | Harvest | N | P | K | Ca | Mg | Fe | Zn | Mn |
|---|---|---|---|---|---|---|---|---|---|
| (%) | g kg−1 | mg kg−1 | |||||||
| Leaves | |||||||||
| 10 years old | 1° | 1.68 | 0.43 | 3.44 | 5.60 | 1.92 | 75.16 | 19.11 | 60.63 |
| 2° | 1.51 | 1.26 | 6.54 | 5.28 | 1.31 | 68.75 | 14.63 | 49.13 | |
| 12 years old | 1° | 1.70 | 0.44 | 3.14 | 5.28 | 2.07 | 74.31 | 14.68 | 69.28 |
| 2° | 1.75 | 1.38 | 6.71 | 3.77 | 1.44 | 64.75 | 11.00 | 53.75 | |
| 17 years old | 1° | 1.50 | 0.33 | 7.49 | 4.40 | 1.76 | 75.65 | 13.48 | 55.53 |
| 2° | 1.67 | 1.13 | 9.57 | 4.36 | 1.32 | 73.13 | 15.13 | 32.63 | |
| Branches | |||||||||
| 10 years old | 1° | 0.61 | 0.34 | 1.79 | 3.52 | 0.41 | 62.92 | 14.09 | 39.30 |
| 2° | 0.79 | 1.65 | 5.23 | 2.85 | 0.64 | 61.13 | 9.88 | 23.38 | |
| 12 years old | 1° | 0.65 | 0.29 | 1.38 | 2.71 | 0.42 | 55.78 | 9.94 | 33.89 |
| 2° | 1.02 | 1.65 | 6.34 | 2.03 | 0.82 | 67.50 | 11.13 | 33.50 | |
| 17 years old | 1° | 0.48 | 0.15 | 3.03 | 2.41 | 0.32 | 54.02 | 9.42 | 25.06 |
| 2° | 0.81 | 1.17 | 8.76 | 1.80 | 0.65 | 62.63 | 13.50 | 21.88 | |
| Stem | |||||||||
| 10 years old | 1° | 0.34 | 0.19 | 1.34 | 2.08 | 0.15 | 27.78 | 2.50 | 10.33 |
| 12 years old | 1° | 0.28 | 0.13 | 0.89 | 1.53 | 0.14 | 18.33 | 0.33 | 9.33 |
| 17 years old | 1° | 0.28 | 0.09 | 1.47 | 1.97 | 0.15 | 38.33 | 1.11 | 15.78 |
| Nutrient Sources | Whole Tree * | Harvest ** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1° | 2° | 1° | 2° | ||||||
| (a) | (b) | (a) | (b) | (a) | (b) | (a) | (b) | ||
| N (Kg ha−1) | Ureia (45% N) | 575 | - | 20.9 | - | 326 | - | 115 | - |
| Ammonium Sulphate (a 21% N) + (b 21% S) | 1.23 exp3 | 259 | 44.8 | 9.41 | 699 | 147 | 247 | 51.8 | |
| Salitre (16% N) | 1.62 exp3 | - | 58.8 | - | 917 | - | 324 | - | |
| Sawdust (2% N) | 1.29 exp4 | - | 470 | - | 7.54 exp3 | - | 2.59 exp3 | - | |
| P (Kg ha−1) | Super Triple Phosphate (a 42% P) + (b 11% Ca) | 24.7 | 2.72 | 2.45 | 0.269 | 11.38 | 1.25 | 16.3 | 1.79 |
| Super Phosphate Simple (a 19% P) + (b 18% Ca) | 54.7 | 9.84 | 5.42 | 0.975 | 25.16 | 4.53 | 35.9 | 6.47 | |
| Monoamonium Phosphate (a 46% P) + (b 10% N) | 22.6 | 2.25 | 2.24 | 0.223 | 10.39 | 1.04 | 14.8 | 1.49 | |
| Natural Araxá Phosphate (36% P) | 28.9 | - | 2.86 | - | 13.28 | - | 18.9 | - | |
| Pottasium Chloride (60% K) | 169 | - | 7.83 | - | 72.0 | - | 41.0 | - | |
| K (Kg ha−1) | Potassium Sulphate (a 50% K) + (b 18% S) | 203 | 36.6 | 9.40 | 1.69 | 86.4 | 15.56 | 49.2 | 8.86 |
| Potassium Nitrate (46% K) + (16% N) | 221 | 35.3 | 10.2 | 1.63 | 93.9 | 15.03 | 53.5 | 8.56 | |
| Ca (Kg ha−1) | Dolomitic Limestone PRNT > 80% (a 20% Ca) + (b 11% Mg) | 593 | 65.2 | 10.5 | 1.16 | 264 | 29.03 | 99.8 | 11.0 |
| Mg (Kg ha−1) | Magnesium Sulphate (a 16% Mg + (b 22 % S) | 133 | 29.4 | 4.44 | 0.98 | 91.7 | 20.17 | 32.4 | 7.14 |
| Fe (g ha−1) | Iron Sulphate (a 19% Fe + b 11% S) | 8.75 | 0.96 | 0.21 | 0.02 | 4.47 | 0.49 | 1.96 | 0.22 |
| Zn (g ha−1) | Zinc Sulphate (a 20% Zn + b 11% S) | 1.26 | 0.14 | 0.04 | 0.00 | 0.89 | 0.10 | 0.31 | 0.03 |
| Mn (g ha−1) | Magnesium Sulphate (a 26% Mn + b 14 % S) | 3.94 | 0.55 | 0.10 | 0.01 | 2.33 | 0.33 | 0.92 | 0.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krainovic, P.M.; Almeida, D.R.A.d.; Desconci, D.; Veiga-Júnior, V.F.d.; Sampaio, P.D.T.B. Sequential Management of Commercial Rosewood (Aniba rosaeodora Ducke) Plantations in Central Amazonia: Seeking Sustainable Models for Essential Oil Production. Forests 2017, 8, 438. https://doi.org/10.3390/f8120438
Krainovic PM, Almeida DRAd, Desconci D, Veiga-Júnior VFd, Sampaio PDTB. Sequential Management of Commercial Rosewood (Aniba rosaeodora Ducke) Plantations in Central Amazonia: Seeking Sustainable Models for Essential Oil Production. Forests. 2017; 8(12):438. https://doi.org/10.3390/f8120438
Chicago/Turabian StyleKrainovic, Pedro Medrado, Danilo Roberti Alves de Almeida, Diego Desconci, Valdir Florêncio da Veiga-Júnior, and Paulo De Tarso Barbosa Sampaio. 2017. "Sequential Management of Commercial Rosewood (Aniba rosaeodora Ducke) Plantations in Central Amazonia: Seeking Sustainable Models for Essential Oil Production" Forests 8, no. 12: 438. https://doi.org/10.3390/f8120438





