Growth, Physiological, Biochemical, and Ionic Responses of Morus alba L. Seedlings to Various Salinity Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Salt Treatments
2.2. Salt Treatment and Determination of Salt Content
2.3. Plant Harvest and Determination of Growth Parameters
2.4. Measurement of Photosynthetic Gas Exchange and Chlorophyll Fluorescence Parameters
2.5. Analysis of Soluble Sugars, Soluble Proteins, and Proline
2.6. Measurement of Antioxidant Enzyme Activities
2.7. Malondialdehyde Concentration and Cell Membrane Stability
2.8. Determination of the Sodium (Na), Potassium (K), Magnesium (Mg), and Calcium (Ca) Contents of Various Organs
2.9. Osmotic Potential
2.10. Statistics
3. Results
3.1. Growth under Increasing Levels of Soil Salinity
3.2. Gas Exchange and Chlorophyll Fluorescence Parameters
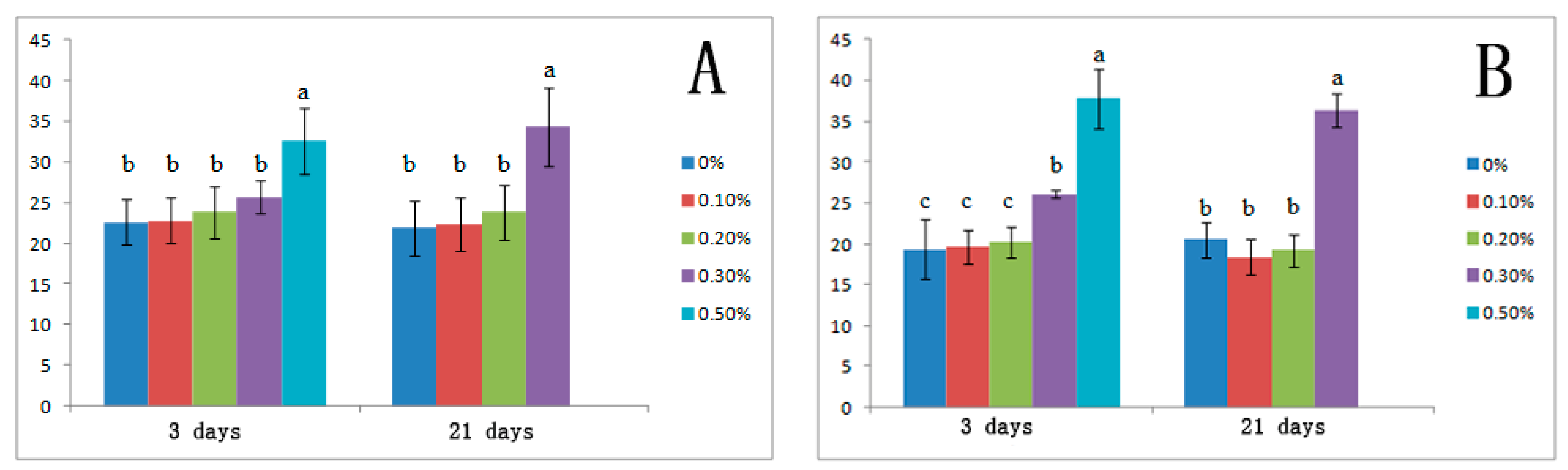
3.3. Effects of Salt Stress on Organic Solutes and Osmotic Potential
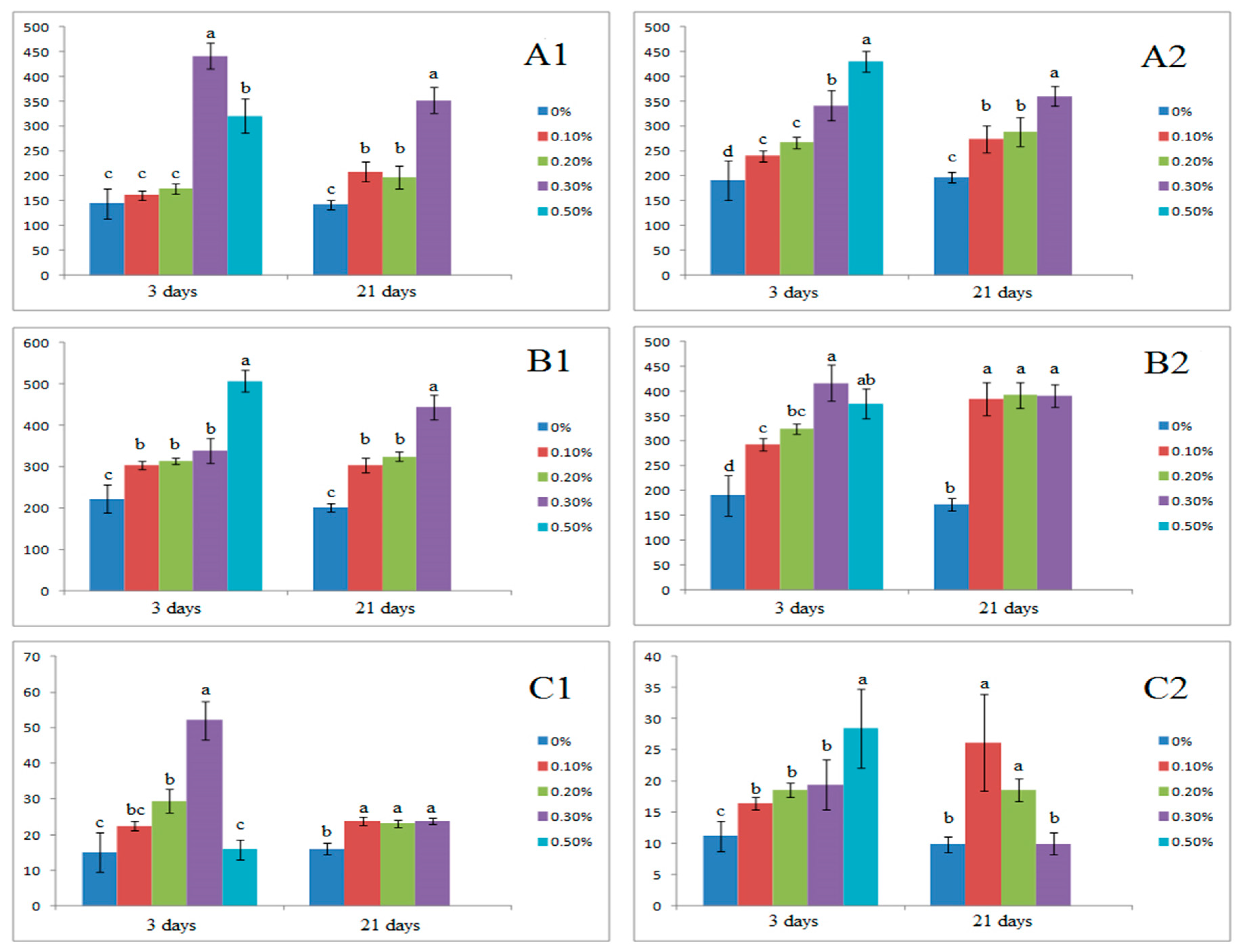
3.4. Effect of Salinity on Inorganic Ion Balance
3.5. Cell Membrane Stability and Malondialdehyde (MDA) Content
3.6. Antioxidant Enzyme Activities
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oba, G.; Nordal, I.; Stenseth, N.C.; Stave, J.; Bjorå, C.S.; Muthondeki, J.K.; Bii, W.K.A. Growth performance of exotic and indigenous tree species in saline soils in Turkana, Kenya. J. Arid Environ. 2001, 47, 499–511. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Ali, Q. Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ. Exp. Bot. 2008, 63, 266–273. [Google Scholar] [CrossRef]
- Hauser, F.; Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shi, D.; Wang, D. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Vaidyanathan, H.; Sivakumar, P.; Chakrabarty, R.; Thomas, G. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.)—Differential response in salt-tolerant and sensitive varieties. Plant Sci. 2003, 165, 1411–1418. [Google Scholar]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Yu, C.; Huang, S.; Hu, X.; Deng, W.; Xiong, C.; Ye, C.; Li, Y.; Peng, B. Changes in photosynthesis, chlorophyll fluorescence, and antioxidant enzymes of mulberry (Morus ssp.) in response to salinity and high-temperature stress. Biologia 2013, 68, 404–413. [Google Scholar]
- Vijayan, K.; Doss, S.G.; Chakraborti, S.P.; Ghosh, P.D. Breeding for salinity resistance in mulberry (Morus spp.). Euphytica 2009, 69, 403–411. [Google Scholar] [CrossRef]
- Kumar, S.G.; Lakshmi, A.; Madhusudhan, K.V.; Ramanjulu, S.; Sudhakar, C. Photosynthesis parameters in two cultivars of mulberry differing in salt tolerance. Photosynthetica 2000, 36, 611–616. [Google Scholar] [CrossRef]
- Gai, Y.P.; Ji, X.L.; Mu, Z.M.; Liu, X.L.; Wang, H.L. Effect of NaCl stress on superoxide dismutase and catalase of mulberry. Canye Kexue 2006, 32, 99–102. [Google Scholar]
- Claes, B.; Dekeyser, R.; Villarroel, R.; Van den Bulcke, M.; Bauw, G.; Van Montagu, M.; Caplan, A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell 1990, 2, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tammam, A.A.; Alhamd, M.A.; Hemeda, M.M. Study of salt tolerance in wheat (Triticum aestivum L.) cultivar Banysoif 1. Aust. J. Crop Sci. 2008, 1, 115–125. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Magné, C.; Saladin, G.; Clément, C. Transient effect of the herbicide flazasulfuron on carbohydrate physiology in Vitis vinifera L. Chemosphere 2006, 62, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Becana, M.; Aparicio-Tejo, P.; Irigoyen, J.J.; Sanchez-Diaz, M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Phys. 1986, 82, 1169–1171. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Civello, P.M.; Martinez, G.A.; Chaves, A.R.; Anon, M.C. Peroxidase from strawberry fruit (Fragaria ananassa Duch.): Partial purification and determination of some properties. J. Agric. Food Chem. 1995, 43, 2596–2601. [Google Scholar]
- Quan, R.D.; Shang, M.; Zhang, H.; Zhao, Y.X.; Zhang, J.R. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]
- Zwiazek, J.J.; Blake, T.J. Early detection of membrane injury in black spruce (Picea mariana). Can. J. For. Res. 1991, 21, 401–404. [Google Scholar] [CrossRef]
- Li, D.Q.; Zou, Q.; Cheng, B.S.; Sun, G.Y. Determining the osmotic potential of plant cell by cryoscopic method. Shandong Agric. Sci. 1988, 1, 43–45. [Google Scholar]
- Harinasut, P.; Poonsopa, D.; Roengmongkol, K.; Charoensataporn, R. Salinity effects on antioxidant enzymes in mulberry cultivar. Sci. Asia 2003, 29, 109–113. [Google Scholar] [CrossRef]
- Hamed, K.B.; Chibani, F.; Abdelly, C.; Magne, C. Growth, sodium uptake and antioxidant responses of coastal plants differing in their ecological status under increasing salinity. Biologia 2014, 69, 193–201. [Google Scholar]
- Hajiboland, R.; Norouzi, F.; Poschenrieder, C. Growth, physiological, biochemical and ionic responses of pistachio seedlings to mild and high salinity. Trees 2014, 28, 1065–1078. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Stanton, D.E.; Schmitz, N.; Farquhar, G.D.; Ball, M.C. Growth responses of the mangrove Avicennia marina to salinity: Development and function of shoot hydraulic systems require saline conditions. Ann. Bot. 2015, 115, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, M.; Syvertsen, J.P. Gas exchange, chlorophyll and nutrient contents in relation to Na+ and Cl− accumulation in ‘Sunburst’ mandarin grafted on different rootstocks. Plant Sci. 2002, 162, 705–712. [Google Scholar]
- Seemann, J.R.; Critchley, C. Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 1985, 164, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.S.; Ashraf, M. Exogenous application of potassium dihydrogen phosphate can alleviate the adverse effects of salt stress on sunflower. J. Plant Nutr. 2011, 34, 1041–1057. [Google Scholar] [CrossRef]
- Agastian, P.; Kingsley, S.J.; Vivekanandan, M. Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 2000, 38, 287–290. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [PubMed]
- Bompy, F.; Lequeue, G.; Imbert, D.; Dulormne, M. Increasing fluctuations of soil salinity affect seedling growth performances and physiology in three Neotropical mangrove species. Plant Soil 2014, 380, 399–413. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Uprety, D.C.; Sarin, M.N. Physiological studies on salt tolerance in Pisum sativum L. iV. tonic composition and nitrogen metabolism. Acta Agron Budap 1976, 25, 455–460. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Mandhania, S.; Madan, S.; Sawhney, V. Antioxidant defense mechanism under salt stress in wheat seedlings. Biol. Plant. 2006, 50, 227–231. [Google Scholar] [CrossRef]
- Demiral, T.; Turkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Sharma, S. Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ. J. Plant Physiol. 2010, 57, 509–517. [Google Scholar] [CrossRef]
- Ahmad, P.; Ozturk, M.; Sharma, S.; Gucel, S. Effect of sodium carbonate-induced salinity–alkalinity on some key osmoprotectants, protein profile, antioxidant enzymes, and lipid peroxidation in two mulberry (Morus alba L.) cultivars. J. Plant Interact. 2012, 9, 460–467. [Google Scholar] [CrossRef]

| Treatment (%) | New Root (DW) | New Shoot (DW) | New Leaf (DW) | Root: Shoot Ratios | Leaf Number Increment | Average Leaf Area Increment | Total Leaf Area Increment |
| Control | 1.83 ± 0.23a | 2.34 ± 0.35a | 2.83 ± 0.80a | 0.35 ± 0.10a | 13.70 ± 4.80a | 2.79 ± 1.28ab | 205.50 ± 77.94a |
| 0.1 | 1.55 ± 0.16b | 1.86 ± 0.41ab | 2.61 ± 0.32a | 0.35 ± 0.06a | 11.00 ± 3.95ab | 4.78 ± 1.94a | 197.15 ± 53.41a |
| 0.2 | 0.36 ± 0.15c | 1.37 ± 0.07b | 1.52 ± 0.17b | 0.26 ± 0.07b | 8.00 ± 6.10ab | 2.63 ± 1.24ab | 122.92 ± 106.87ab |
| 0.3 | 0.15 ± 0.09d | 0.68 ± 0.04c | 1.24 ± 0.34b | 0.13 ± 0.05c | 8.60 ± 5.82ab | 2.01 ± 0.49bc | 115.15 ± 42.34ab |
| 0.5 | 0.13 ± 0.08d | 0.36 ± 0.08d | 0.68 ± 0.36c | 0.07 ± 0.03d | 7.50 ± 0.84ab | 1.89 ± 0.09bc | 89.18 ± 6.74b |
| Treatment (%) | Lateral Root Number | Lateral Root Length (1st longest) | Average Lateral Root Length | Lateral Root Diameter (1st biggest) | Average Lateral Root Diameter | Total Shoot Length | Average Shoot Length |
| Control | 47.50 ± 13.13a | 31.20 ± 8.09a | 20.08 ± 6.05a | 1.65 ± 0.36a | 1.18 ± 0.14a | 34.63 ± 9.25a | 9.90 ± 3.23a |
| 0.1 | 40.00 ± 8.91a | 32.25 ± 7.85a | 22.00 ± 6.65a | 1.88 ± 0.85a | 1.26 ± 0.46a | 21.54 ± 6.25ab | 7.72 ± 2.45ab |
| 0.2 | 32.75 ± 13.23a | 23.75 ± 1.71ab | 15.38 ± 1.60b | 0.93 ± 0.20ab | 0.65 ± 0.09b | 14.87 ± 6.13b | 5.13 ± 3.10bc |
| 0.3 | 19.25 ± 2.22b | 13.95 ± 2.89b | 8.38 ± 3.40b | 0.69 ± 0.19b | 0.48 ± 0.17b | 16.35 ± 5.12b | 4.94 ± 2.41bc |
| 0.5 | 13.42 ± 0.35b | 10.34 ± 3.78b | 7.98 ± 4.78b | 0.54 ± 0.07b | 0.39 ± 0.11b | 10.53 ± 6.48ab | 2.58 ± 1.42c |
| Treatment (%) | Pn | Gs | Tr | Ci | iWUE |
|---|---|---|---|---|---|
| 3 days | |||||
| 0 | 10.79 ± 4.10a | 0.14 ± 0.07a | 2.37 ± 1.02a | 228.35 ± 21.29ab | 4.63 ± 0.52a |
| 0.1 | 10.55 ± 3.10a | 0.11 ± 0.04a | 2.63 ± 0.8a | 206.12 ± 40.50b | 4.10 ± 0.85a |
| 0.2 | 8.32 ± 3.12ab | 0.11 ± 0.05a | 2.22 ± 0.41a | 214.54 ± 28.22ab | 3.75 ± 0.88ab |
| 0.3 | 5.72 ± 4.16b | 0.05 ± 0.02b | 1.41 ± 0.56b | 224.27 ± 88.32ab | 3.55 ± 2.06ab |
| 0.5 | 1.09 ± 1.03c | 0.02 ± 0.02c | 0.67 ± 0.38b | 229.34 ± 87.85a | 1.30 ± 2.96b |
| 21 days | |||||
| 0 | 9.28 ± 2.47a | 0.17 ± 0.07a | 1.50 ± 0.52a | 280.80 ± 22.17a | 6.38 ± 1.10b |
| 0.1 | 11.08 ± 2.04a | 0.17 ± 0.07a | 1.57 ± 0.47a | 262.00 ± 29.21a | 7.38 ± 1.47a |
| 0.2 | 8.52 ± 2.08ab | 0.13 ± 0.05a | 1.28 ± 0.52a | 258.90 ± 23.54a | 6.65 ± 1.24b |
| 0.3 | 6.10 ± 1.80b | 0.11 ± 0.05a | 1.14 ± 0.43a | 290.75 ± 21.16a | 5.58 ± 1.06c |
| 0.5 | - | - | - | - | - |
| Treatment | Fv/Fm | Fv/F0 | ΦPSII | qP | NPQ |
|---|---|---|---|---|---|
| 3 days | |||||
| 0 | 0.76 ± 0.02a | 3.08 ± 0.25a | 0.59 ± 0.12a | 0.72 ± 0.17a | 1.11 ± 0.02d |
| 0.1 | 0.75 ± 0.04a | 2.96 ± 0.17a | 0.55 ± 0.13a | 0.71 ± 0.21a | 1.68 ± 0.03a |
| 0.2 | 0.72 ± 0.08a | 2.68 ± 0.45ab | 0.52 ± 0.11a | 0.68 ± 0.28a | 1.51 ± 0.08b |
| 0.3 | 0.64 ± 0.10ab | 2.18 ± 0.45bc | 0.36 ± 0.31ab | 0.45 ± 0.33b | 1.37 ± 0.08c |
| 0.5 | 0.56 ± 0.03b | 1.63 ± 0.32c | 0.30 ± 0.25b | 0.38 ± 0.31b | 1.30 ± 0.06c |
| 21 days | |||||
| 0 | 0.76 ± 0.03a | 3.48 ± 0.57a | 0.60 ± 0.32a | 0.72 ± 0.38a | 0.78 ± 0.18b |
| 0.1 | 0.75 ± 0.04a | 3.30 ± 0.24a | 0.55 ± 0.27a | 0.70 ± 0.41a | 1.18 ± 0.07a |
| 0.2 | 0.74 ± 0.05a | 3.03 ± 0.35a | 0.48 ± 0.22a | 0.68 ± 0.34a | 1.10 ± 0.08a |
| 0.3 | 0.63 ± 0.08b | 2.67 ± 0. 51a | 0.44 ± 0.40b | 0.56 ± 0.46b | 0.98 ± 0.04ab |
| 0.5 | - | - | - | - | - |
| Treatments (%) | Soluble Sugar | Proline | Soluble Protein |
|---|---|---|---|
| Leaf | |||
| 3 days | |||
| Control | 31.03 ± 1.45a | 0.25 ± 0.02d | 18.58 ± 1.64a |
| 0.1 | 31.18 ± 2.45a | 0.76 ± 0.14c | 17.22 ± 5.67a |
| 0.2 | 31.27 ± 1.77a | 0.83 ± 0.16c | 18.81 ± 0.96a |
| 0.3 | 32.47 ± 1.55a | 1.31 ± 0.13b | 20.25 ± 0.92a |
| 0.5 | 33.85 ± 1.88a | 2.13 ± 0.09a | 11.24 ± 3.57b |
| 21 days | |||
| Control | 32.63 ± 2.47a | 0.26 ± 0.03c | 19.71 ± 0.11b |
| 0.1 | 34.60 ± 1.44a | 0.68 ± 0.12b | 23.22 ± 0.20a |
| 0.2 | 34.27 ± 1.77a | 0.73 ± 0.16b | 24.81 ± 0.96a |
| 0.3 | 35.07 ± 2.95a | 1.39 ± 0.14a | 16.95 ± 0.46c |
| 0.5 | - | - | - |
| Root | |||
| 3 days | |||
| Control | 12.46 ± 1.20a | 0.16 ± 0.03b | 13.03 ± 2.68a |
| 0.1 | 15.30 ± 1.97a | 0.15 ± 0.03b | 12.67 ± 0.70a |
| 0.2 | 15.02 ± 3.50a | 0.18 ± 0.06b | 12.81 ± 0.36a |
| 0.3 | 14.04 ± 2.08a | 0.25 ± 0.02a | 13.81 ± 0.51a |
| 0.5 | 13.00 ± 1.40a | 0.29 ± 0.03a | 13.45 ± 1.78a |
| 21 days | |||
| Control | 16.22 ± 3.75a | 0.15 ± 0.02c | 10.78 ± 1.54c |
| 0.1 | 16.97 ± 1.27a | 0.22 ± 0.02b | 20.31 ± 4.79a |
| 0.2 | 16.02 ± 2.50a | 0.30 ± 0.02a | 14.86 ± 1.30b |
| 0.3 | 15.12 ± 2.12a | 0.38 ± 0.09a | 9.14 ± 2.14c |
| 0.5 | - | - | - |
| Treatment (%) | Leaf | Root |
|---|---|---|
| 3 days | ||
| Control | −11.50 ± 0.15a | −0.66 ± 0.05a |
| 0.1 | −1.53 ± 0.11a | −0.77 ± 0.04a |
| 0.2 | −2.10 ± 0.12b | −0.88 ± 0.11b |
| 0.3 | −2.32 ± 0.23b | −0.92 ± 0.15b |
| 0.5 | −4.85 ± 0.32c | −1.32 ± 0.15c |
| 21 days | ||
| Control | −1.73 ± 0.15a | −0.87 ± 0.05a |
| 0.1 | −2.14 ± 0.11b | −0.92 ± 0.04a |
| 0.2 | −2.12 ± 0.12b | −0.96 ± 0.11a |
| 0.3 | −2.39 ± 0.23b | −1.02 ± 0.15a |
| 0.5 | - | - |
| Treatments (%) | Na | K | Mg | Ca | K:Na ratio | Mg:Na Ratio | Ca:Na Ratio |
|---|---|---|---|---|---|---|---|
| Leaf | |||||||
| 3 days | |||||||
| Control | 0.12 ± 0.05d | 11.13 ± 0.46a | 4.74 ± 0.44ab | 22.57 ± 3.47b | 92.24 ± 16.69a | 39.29 ± 8.70a | 187.07 ± 70.20a |
| 0.1 | 0.16 ± 0.07d | 10.36 ± 0.51a | 5.13 ± 0.25a | 23.19 ± 8.34b | 63.85 ± 15.64b | 31.60 ± 3.77a | 142.95 ± 53.94a |
| 0.2 | 0.46 ± 0.15c | 11.01 ± 0.44a | 4.85 ± 0.15ab | 22.64 ± 5.13b | 23.93 ± 5.54bc | 16.25 ± 3.12b | 48.83 ± 10.69b |
| 0.3 | 2.20 ± 0.89b | 12.01 ± 0.72a | 4.57 ± 0.27ab | 19.97 ± 6.34b | 5.45 ± 0.23c | 2.07 ± 0.14c | 9.06 ± 2.90c |
| 0.5 | 16.28 ± 2.36a | 10.11 ± 0.33a | 4.32 ± 0.29b | 32.29 ± 8.78a | 0.62 ± 0.07d | 0.27 ± 0.05c | 1.98 ± 0.87d |
| 21 days | |||||||
| Control | 0.31 ± 0.12b | 8.85 ± 0.34a | 5.43 ± 0.25a | 56.66 ± 12.34a | 28.37 ± 2.34a | 17.40 ± 2.34a | 181.67 ± 52.34a |
| 0.1 | 0.59 ± 0.33b | 10.10 ± 0.55a | 5.25 ± 0.36a | 61.41 ± 6.32a | 17.04 ± 4.32b | 8.86 ± 1.03b | 103.60 ± 31.03b |
| 0.2 | 0.81 ± 0.12b | 8.81 ± 0.21a | 4.81 ± 0.21b | 62.55 ± 5.21a | 10.87 ± 5.21b | 5.94 ± 2.21b | 77.22 ± 25.40b |
| 0.3 | 3.59 ± 1.13a | 4.87 ± 0.78b | 4.21 ± 0.27b | 63.39 ± 7.55a | 1.36 ± 0.55c | 1.17 ± 0.24c | 17.66 ± 7.24c |
| 0.5 | - | - | - | - | - | - | - |
| Shoot | |||||||
| 3 days | |||||||
| Control | 0.08 ± 0.05e | 4.38 ± 0.51b | 1.76 ± 0.21b | 6.49 ± 2.15b | 54.73 ± 8.32a | 21.97 ± 4.18a | 81.12 ± 23.43a |
| 0.1 | 0.36 ± 0.23d | 6.72 ± 0.27a | 2.40 ± 0.34a | 10.00 ± 2.43a | 18.66 ± 3.21b | 6.66 ± 1.21b | 27.79 ± 7.75b |
| 0.2 | 1.04 ± 0.25c | 6.25 ± 0.34 a | 2.58 ± 0.33a | 9.58 ± 4.65a | 6.01 ± 1.02c | 2.31 ± 0.87c | 10.65 ± 2.31c |
| 0.3 | 4.07 ± 1.14b | 5.48 ± 0.49ab | 2.48 ± 0.42a | 8.81 ± 2.15ab | 1.23 ± 0.11d | 0.55 ± 0.28c | 2.16 ± 0.56d |
| 0.5 | 12.17 ± 5.24a | 5.57 ± 0.54ab | 2.41 ± 0.18a | 8.25 ± 1.35ab | 0.46 ± 0.10e | 0.20 ± 0.08c | 0.68 ± 0.12e |
| 21 days | |||||||
| Control | 0.11 ± 0.03b | 6.59 ± 0.47a | 2.38 ± 0.24a | 10.82 ± 6.12a | 57.64 ± 15.66a | 20.82 ± 2.34a | 94.55 ± 22.34a |
| 0.1 | 0.23 ± 0.14b | 5.31 ± 0.52ab | 1.59 ± 0.32b | 7.74 ± 1.33a | 23.11 ± 3.52b | 6.92 ± 2.32b | 33.64 ± 8.03b |
| 0.2 | 0.53 ± 0.16b | 4.81 ± 0.12b | 1.81 ± 0.21b | 7.81 ± 0.21a | 9.08 ± 5.21bc | 3.42 ± 1.21b | 14.75 ± 5.21bc |
| 0.3 | 5.29 ± 0.78a | 4.17 ± 0.33b | 1.79 ± 0.34b | 8.13 ± 2.36a | 0.79 ± 0.15c | 0.34 ± 0.25c | 1.54 ± 0.24c |
| 0.5 | - | - | - | - | - | - | - |
| Taproot | |||||||
| 3 days | |||||||
| Control | 0.71 ± 0.23d | 5.22 ± 0.29c | 1.84 ± 0.15ab | 3.84 ± 0.89b | 7.38 ± 1.23a | 2.60 ± 0.52a | 5.43 ± 2.12a |
| 0.1 | 0.96 ± 0.34d | 5.95 ± 0.44a | 1.91 ± 0.34a | 4.62 ± 1.25ab | 6.20 ± 1.67a | 1.99 ± 0.98ab | 4.79 ± 0.54ab |
| 0.2 | 1.58 ± 0.54c | 5.71 ± 0.53a | 1.83 ± 0.66ab | 4.44 ± 1.03ab | 3.61 ± 0.31b | 1.16 ± 0.42bc | 2.81 ± 1.08bc |
| 0.3 | 2.14 ± 1.32b | 5.68 ± 0.38a | 1.66 ± 0.10b | 4.38 ± 1.22ab | 2.65 ± 0.34bc | 0.78 ± 0.18b | 2.04 ± 0.32b |
| 0.5 | 3.28 ± 1.11a | 5.94 ± 0.47a | 1.94 ± 0.15a | 5.57 ± 2.34a | 1.82 ± 0.31c | 0.59 ± 0.08b | 1.71 ± 0.12b |
| 21 days | |||||||
| Control | 0.88 ± 0.58b | 6.25 ± 0.21a | 1.93 ± 0.17a | 5.27 ± 2.34b | 6.13 ± 1.75a | 1.90 ± 0.34ab | 5.17 ± 2.36a |
| 0.1 | 1.02 ± 0.35b | 5.55 ± 0.37b | 1.80 ± 0.35a | 3.50 ± 2.15b | 5.12 ± 1.32a | 2.04 ± 0.92a | 3.96 ± 1.03a |
| 0.2 | 1.21 ± 0.12b | 5.31 ± 0.12b | 1.81 ± 0.21a | 3.81 ± 0.21b | 4.39 ± 1.21ab | 1.50 ± 0.75ab | 3.15 ± 1.21a |
| 0.3 | 2.64 ± 1.13a | 5.15 ± 0.33b | 2.05 ± 0.25a | 10.37 ± 2.44a | 1.95 ± 0.32b | 0.78 ± 0.22b | 3.93 ± 1.25a |
| 0.5 | - | - | - | - | - | - | - |
| Lateral root | |||||||
| 3 days | |||||||
| Control | 2.45 ± 0.76c | 7.95 ± 0.65 b | 2.85 ± 0.31ab | 7.60 ± 1.24b | 3.24 ± 0.56a | 1.16 ± 0.34a | 3.10 ± 0.35a |
| 0.1 | 4.37 ± 1.07b | 9.19 ± 0.72a | 3.34 ± 0.56a | 9.04 ± 2.36ab | 2.10 ± 0.43b | 0.76 ± 0.12b | 2.07 ± 0.26bc |
| 0.2 | 4.12 ± 1.67b | 9.88 ± 0.24a | 3.28 ± 0.77a | 9.03 ± 0.88ab | 2.40 ± 0.32b | 0.80 ± 0.16b | 2.19 ± 0.52b |
| 0.3 | 4.57 ± 2.31b | 9.74 ± 0.46a | 2.71 ± 0.32b | 12.01 ± 2.37a | 2.13 ± 0.20b | 0.54 ± 0.11b | 2.37 ± 0.08b |
| 0.5 | 5.87 ± 0.78a | 7.28 ± 0.23b | 2.90 ± 0.32ab | 9.07 ± 1.33ab | 1.24 ± 0.05c | 0.49 ± 0.08b | 1.55 ± 0.05c |
| 21 days | |||||||
| Control | 2.59 ± 0.61b | 10.37 ± 0.55a | 2.55 ± 0.10b | 5.62 ± 1.78b | 4.00 ± 0.78a | 0.98 ± 0.17a | 2.17 ± 0.34a |
| 0.1 | 4.34 ± 0.98b | 9.57 ± 0.24ab | 2.16 ± 0.11c | 5.37 ± 3.33a | 2.21 ± 0.33b | 0.50 ± 0.12b | 1.24 ± 1.03a |
| 0.2 | 4.81 ± 0.45b | 8.81 ± 0.12b | 2.11 ± 0.10c | 5.41 ± 2.10a | 1.83 ± 0.27b | 0.44 ± 0.22b | 1.12 ± 0.23a |
| 0.3 | 5.89 ± 1.41a | 8.31 ± 0.42b | 2.89 ± 0.16a | 6.99 ± 2.33a | 1.95 ± 0.43b | 0.49 ± 0.25b | 1.19 ± 0.24a |
| 0.5 | - | - | - | - | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Luo, Z.; Ke, Y.; Dai, L.; Duan, H.; Hou, R.; Cui, B.; Dou, S.; Zhang, Y.; Sun, Y.; et al. Growth, Physiological, Biochemical, and Ionic Responses of Morus alba L. Seedlings to Various Salinity Levels. Forests 2017, 8, 488. https://doi.org/10.3390/f8120488
Lu N, Luo Z, Ke Y, Dai L, Duan H, Hou R, Cui B, Dou S, Zhang Y, Sun Y, et al. Growth, Physiological, Biochemical, and Ionic Responses of Morus alba L. Seedlings to Various Salinity Levels. Forests. 2017; 8(12):488. https://doi.org/10.3390/f8120488
Chicago/Turabian StyleLu, Nan, Zijing Luo, Yuzhou Ke, Li Dai, Hongjing Duan, Rongxuan Hou, Binbin Cui, Suhan Dou, Yadong Zhang, Yuhan Sun, and et al. 2017. "Growth, Physiological, Biochemical, and Ionic Responses of Morus alba L. Seedlings to Various Salinity Levels" Forests 8, no. 12: 488. https://doi.org/10.3390/f8120488





