Mortality and Recovery of Hemlock Woolly Adelgid (Adelges tsugae) in Response to Winter Temperatures and Predictions for the Future
Abstract
:1. Introduction
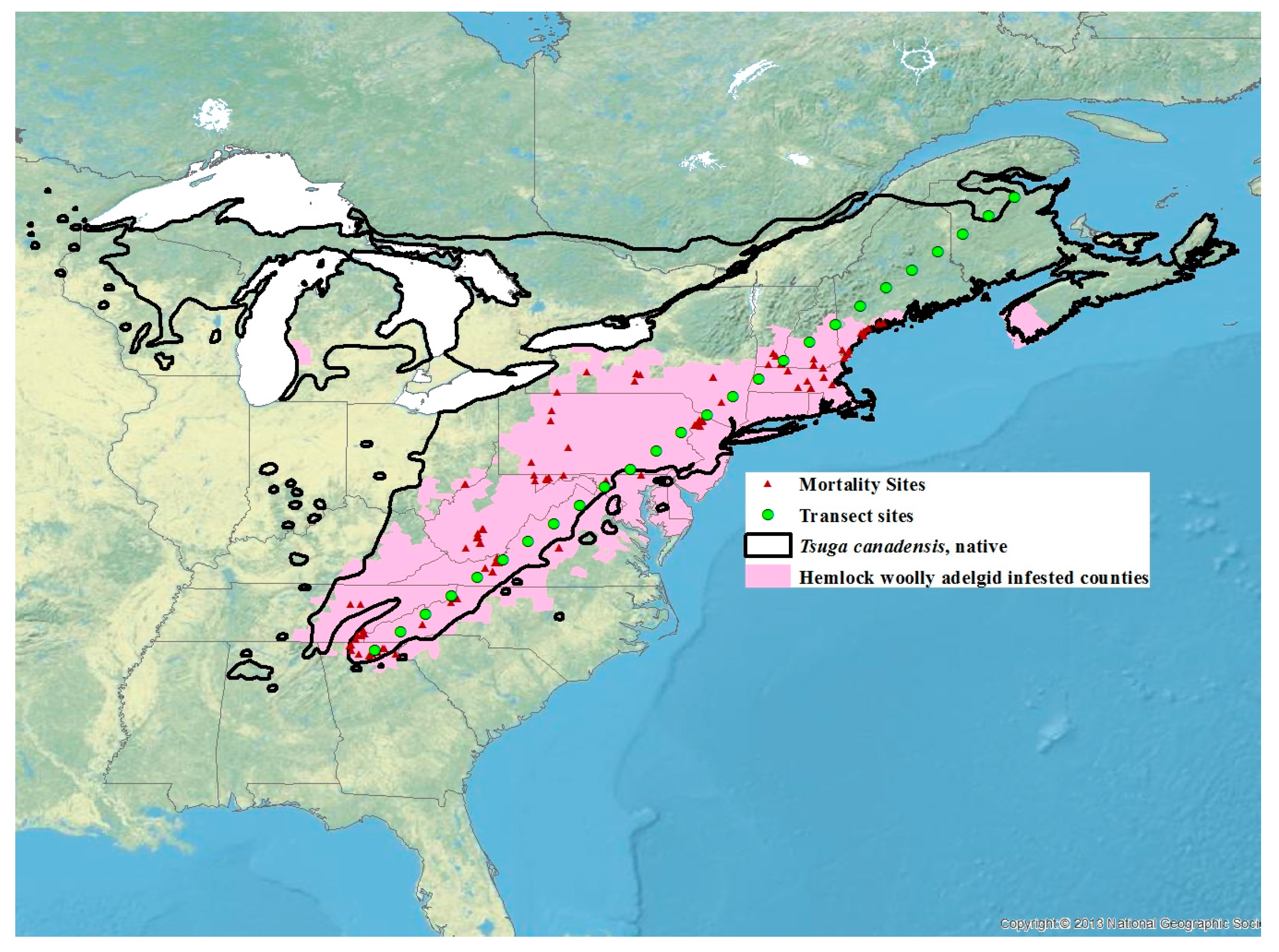
2. Materials and Methods
2.1. Winter Mortality
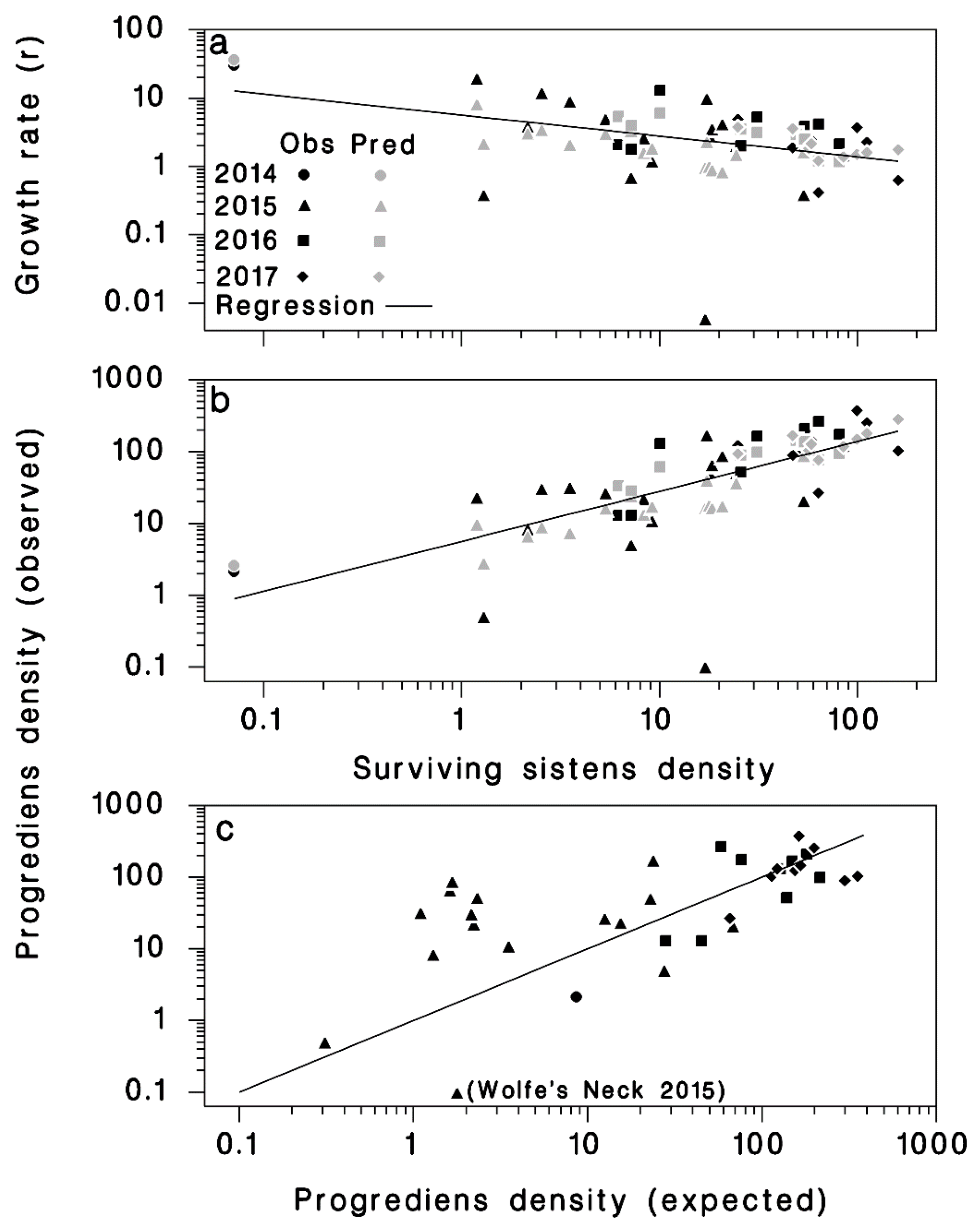
2.2. Progrediens Recovery
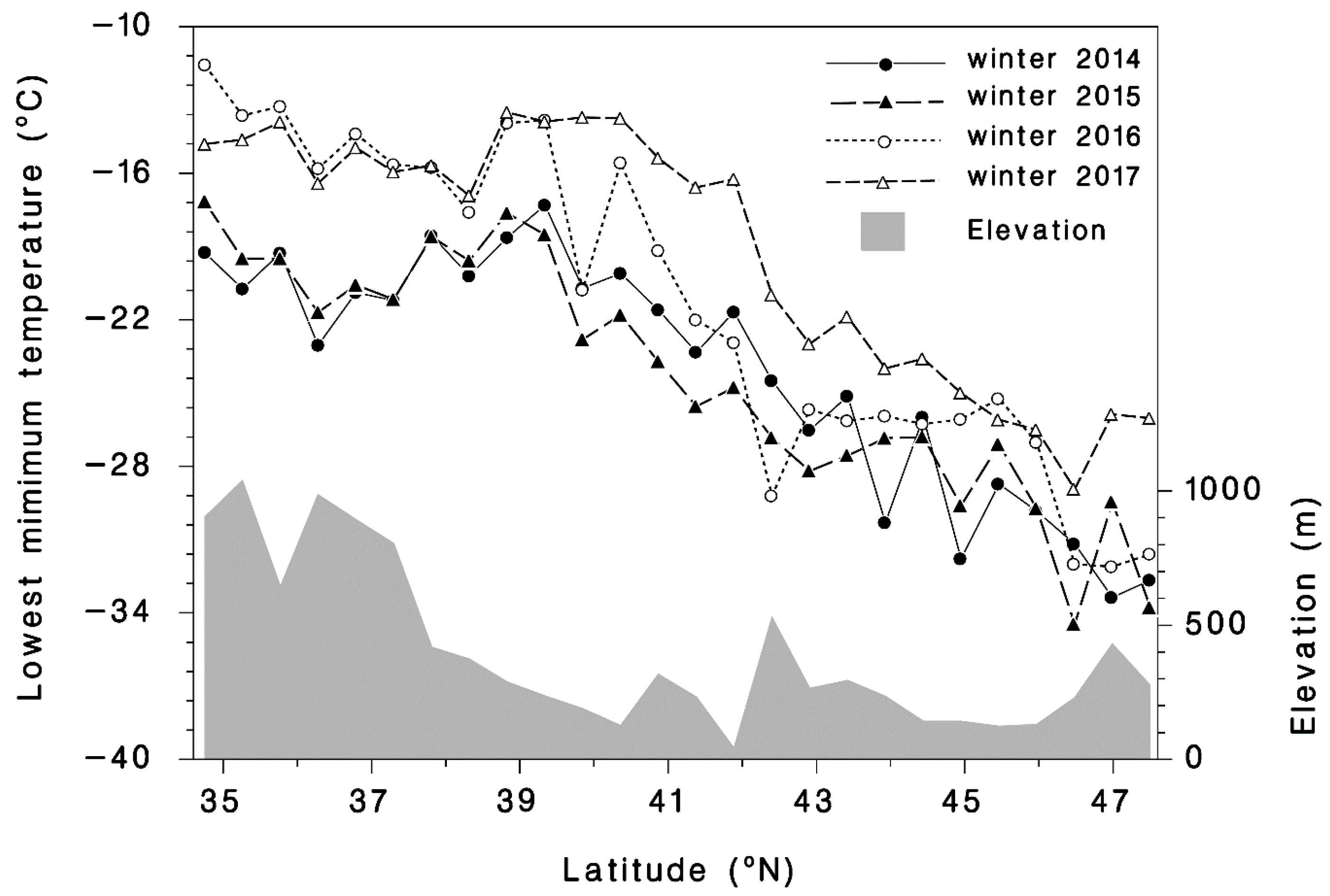
2.3. Temperature Data
2.4. Data Analysis
2.5. Climate Change Projections
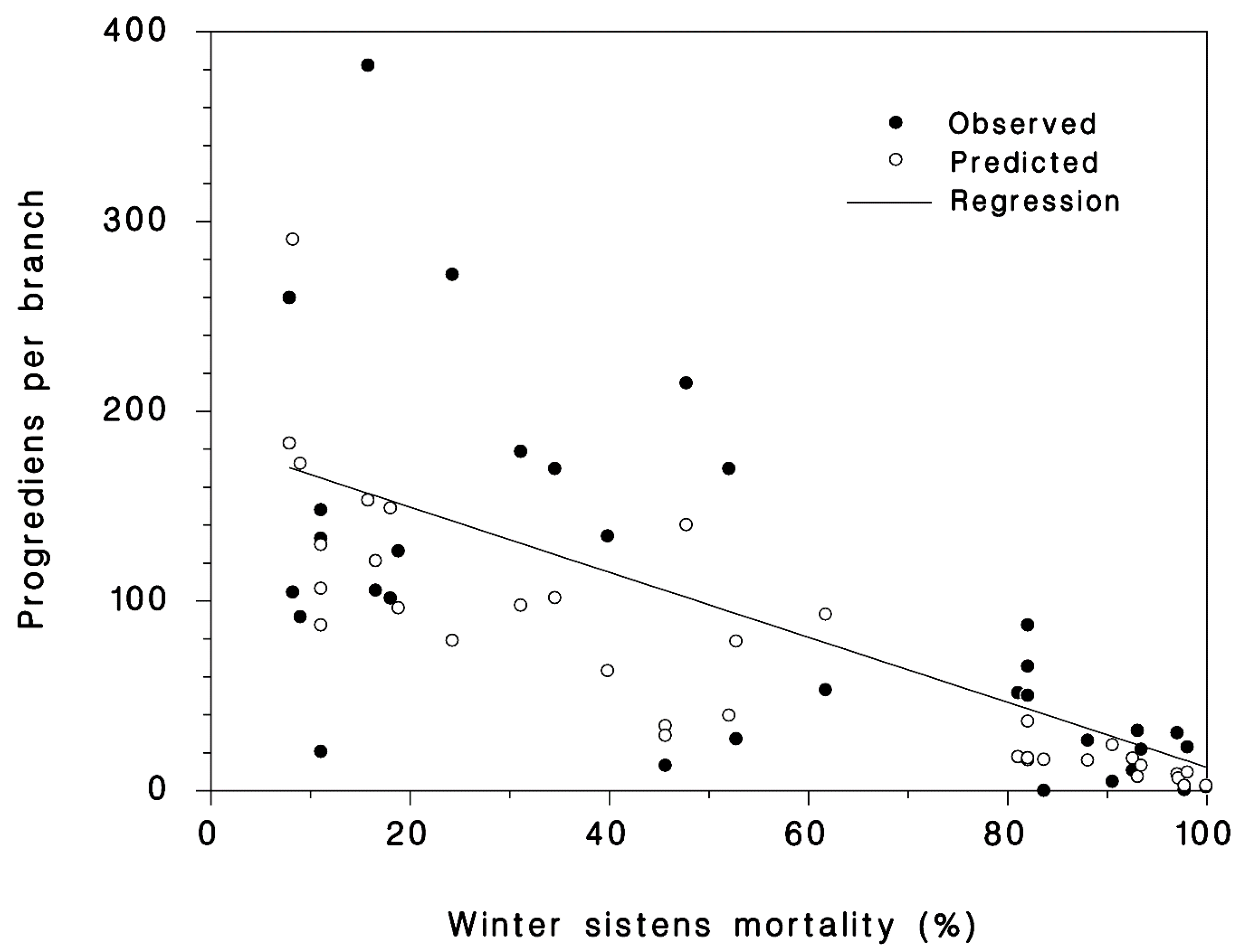
3. Results
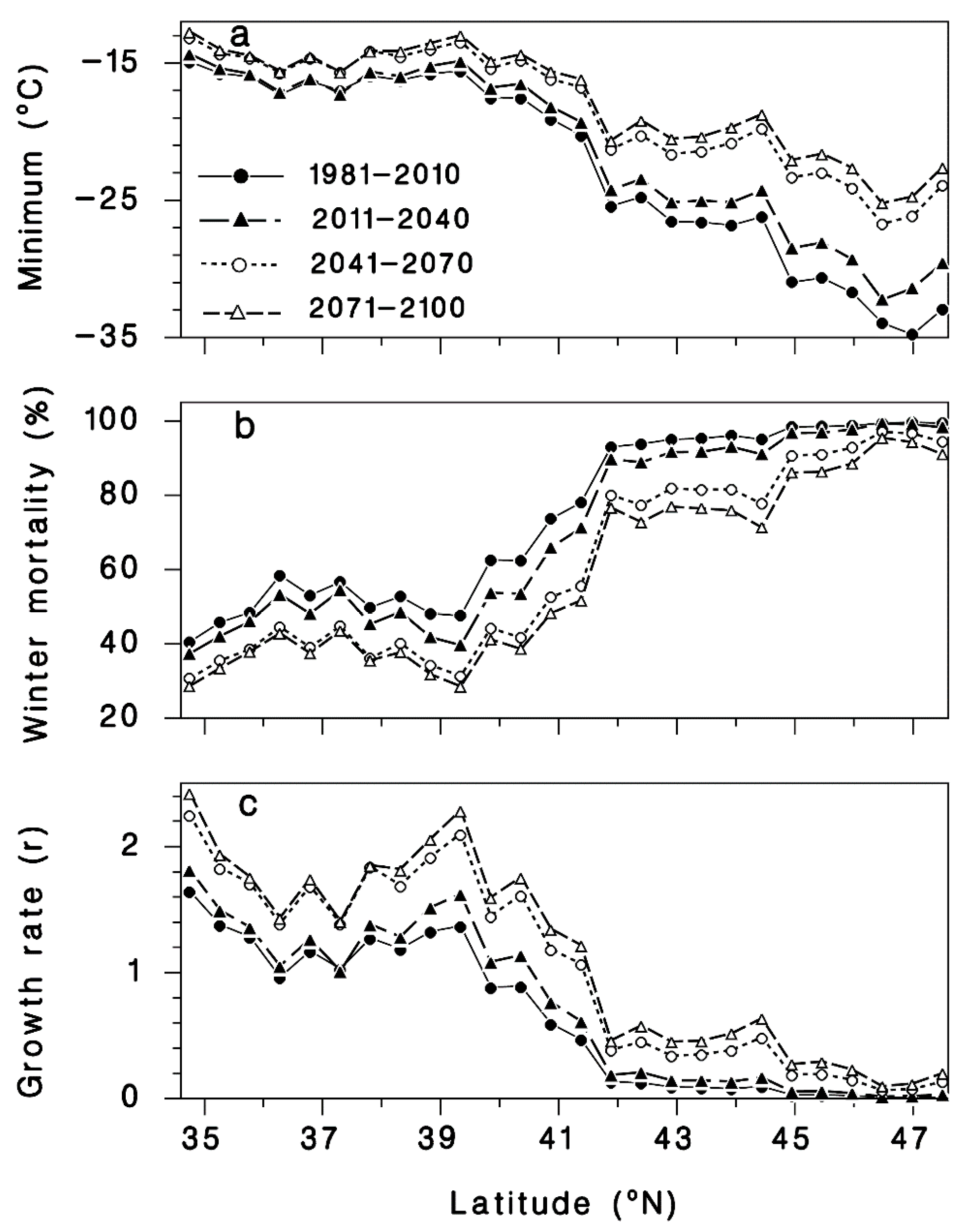
3.1. Winter Mortality
3.2. Progrediens Recovery
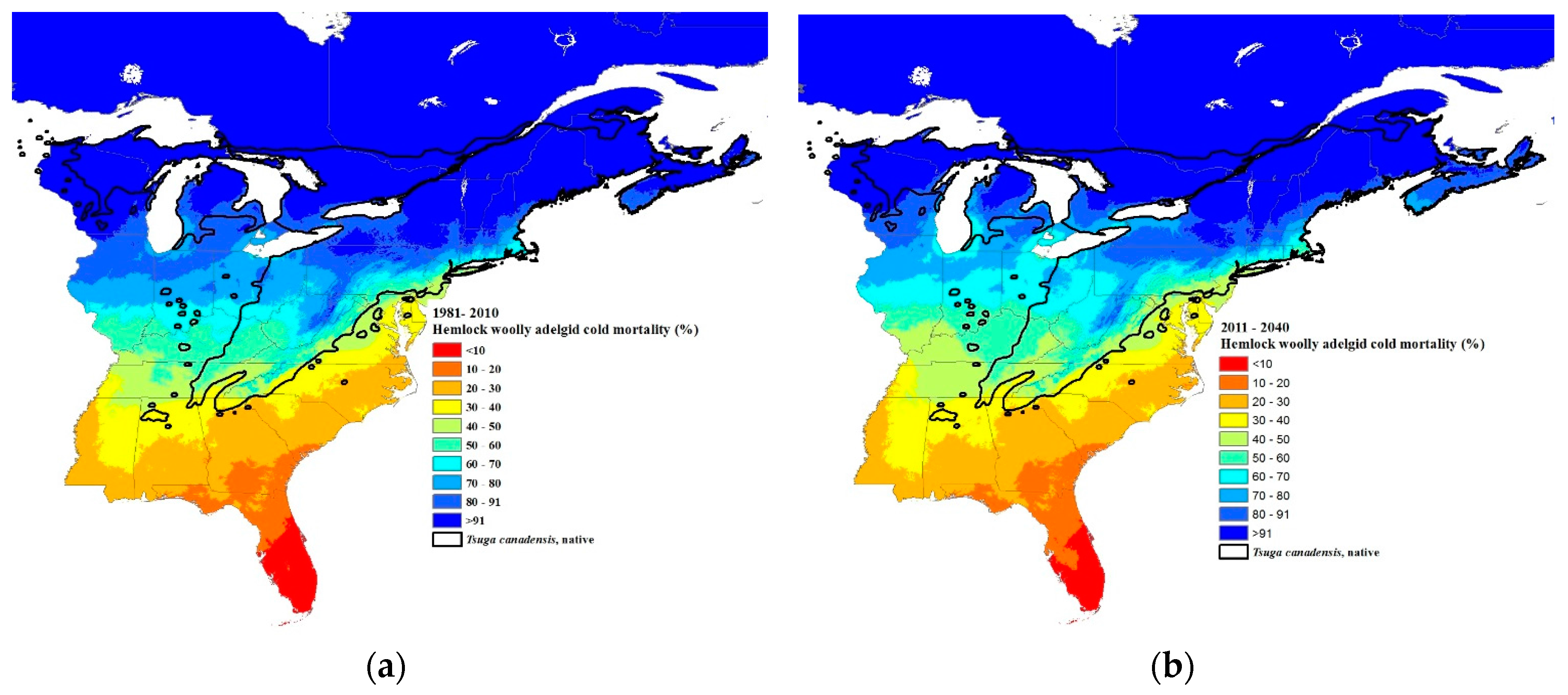
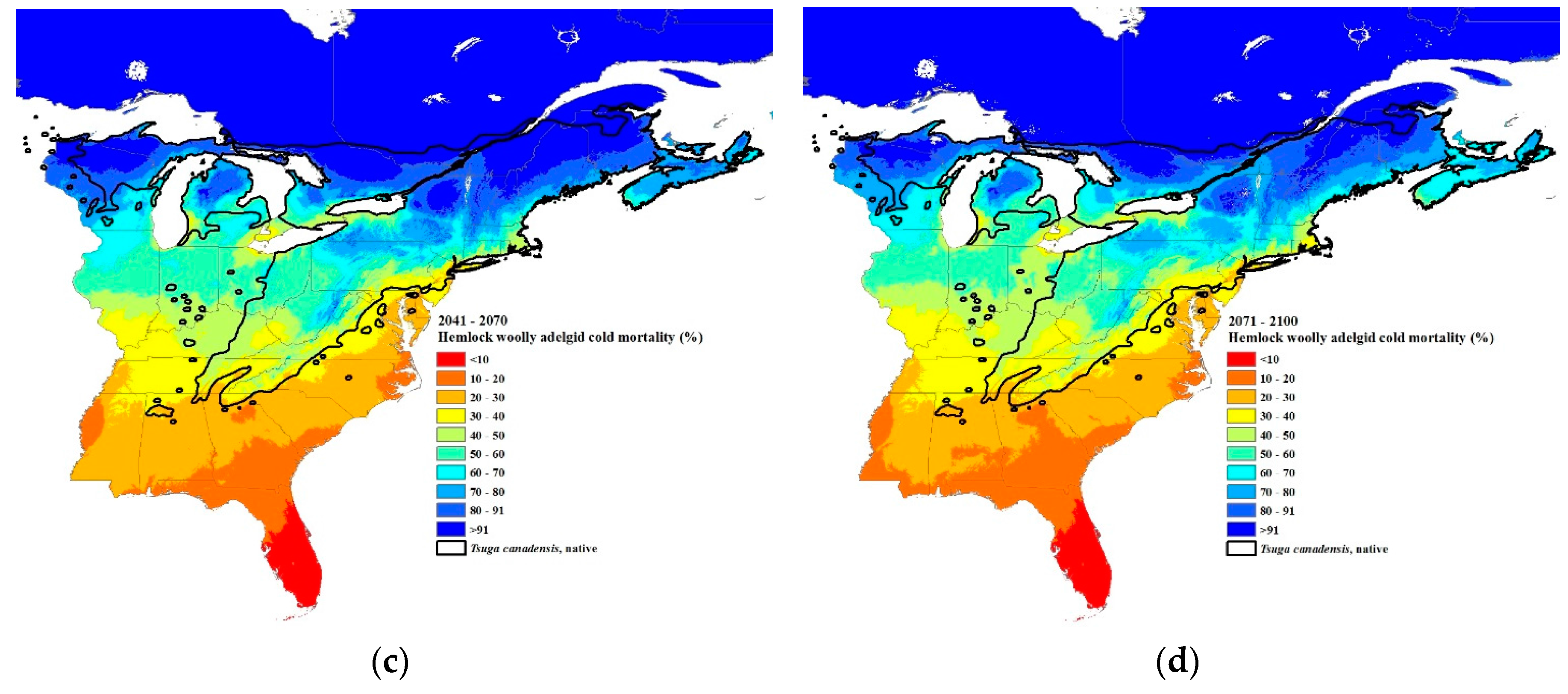
3.3. Climate Change Projections
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Godman, R.M.; Lancaster, K. Tsuga canadensis (L.) Carriere. In Silvics of North America: Conifers; Agriculture Handbook 654; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 605–612. [Google Scholar]
- Fowells, H.A. Silvics of Forest Trees of the United States; Agriculture Handbook No. 271; U.S. Department of Agriculture: Washington, DC, USA, 1965; 760p.
- Jetton, R.M.; Dvorak, W.S.; Whittier, W.A. Ecological and genetic factors that define the natural distribution of Carolina hemlock in the Southeastern United States and their role in ex situ conservation. For. Ecol. Manag. 2008, 255, 3212–3221. [Google Scholar] [CrossRef]
- Kessell, S.R. Adaption and dimorphism in eastern hemlock, Tsuga canadensis (L.) Carr. Am. Nat. 1979, 113, 333–350. [Google Scholar] [CrossRef]
- McWilliams, W.H.; Schmidt, T.L. Composition, structure, and sustainability of hemlock ecosystems in Eastern North America. In Proceedings of the Symposium on Sustainable Management of Hemlock Ecosystems in Eastern North America, Durham, NH, USA, 22–24 June 1999; McManus, K.A., Shields, K.S., Souto, D.R., Eds.; USDA Forest Service: Washington, DC, USA, 1999. [Google Scholar]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Narayanaraj, P.V.; Bolstad, P.V.; Elliott, K.J.; Vose, J.M. Terrain and Landforms Influence on Tsuga canadensis (L.) Carrière (Eastern Hemlock) Distribution in the Southern Appalachian Mountains. Castanea 2010, 75, 1–8. [Google Scholar] [CrossRef]
- Tingley, M.W.; Orwig, D.A.; Field, R.; Motzkin, G. Avian response to removal of a forest dominant: Consequences of hemlock woolly adelgid infestations. J. Biogeogr. 2002, 29, 1505–1516. [Google Scholar] [CrossRef]
- Snyder, C.D.; Young, J.A.; Lemarié, D.P.; Smith, D.R. Influence of eastern hemlock (Tsuga canadensis) forests on aquatic invertebrate assemblages in headwater streams. Can. J. Fish. Aquat. Sci. 2002, 59, 262–275. [Google Scholar] [CrossRef]
- Souto, D.; Luther, T.; Chianese, B. Past and current status of HWA in eastern and Carolina hemlock stands. In Proceedings of the First Hemlock Woolly Adelgid Review, Charlottesville, VA, USA, 12 October 1995; Salom, S.M., Tigner, T.C., Reardon, R.C., Eds.; USDA Forest Service: Morgantown, WV, USA, 1996; pp. 9–15. [Google Scholar]
- McClure, M.S. Density-dependent feedback and population cycles in Adelges tsugae (Homoptera: Adelgidae) on Tsuga canadensis. Environ. Entomol. 1991, 20, 258–264. [Google Scholar] [CrossRef]
- Orwig, D.A.; Foster, D.R. Forest response to the introduced hemlock woolly adelgid in southern New England. J. Torrey Bot. Soc. 1998, 125, 60–73. [Google Scholar] [CrossRef]
- Morin, R.S.; Oswalt, S.N.; Trotter, R.T., III; Liebhold, A.W. Status of Hemlock in the Eastern United States Forest Inventory and Analysis Factsheet; e-Science Update SRS-038; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2011; p. 4.
- Orwig, D.A.; Thompson, J.R.; Povak, N.A.; Manner, M.; Niebyl, D.; Foster, D.R. A foundation tree at the precipice: Tsuga canadensis health after the arrival of Adelges tsugae in central New England. Ecosphere 2012, 3, 1–16. [Google Scholar] [CrossRef]
- Havill, N.P.; Montgomery, M.E.; Yu, G.; Shiyake, S.; Caccone, A. Mitochondrial DNA from hemlock woolly adelgid (Hemiptera: Adelgidae) suggest cryptic speciation and pinpoints the source of the introduction to Eastern North America. Ann. Entomol. Soc. Am. 2006, 9, 195–203. [Google Scholar] [CrossRef]
- Orwig, D.A.; Foster, D.R.; Mausel, D.L. Landscape patterns of hemlock decline in New England due to introduced hemlock woolly adelgid. J. Biogeogr. 2002, 29, 1475–1487. [Google Scholar] [CrossRef]
- Eschtruth, A.; Cleavitt, N.L.; Battles, J.J.; Evans, R.A.; Fahey, T.J. Vegetation dynamics in declining eastern hemlock stands: 9 years of forest response to hemlock woolly adelgid infestation. Can. J. For. Res. 2006, 36, 1435–1450. [Google Scholar] [CrossRef]
- McClure, M.S. Biology of Adelges tsugae and its potential for spread in the Northeastern United States. In Proceedings of the First Hemlock Woolly Adelgid Review, Charlottesville, VA, USA, 12 October 1995; FHTET 96-10. Salom, S.M., Tigner, T.C., Reardon, R.C., Eds.; USDA Service Center: Morgantown, WV, USA, 1996; pp. 16–25. [Google Scholar]
- Havill, N.P.; Foottit, R.G. Biology and evolution of Adelgidae. Annu. Rev. Entomol. 2007, 52, 325–349. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.R.; Salom, S.M. Biology of hemlock woolly adelgid in the southern Appalachians. In Proceedings of the First Hemlock Woolly Adelgid Review, Charlottesville, VA, USA, 12 October 1995; Salom, S.M., Tigner, T.C., Reardon, R.C., Eds.; USDA Forest Service: Morgantown, WV, USA, 1996; pp. 26–35. [Google Scholar]
- Jones, A.C.; Mullins, D.E.; Jones, T.H.; Salom, S.M. Potential feeding deterrents found in hemlock woolly adelgid, Adelges tsugae. Naturwissenschaften 2012, 99, 583–586. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.S. Role of wind, birds, deer, and humans in the dispersal of hemlock woolly adelgid (Homoptera: Adelgidae). Environ. Entomol. 1990, 19, 36–43. [Google Scholar] [CrossRef]
- Russo, N.J.; Cheah, C.A.S.-J.; Tingley, M.W. Experimental evidence for branch-to-bird transfer as a mechanism for avian dispersal of the hemlock woolly adelgid (Hemiptera: Adelgidae). Environ. Entomol. 2016, 45, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Gregoire, T.G. A geographical variable model of hemlock woolly adelgid spread. Biol. Invasions 2007, 9, 369–382. [Google Scholar] [CrossRef]
- Trotter, R.T., III; Shields, K.S. Variation in winter survival of the invasive hemlock woolly adelgid (Hemiptera: Adelgidae) across the Eastern United States. Environ. Entomol. 2009, 38, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Mullins, D.E.; Brewster, C.; Rhea, J.P.; Salom, S.M. Fitness and physiology of Adelges tsugae (Hemiptera: Adelgidae) in relation to the health of the eastern hemlock. Insect Sci. 2016, 23, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.E.; Lyon, S.M. Natural enemies of adelgids in North America: Their prospect for biological control of Adelges tsugae (Homoptera: Adelgidae). In Proceedings of the First Hemlock Woolly Adelgid Review, Charlottesville, VA, USA, 12 October 1995; Salom, S.M., Tigner, T.C., Reardon, R.C., Eds.; USDA Forest Service: Morgantown, WV, USA, 1996; pp. 89–102. [Google Scholar]
- Wallace, M.S.; Hain, F.P. Field surveys and evaluation of native and established predators of the hemlock woolly adelgid (Homoptera: Adelgidae) in the Southeastern United States. Environ. Entomol. 2000, 29, 638–644. [Google Scholar] [CrossRef]
- Onken, B.P.; Reardon, R.C.; Technical Coordinators. Implementation and Status of Biological Control of the Hemlock Woolly Adelgid; Publication FHTET-2011-04; USDA Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2011; p. 230.
- Lagalante, A.F.; Lewis, N.; Montgomery, M.E.; Shields, K.S. Temporal and spatial variation in eastern hemlock (Tsuga canadensis) in relation to feeding by Adelges tsugae. J. Chem. Ecol. 2006, 32, 2389–2403. [Google Scholar] [CrossRef] [PubMed]
- Bentz, S.E.; Montgomery, M.E.; Olsen, R.T. Resistance of hemlock species and hybrids to hemlock woolly adelgid. In Proceedings of the Fourth Symposium on Hemlock Woolly Adelgid in the Eastern United States, Hartford, CN, USA, 12–14 Feburary 2008; FHTET-2008-01. Onken, B., Reardon, R.C., Eds.; USDA Forest Service: Morgantown, WV, USA, 2008; pp. 137–139. [Google Scholar]
- Ayayee, P.; Yang, F.; Rieske, L.K. Biomechanical properties of hemlocks: A novel approach to evaluating physical barriers of the plant-insect interface and resistance to a phloem-feeding herbivore. Insects 2014, 5, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Mech, A.M.; Tobin, P.C.; Teskey, R.O.; Rhea, J.R.; Gandhi, K.J.K. Increases in summer temperatures decrease the survival of an invasive forest insect. Bio. Invasions 2017. [Google Scholar] [CrossRef]
- Brantley, T.S.; Mayfield, A.E., III; Jetton, R.M.; Miniat, C.F.; Zielow, D.R.; Brown, C.L.; Rhea, J.R. Elevated light levels reduce hemlock woolly adelgid infestation and improve carbon balance of infested eastern hemlock seedlings. For. Ecol. Manag. 2017, 385, 150–160. [Google Scholar] [CrossRef]
- McAvoy, T.J.; Mays, R.; Johnson, N.G.; Salom, S.M. The effects of shade, fertilizer, and pruning on eastern hemlock trees and hemlock woolly adelgid. Forests 2017, 8, 156. [Google Scholar] [CrossRef]
- Shields, K.S.; Cheah, A.S.-J. Winter mortality in Adelges tsugae populations in 2003 and 2004. In Proceedings of the Third Symposium on Hemlock Woolly Adelgid in the Eastern United States, Asheville, NC, USA, 1–3 Feburary 2005; Onken, B., Reardon, R., Eds.; USDA Forest Service: Morgantown, WV, USA, 2005; pp. 354–356. [Google Scholar]
- Paradis, A.; Elkinton, J.; Hayhoe, K.; Buonaccorsi, J. Role of winter temperature and climate change on the survival and future range expansion of the hemlock woolly adelgid (Adelges tsugae) in Eastern North America. Mitig. Adapt. Strat. Glob. Chang. 2008, 13, 541–554. [Google Scholar] [CrossRef]
- Cheah, C.A.S.-J. Predicting hemlock woolly adelgid winter mortality in Connecticut forests by climate divisions. Northeast. Nat. 2017, 24, B90–B118. [Google Scholar] [CrossRef]
- Tobin, P.C.; Turcotte, R.M.; Blackburn, L.M.; Juracko, J.A.; Simpson, B.T. The big chill: Quantifying the effect of the 2014 North American cold wave on hemlock woolly adelgid populations in the central Appalachian Mountains. Popul. Ecol. 2017, 59, 251–258. [Google Scholar] [CrossRef]
- Costa, S.D.; Trotter, R.T.; Montgomery, M.; Fortney, M. Low temperature in the hemlock woolly adelgid system. In Proceedings of the Fourth Symposium on Hemlock Woolly Adelgid in the Eastern United States, Hartford, CN, USA, 12–14 Feburary 2008; FHTET-2008-01. Onken, B., Reardon, R.C., Eds.; USDA Forest Service: Morgantown, WV, USA, 2008; pp. 47–52. [Google Scholar]
- Elkinton, J.S.; Lombardo, J.A.; Roehrig, A.D.; McAvoy, T.J.; Mayfield, A.; Whitmore, M. Induction of cold hardiness in an invasive herbivore: The case of hemlock woolly adelgid (Hemiptera: Adelgidae). Environ. Entomol. 2017, 46, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.L.; Skinner, M.; Gouli, S.; Ashikaga, T.; Teillon, H.B. Survival of hemlock woolly adelgid (Homoptera: Adelgidae) at low temperatures. For. Sci. 1998, 44, 414–420. [Google Scholar]
- Parker, B.L.; Skinner, M.; Gouli, S.; Ashikaga, T.; Teillon, H.B. Low lethal temperature for hemlock woolly adelgid (Homoptera: Adelgidae). Environ. Entomol. 1999, 28, 1085–1091. [Google Scholar] [CrossRef]
- Butin, E.; Porter, A.H.; Elkinton, J. Adaption during biological invasions and the case of Adelges tsugae. Evol. Ecol. Res. 2005, 7, 887–900. [Google Scholar]
- Skinner, M.; Parker, B.L.; Gouli, S.; Ashikaga, T. Regional responses of hemlock woolly adelgid (Homoptera: Adelgidae) to low temperatures. Environ. Entomol. 2003, 32, 523–528. [Google Scholar] [CrossRef]
- Lombardo, J.A.; Elkinton, J.S. Environmental adaption in an asexual invasive insect. Ecol. Evol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tobin, P.C.; Turcotte, R.M.; Snider, D.A. When one is not necessarily a lonely number: Initial colonization dynamics of Adelges tsugae on eastern hemlock, Tsuga canadensis. Biol. Invasions 2013, 15, 1925–1932. [Google Scholar] [CrossRef]
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.L.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.M.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354. [Google Scholar] [CrossRef] [PubMed]
- Kharuk, V.L.; Im, S.T.; Ranson, K.J.; Yagunov, M.N. Climate-Induced Northerly Expansion of Siberian Silkmoth Range. Forests 2017. [Google Scholar] [CrossRef]
- Thomson, A.J.; Benton, R. A 90-year sea warming trend explains outbreak patterns of western spruce budworm on Vancouver Island. For. Chron. 2007, 83, 867–869. [Google Scholar] [CrossRef]
- Régnière, J.; St.-Amant, R.; Duval, P. Predicting insect distributions under climate change from physiological responses: Spruce budworm as an example. Biol. Invasions 2012, 14, 1571–1586. [Google Scholar] [CrossRef]
- Bentz, B.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Lundquist, J.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the Western US and Canada: Direct and indirect effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Wang, C.; Hawthorne, D.; Qin, Y.; Pan, X.; Li, Z.; Zhu, S. Impact of climate and host availability on future distribution of Colorado potato beetle. Sci. Rep. 2017, 7, 4489. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, C.; Simberloff, D. Implications of early production in an invasive forest pest. Agric. For. Entomol. 2017, 19, 217–224. [Google Scholar] [CrossRef]
- Yee, D.A.; Ezeakacha, N.F.; Abbott, K.C. The interactive effects of photoperiod and future climate change may have negative consequences for a wide-spread invasive insect. Oikos 2017, 126, 40–51. [Google Scholar] [CrossRef]
- Dukes, J.S.; Pontius, J.; Orwig, D.; Garnas, J.R.; Rodgers, V.L.; Brazee, N.; Cooke, B.; Theoharides, K.A.; Stange, E.E.; Harrington, R.; et al. Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of Northeastern North America: What can we predict? Can. J. For. Res. 2009, 39, 231–248. [Google Scholar] [CrossRef]
- Gray, D.R. Quantifying the sources of epistemic uncertainty in model predictions of insect disturbances in an uncertain climate. Ann. For. Sci. 2017, 74. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zverev, V.; Zvereva, E.L. Combined effects of environmental disturbance and climate warming on insect herbivory in mountain birch in subarctic forests: Results of 26-year monitoring. Sci. Total Eviron. 2017, 601, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, X. A physical analysis of the severe 2013/2014 cold winter in North America. J. Geophys. Res. Atmos. 2015, 120, 149–165. [Google Scholar] [CrossRef]
- Davis, G.A.; Salom, S.M.; Brewster, C.C.; Onken, B.P.; Kok, L.T. Spatiotemporal distribution of the hemlock woolly adelgid predator Laricobius nigrinus after release in eastern hemlock forests. Agric. For. Entomol. 2012, 14, 408–418. [Google Scholar] [CrossRef]
- Régnière, J.; St.-Amant, R.; Béchard, A. BioSIM 10.0 User’s Manual; Information Report LAU-X-137E; Natural Resources Canada, Canadian Forest Service: Ottawa, ON, Canada, 2016. Available online: ftp://ftp.cfl.scf.rncan.gc.ca/regniere/software/BioSIM/BioSIM10/Doc/LAU-X-137E.ZIP (accessed on 26 May 2017).
- Chuine, I.; Cour, P.; Rousseau, D.D. Fitting models predicting dates of flowering of temperate-zone trees using simulated annealing. Plant Cell Environ. 1998, 21, 455–466. [Google Scholar] [CrossRef]
- SAS Institute Inc. User’s Guide; release 9.2; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- United States Department of Agriculture. Available online: http://planthardiness.ars.usda.gov/PHZMWeb (accessed on 27 October 2017).
- Régnière, J.; Bolstad, P. Statistical simulation of daily air temperature patterns in Eastern North America to forecast seasonal events in insect pest management. Environ. Entmol. 1994, 23, 1368–1380. [Google Scholar] [CrossRef]
- United States Forest Service. Available online: https://www.na.fs.fed.us/fhp/hwa/maps/2015_HWA_Infestation_Map_20160502.pdf (accessed on 25 October 2017).
- Michigan Department of Agriculture and Rural Development. Available online: http://www.michigan.gov/mdard/0,4610,7-125-1572_3628-424513--m_2017_6,00.html (accessed on 25 October 2017).
- Canada Agriculture. Available online: https://www.canada.ca/en/food-inspection-agency/news/2017/08/hemlock_woolly_adelgidconfirmedinnovascotia.html (accessed on 17 August 2017).
- Sussky, E.M.; Elkinton, J.S. Density dependent survival and fecundity of hemlock woolly adelgid (Hemiptera: Adelgidae). Environ. Entomol. 2014, 43, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, A., III; Jetton, R.M. A shady situation: Evaluating the effect of shade on hemlock woolly adelgid densities on potted hemlock seedlings. In Proceedings of the 55th Southern Forest Insect Work Conference, New Orleans, LA, USA, 23–26 July 2013; Shepherd, W.P., Ed.; p. 43. [Google Scholar]
- Hickin, M.; Preisser, E.L. Effects of light and water availability on the performance of hemlock woolly adelgid (Hemiptera: Adelgidae). Environ. Entomol. 2015, 44, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.M.; Potter, K.M. Macro-scale assessment of demographic and environmental variation within genetically derived evolutionary lineages of eastern hemlock (Tsuga canadensis), an imperiled conifer of the eastern United States. Biodivers. Conserv. 2017, 26, 2223–2249. [Google Scholar] [CrossRef]
- Dunckel, K.; Weiskittel, A.; Fiske, G. Projected future distribution of Tsuga canadensis across alternative climate scenarios in Maine, U.S. Forests 2017, 8, 285. [Google Scholar] [CrossRef]
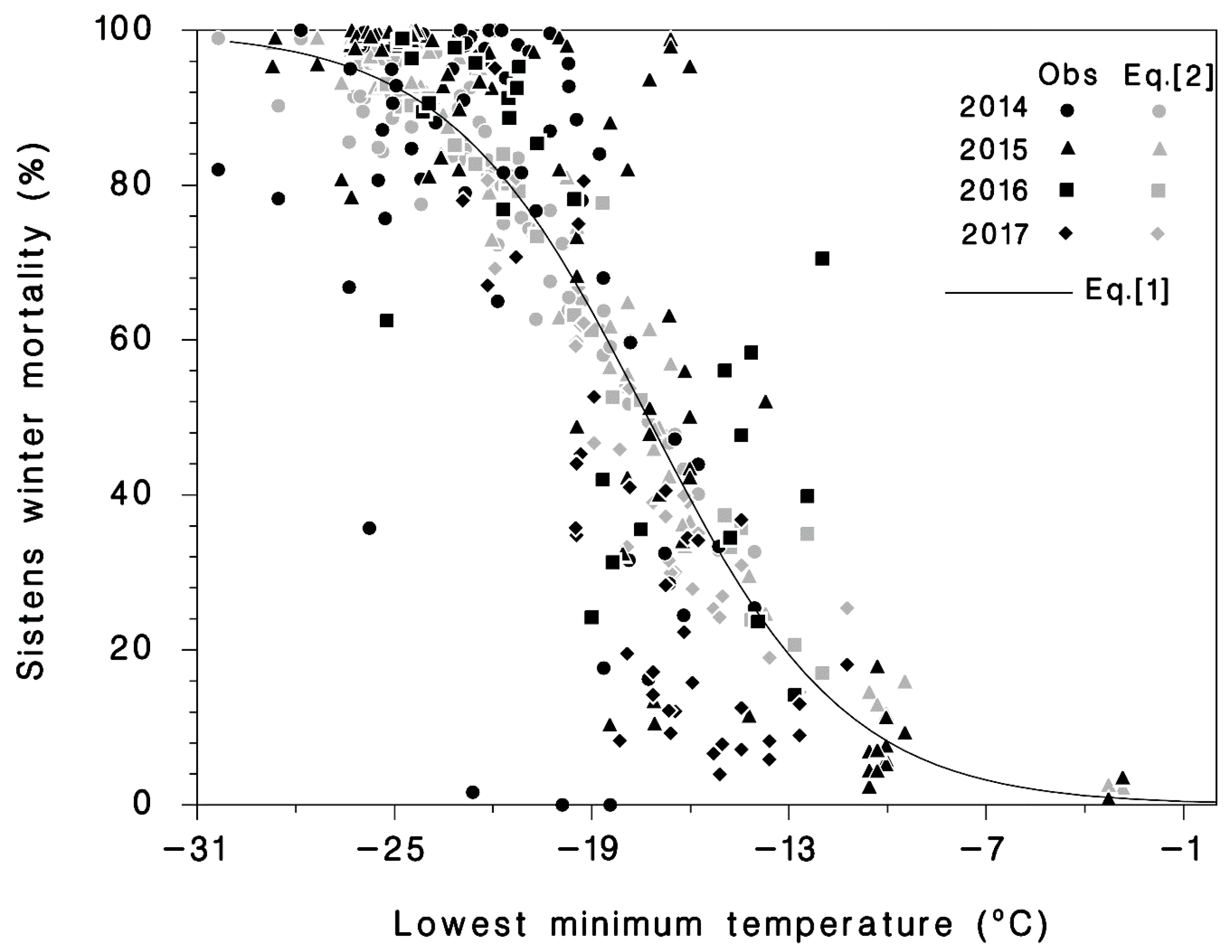

| Equation | Coefficient | Standard Error | Wald χ2 | p | AIC | R2 |
|---|---|---|---|---|---|---|
| (1) | 27,655 | 0.631 | ||||
| p0 | −5.7290 | 0.0443 | 16,723 | <0.0001 | ||
| p1Tmin | −0.3311 | 0.0023 | 21,389 | <0.0001 | ||
| (2) | 24,944 | 0.669 | ||||
| p0 | −4.9946 | 0.0526 | 9,008 | <0.0001 | ||
| p1Tmin | −0.2760 | 0.0035 | 6,163 | <0.0001 | ||
| p2N−1 | 0.0549 | 0.0013 | 1,829 | <0.0001 | ||
| p3Q3 | 0.1416 | 0.0032 | 1,968 | <0.0001 | ||
| (3) | 22,554 | 0.647 | ||||
| p0,2014 | −2.3225 | 0.1760 | 1,089 | <0.0001 | ||
| p0,2015 | −5.8618 | 0.1640 | 192 | <0.0001 | ||
| p0,2016 | −4.8990 | 0.1887 | 293 | <0.0001 | ||
| p0,2017 | −8.1307 | 0.1452 | 3,135 | <0.0001 | ||
| p1,2014 Tmin | −0.1767 | 0.0092 | 685 | <0.0001 | ||
| p1,2015 Tmin | −0.3666 | 0.0090 | 31 | <0.0001 | ||
| p1,2016 Tmin | −0.2975 | 0.0101 | 141 | <0.0001 | ||
| p1,2017 Tmin | −0.4168 | 0.0080 | 2,714 | <0.0001 | ||
| (4) | 26,159 | 0.605 | ||||
| p0,5 | −2.7359 | 0.2194 | 197 | <0.0001 | ||
| p0,6 | −6.6776 | 0.1705 | 26 | <0.0001 | ||
| p0,7 | −5.8135 | 0.1571 | 1,369 | <0.0001 | ||
| p1,5 Tmin | −0.1898 | 0.0115 | 169 | <0.0001 | ||
| p1,6 Tmin | −0.3852 | 0.0102 | 20 | <0.0001 | ||
| p1,7 Tmin | −0.3397 | 0.0096 | 1,262 | <0.0001 |
| Equation | Coefficient | Standard Error | Student’s T | p | R2 |
|---|---|---|---|---|---|
| (5) | 0.336 | ||||
| p0 | 8.16900 | 2.71100 | 3.01 | 0.006 | |
| p1ln(S) | −0.65160 | 0.23190 | −2.81 | 0.009 | |
| p2Tmin | 0.23890 | 0.13790 | 1.73 | 0.095 | |
| p3N−1 | 0.01540 | 0.12110 | 0.13 | 0.899 | |
| p4Q3 | 0.00782 | 0.02736 | 0.29 | 0.777 | |
| p5Tmin,pro | 0.04580 | 0.13540 | 0.34 | 0.738 | |
| p6N−1,pro | −0.05530 | 0.11900 | −0.46 | 0.646 | |
| p7DDpro | −0.00131 | 0.00100 | −1.30 | 0.203 | |
| (6) | 0.251 | ||||
| p0 | 5.074 | 1.445 | 3.83 | 0.001 | |
| p1ln(S) | −0.5187 | 0.1651 | −3.75 | 0.004 | |
| p2Tmin | 0.14227 | 0.05831 | 3.09 | 0.020 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAvoy, T.J.; Régnière, J.; St-Amant, R.; Schneeberger, N.F.; Salom, S.M. Mortality and Recovery of Hemlock Woolly Adelgid (Adelges tsugae) in Response to Winter Temperatures and Predictions for the Future. Forests 2017, 8, 497. https://doi.org/10.3390/f8120497
McAvoy TJ, Régnière J, St-Amant R, Schneeberger NF, Salom SM. Mortality and Recovery of Hemlock Woolly Adelgid (Adelges tsugae) in Response to Winter Temperatures and Predictions for the Future. Forests. 2017; 8(12):497. https://doi.org/10.3390/f8120497
Chicago/Turabian StyleMcAvoy, Thomas. J., Jacques Régnière, Rémi St-Amant, Noel F. Schneeberger, and Scott M. Salom. 2017. "Mortality and Recovery of Hemlock Woolly Adelgid (Adelges tsugae) in Response to Winter Temperatures and Predictions for the Future" Forests 8, no. 12: 497. https://doi.org/10.3390/f8120497





