Elevated Atmospheric CO2 and Warming Stimulates Growth and Nitrogen Fixation in a Common Forest Floor Cyanobacterium under Axenic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1: Culture Growth and N Fixation under Elevated CO2
2.2. Experiment 2: N Fixation under Elevated Warming and Atmospheric CO2
2.3. Statistical Analysis
3. Results
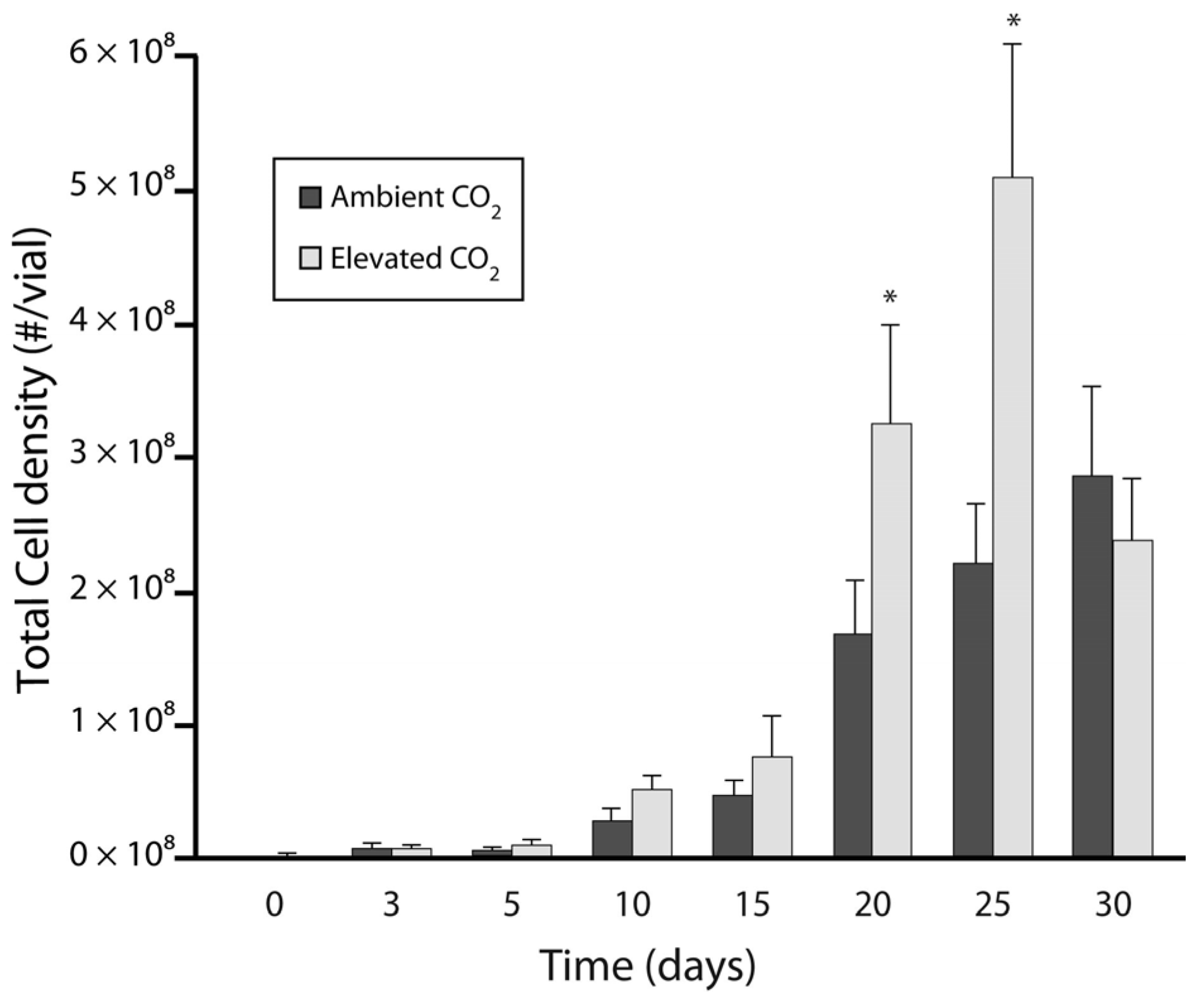
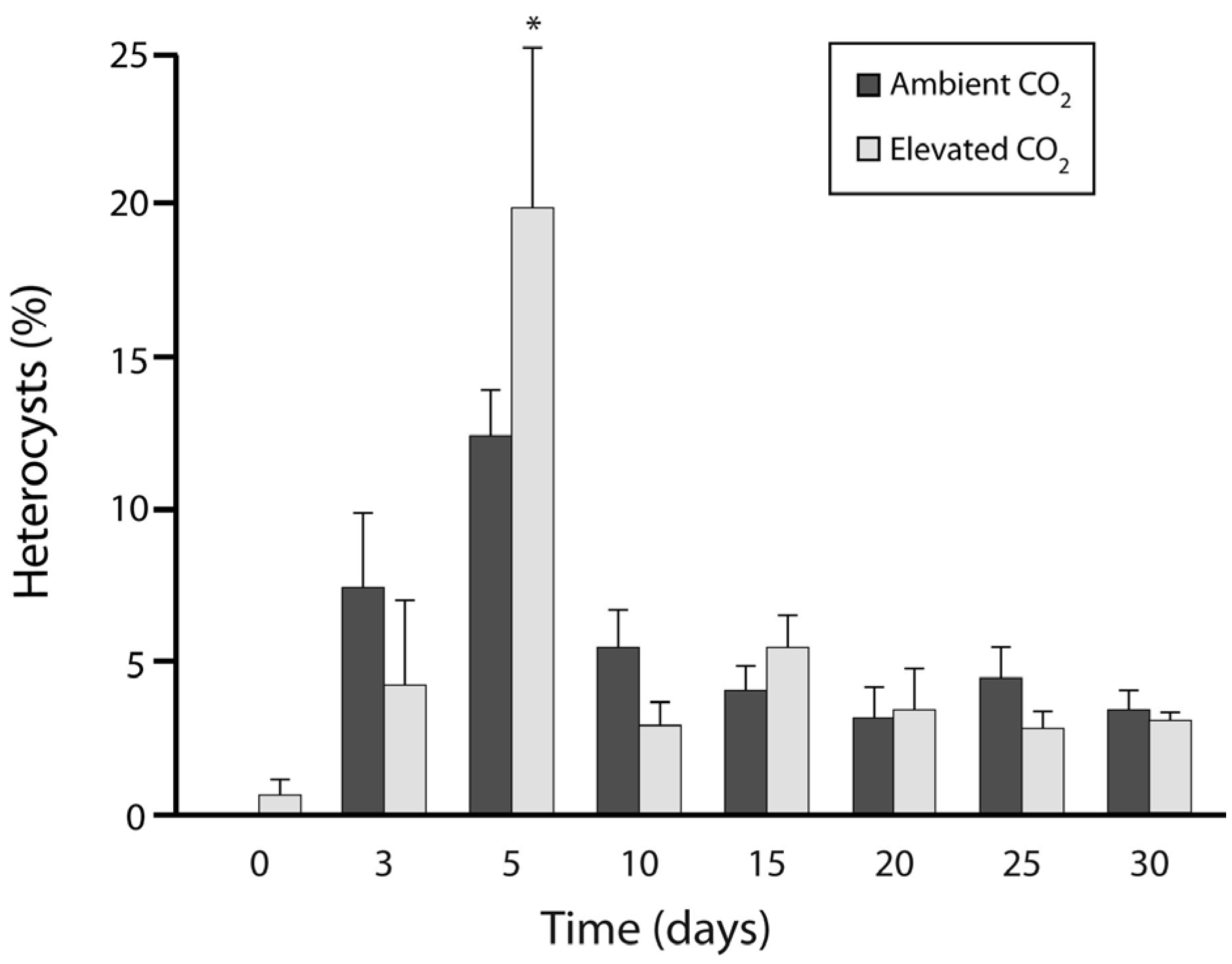
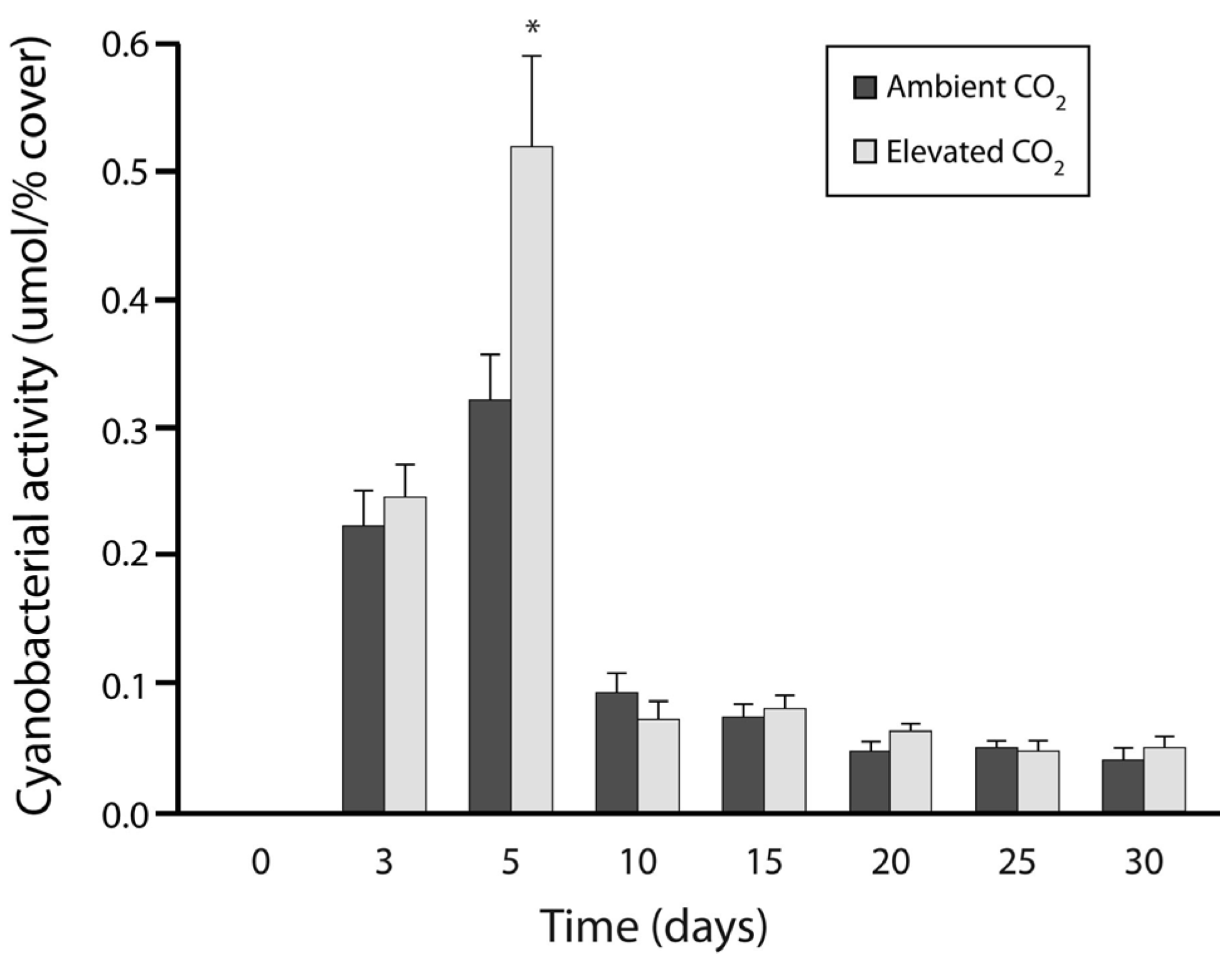
3.1. Experiment 1: Culture Growth and N Fixation under Elevated CO2
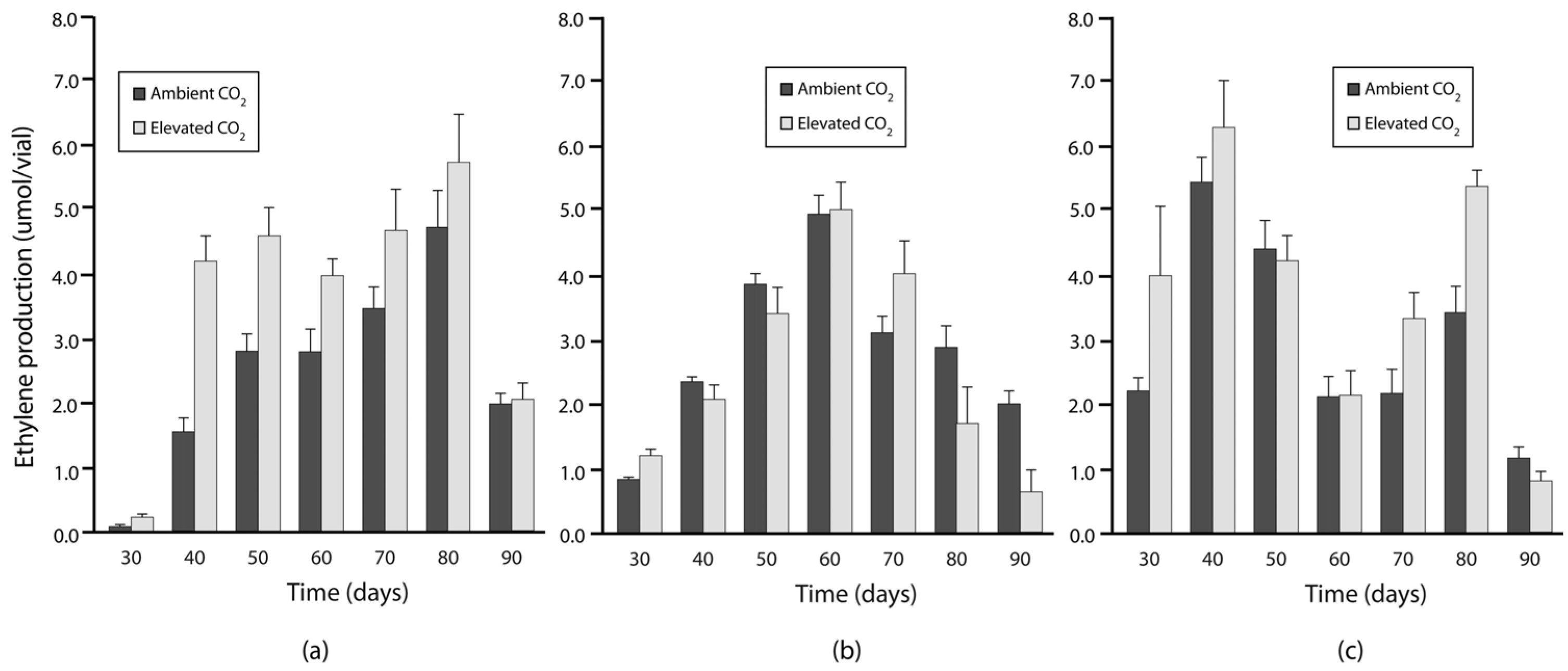
3.2. Experiment 2: N Fixation under Elevated Warming and Atmospheric CO2
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.; Field, C.B.; Grimm, N.B.; Horwarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B.; et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 2002, 57, 1–45. [Google Scholar] [CrossRef]
- Zielke, M.; Solheim, B.; Spjelkavik, S.; Olsen, R.A. Nitrogen fixation in the high arctic: Role of vegetation and environmental conditions. Arct. Antarct. Alp. Res. 2005, 37, 372–378. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zackrisson, O.; Gentili, F.; Sellstedt, A.; Nilsson, M.-C. Ecosystem controls of nitrogen fixation in boreal feather moss communities. Oecologia 2007, 152, 121–130. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, T.H.; Zackrisson, O.; Gundale, M.J.; Nilsson, M.-C. Ecosystem feedbacks and nitrogen fixation in boreal forests. Science 2008, 320, 1181. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.L.; Michelsen, A. Long-term warming and litter addition affects nitrogen fixation in a subarctic heath. Glob. Chang. Biol. 2011, 17, 528–537. [Google Scholar] [CrossRef]
- Rousk, K.; Jones, D.L.; DeLuca, T.H. Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Front. Microbiol. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lindo, Z.; Whiteley, J.A. Old trees contribute to bio-available nitrogen through canopy bryophytes. Plant Soil 2011, 342, 141–148. [Google Scholar] [CrossRef]
- Lindo, Z.; Nilsson, M.-C.; Gundale, M.J. Bryophyte-cyanobacteria associations as regulators of northern latitude carbon balance in response to global change. Glob. Chang. Biol. 2013, 19, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Intergovernmental Panel on Climate Change Fourth Assessment Report; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Gentili, F.; Nilsson, M.-C.; Zackrisson, O.; DeLuca, T.H.; Sellstedt, A. Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria. J. Exp. Bot. 2005, 56, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Gundale, M.J.; Nilsson, M.-C.; Bansal, S.; Jäderlund, A. The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol. 2012, 194, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.C.; Elhai, J.; Thiel, T.; Potts, M.; Larimer, F.; Lamerdin, J.; Predki, P.; Atlas, R. An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium. Photosynth. Res. 2001, 70, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Houlton, B.Z.; Wang, Y.P.; Vitousek, P.M.; Field, C.B. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 2008, 454, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.R. Effects of abiotic factors on acetylene reduction by cyanobacteria epiphytic on moss at a subantarctic island. Appl. Environ. Microbiol. 1984, 48, 594–600. [Google Scholar] [PubMed]
- Hutchins, D.A.; Fu, F.-X.; Zhang, Y.; Warner, M.E.; Feng, Y.; Portune, K.; Bernhardt, P.W.; Mulholland, M.R. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: Implications for past, present, and future ocean biogeochemistry. Limnol. Oceanogr. 2007, 52, 1293–1304. [Google Scholar] [CrossRef]
- Levitan, O.; Rosenberg, G.; Setlik, I.; Setlikova, E.; Grigel, J.; Klepetar, J.; Prasil, O.; Berman-Frank, I. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Chang. Biol. 2007, 13, 531–538. [Google Scholar] [CrossRef]
- Sohm, J.A.; Webb, E.A.; Capone, D.G. Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 2011, 9, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Czerny, J.; Barcelos e Ramos, J.; Riebesell, U. Influence of elevated CO2 concentrations on cell division and nitrogen fixation rates in the bloom-forming cyanobacterium Nodularia spumigena. Biogeosciences 2009, 6, 1865–1875. [Google Scholar] [CrossRef]
- Dodds, W.K.; Gudder, D.A.; Mollenhauer, D. The ecology of Nostoc. J. Phycol. 1995, 31, 2–18. [Google Scholar] [CrossRef]
- Meeks, J.C.; Elhai, J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. Rev. 2002, 66, 94–121. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.W.F.; Holsten, R.D.; Jackson, E.K.; Burns, R.C. The acetylene-ethylene assay for N2 fixation: Laboratory and field evaluation. Plant Physiol. 1968, 43, 1185–1207. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, T.H.; Zackrisson, O.; Nilsson, M.-C.; Sellstedt, A. Quantifying nitrogen fixation in feather moss carpets of boreal forests. Nature 2002, 419, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, S.M.; Salemaa, M.; Smolander, A.; Mäkipää, R.; Tiirola, M. Nitrogen fixation and methanotrophy in forest mosses along a N deposition gradient. Environ. Exp. Bot. 2013, 90, 62–69. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://www.R-project.org/ (accessed on 23 January 2017).
- Campbell, E.L.; Summers, M.L.; Christman, H.; Martin, M.E.; Meeks, C.J. Global gene expression patterns of Nostoc punctiforme in steady-state dinitrogen grown heterocyst-containing cultures and at single time points during the differentiation of akinetes and hormogonia. J. Bacteriol. 2007, 189, 5247–5256. [Google Scholar] [CrossRef] [PubMed]
- Hungate, B.A.; Stiling, P.D.; Dijkstra, P.; Johnson, D.W.; Ketterer, M.E.; Hymus, G.J.; Hinkle, C.R.; Drake, B.G. CO2 elicits long-term decline in nitrogen fixation. Science 2004, 304, 1291. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, S.; Ramakrishnan, B.; Bhatnagar, A.; Goyal, S.K.; Das, K.C. Carbon and nitrogen fixation by Anabaena fertilissima under elevated CO2 and temperature. J. Freshw. Ecol. 2009, 24, 587–596. [Google Scholar] [CrossRef]
- Zarzycki, J.; Axen, S.D.; Kinney, J.N.; Kerfeld, C.A. Cyanobacterial-based approaches to improving photosynthesis in plants. J. Exp. Bot. 2013, 64, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.L.; Lett, S.; Michelsen, A. Moss-specific changes in nitrogen fixation following two decades of warming, shading, and fertilizer addition. Plant Ecol. 2012, 213, 695–706. [Google Scholar] [CrossRef]
- Lang, S.I.; Cornelissen, J.H.C.; Shaver, G.R.; Ahrens, M.; Callaghan, T.V.; Molau, U.; Ter Braak, C.J.F.; Hölzer, A.; Aerts, R. Arctic warming on two continents has consistent negative effects on lichen diversity and mixed effects on bryophyte diversity. Glob. Chang. Biol. 2012, 18, 1096–1107. [Google Scholar] [CrossRef]
- Jean, M.-E.; Cassar, N.; Setzer, C.; Bellenger, J.-P. Short-term N2 fixation kinetics in a moss-associated cyanobacteria. Environ. Sci. Technol. 2012, 46, 8667–8671. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Danielsson, A.; Svensson, B.H. Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant Soil 2013, 362, 271–278. [Google Scholar] [CrossRef]
- Bay, G.; Nahar, N.; Oubre, M.; Whitehouse, M.J.; Wardle, D.A.; Zackrisson, O.; Nilccon, M.-C.; Rasmussen, U. Boreal feather mosses secrete chemical signals to gain nitrogen. New Phytol. 2013, 200, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Carleton, T.J.; Read, D.J. Ectomycorrhizas and nutrient transfer in conifer feather moss ecosystems. Can. J. Bot. 1991, 69, 778–785. [Google Scholar] [CrossRef]
- Kauserud, H.; Mathiesen, C.; Ohlson, M. High diversity of fungi associated with living parts of boreal forest bryophytes. Botany 2008, 86, 1326–1333. [Google Scholar] [CrossRef]
- Davey, M.L.; Nybakken, L.; Kauserud, H.; Ohlson, M. Fungal biomass in the phyllosphere of bryophytes and vascular plants. Mycol. Res. 2009, 113, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.L.; Heegaard, E.; Halvorsen, R.; Ohlson, M.; Kauserud, H. Seasonal trends in the biomass and structure of bryophyte associated fungal communities explored by 454 pyrosequencing. New Phytol. 2012, 195, 844–856. [Google Scholar] [CrossRef] [PubMed]


| Treatment Effect (Degrees of Freedom) | F Statistic | p Value |
|---|---|---|
| CO2 (1,30) | 21.77 | <0.001 |
| temperature (2,30) | 9.77 | <0.001 |
| CO2 × temperature (2,30) | 4.71 | 0.017 |
| time (6,180) | 53.83 | <0.001 |
| time × CO2 (6,180) | 1.41 | 0.204 |
| time × temperature (12,180) | 30.95 | <0.001 |
| time × CO2 × temperature (12,180) | 2.34 | 0.008 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindo, Z.; Griffith, D.A. Elevated Atmospheric CO2 and Warming Stimulates Growth and Nitrogen Fixation in a Common Forest Floor Cyanobacterium under Axenic Conditions. Forests 2017, 8, 73. https://doi.org/10.3390/f8030073
Lindo Z, Griffith DA. Elevated Atmospheric CO2 and Warming Stimulates Growth and Nitrogen Fixation in a Common Forest Floor Cyanobacterium under Axenic Conditions. Forests. 2017; 8(3):73. https://doi.org/10.3390/f8030073
Chicago/Turabian StyleLindo, Zoë, and Danielle A. Griffith. 2017. "Elevated Atmospheric CO2 and Warming Stimulates Growth and Nitrogen Fixation in a Common Forest Floor Cyanobacterium under Axenic Conditions" Forests 8, no. 3: 73. https://doi.org/10.3390/f8030073
APA StyleLindo, Z., & Griffith, D. A. (2017). Elevated Atmospheric CO2 and Warming Stimulates Growth and Nitrogen Fixation in a Common Forest Floor Cyanobacterium under Axenic Conditions. Forests, 8(3), 73. https://doi.org/10.3390/f8030073






