Effect of Auxins and Associated Metabolic Changes on Cuttings of Hybrid Aspen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Stem Cuttings
2.2. Anatomical Procedure
2.3. Assay of Endogenous Hormones
2.4. IAA Oxidase (IAAO) Assay
2.5. Polyphenol Oxidase (PPO) Assay
2.6. Peroxidase (POD) Assay
2.7. Quantification of PCR Products
2.8. Statistical Analysis
3. Results
3.1. Effects of a Range of Auxin Types on Rooting of Hybrid Aspen
3.2. Morphological and Anatomical Observations of Rooting Process of Cuttings
3.3. Changes in the Level of Endogenous Hormones
3.4. Biochemical Changes during Rooting
3.5. Gene Expression during Rooting
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haus, S.; Gustavsson, L.; Sathre, R. Climate mitigation comparison of woody biomass systems with the inclusion of land-use in the reference fossil system. Biomass Bioenerg. 2014, 65, 136–144. [Google Scholar] [CrossRef]
- Lutter, R.; Tullus, A.; Kanal, A.; Tullus, T.; Tullus, H. The impact of former land-use type to above- and below-ground C and N pools in short-rotation hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantations in hemiboreal conditions. For. Ecol. Manag. 2016, 378, 79–90. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The role of plantations in managing the world’s forests in the Anthropocene. Front. Ecol. Environ. 2010, 8, 27–34. [Google Scholar] [CrossRef]
- Rytter, L.; Stener, L.G. Growth and thinning effects during a rotation period of hybrid aspen in southern Sweden. Scand. J. For. Res. 2014, 29, 747–756. [Google Scholar] [CrossRef]
- Johansson, T. Biomass production of hybrid aspen growing on former farm land in Sweden. J. For. Res. 2013, 24, 237–246. [Google Scholar] [CrossRef]
- Lutter, R.; Tullus, A.; Kanal, A.; Tullus, T.; Tullus, H. The impact of short-rotation hybrid aspen (Populus tremula L. × P. tremuloides Michx.) plantations on nutritional status of former arable soils. For. Ecol. Manag. 2016, 362, 184–193. [Google Scholar] [CrossRef]
- Kristiansen, K.; Bredmose, N.; Nielsen, B. Influence of propagation temperature, photosynthetic photon flux density, auxin treatment and cutting position on root formation, axillary bud growth and shoot development in Schlumbergera ‘Russian Dancer’. J. Hortic. Sci. Biotechnol. 2005, 80, 297–302. [Google Scholar]
- Leakey, R.R.B. Physiology of vegetative propagation. In Encyclopaedia of Forest Sciences; Burley, J., Evans, J., Younquist, J.A., Eds.; Academic Press: London, UK, 2004; pp. 1655–1668. [Google Scholar]
- Fogaça, C.M.; Fett-Neto, A.G. Role of auxin and its modulators in the adventitious rooting of Eucalyptus species differing in recalcitrance. Plant Growth Regul. 2005, 45, 1–10. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Hausman, J.F. Indissociable chief factors in the inductive phase of adventitious rooting. In Biology of Root Formation and Development; Altman, A., Waisel, Y., Eds.; Plenum Press: New York, NY, USA, 1997; pp. 55–63. [Google Scholar]
- Da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Arabidopsis SHR and SCR transcription factors and AUX1 auxin influx carrier control the switch between adventitious rooting and xylogenesis in planta and in in vitro cultured thin cell layers. Ann. Bot. 2015, 115, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Legué, V.; Rigal, A.; Bhalerao, R.P. Adventitious root formation in tree species: Involvement of transcription factors. Physiol. Plant 2014, 151, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant 2014, 151, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Welander, M.; Geier, T.; Smolka, A.; Ahlman, A.; Fan, J.; Zhu, L.H. Origin, timing, and gene expression profile of adventitious rooting in Arabidopsis hypocotyls and stems. Am. J. Bot. 2014, 101, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant Hormone Homeostasis, Signaling, and Function during Adventitious Root Formation in Cuttings. Front. Plant Sci. 2016, 7, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Lodama, K.E.; du Toit, E.S.; Steyn, J.M.; Araya, H.T.; Prinsloo, G.; du Plooy, C.P.; Robbertse, P.J. Improving rooting of Lobostemon fruticosus L. cuttings with delayed auxin treatment. S. Afr. J. Bot. 2016, 105, 111–115. [Google Scholar] [CrossRef]
- Cao, W.X.; Wang, Z.L.; Dai, T.B. Changes in levels of endogenous plant hormones during floret development in wheat genotypes of different spike sizes. Acta Bot. Sin. 2000, 42, 1026–1032. [Google Scholar]
- Beffa, R.; Martin, H.V.; Pilet, P.E. In vitro oxidation of indoleacetic acid by soluble auxin-oxidases and peroxidases from maize roots. Plant Physiol. 1990, 94, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Ma, X. Different response on drought tolerance and postdrought recovery between the small-leafed and the large-leafed white clover (Trifolium repens L.) associated with antioxidative enzyme protection and lignin metabolism. Acta Physiol. Plant 2013, 35, 213–222. [Google Scholar] [CrossRef]
- Tchoundjeu, Z.; Ngo Mpeck, M.L.; Asaah, E.; Amougou, A. The role of vegetative propagation in the domestication of Pausinystalia johimbe (K. Schum), a highly threatened medicinal species of West and Central Africa. For. Ecol. Manag. 2004, 188, 175–183. [Google Scholar] [CrossRef]
- Ludwig-Mu¨ller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000, 32, 219–230. [Google Scholar] [CrossRef]
- Kochhar, V.K.; Singh, S.P.; Katiyar, R.S.; Pushpangadan, P. Differential rooting and sprouting behaviour of two Jatropha species and associated physiological and biochemical changes. Curr. Sci. 2005, 89, 936–939. [Google Scholar]
- Ricci, A.; Rolli, E.; Brunoni, F.; Dramis, L.; Sacco, E.; Fattorini, L.; Ruffoni, B.; Díaz-Sala, C.; Altamura, M.M. 1,3-di(benzo[d]oxazol-5-yl)urea acts as either adventitious rooting adjuvant or xylogenesis enhancer in carob and pine microcuttings depending on the presence/absence of exogenous indole-3-butyric acid. Plant Cell Tissue Organ Cult. 2016, 126, 411–427. [Google Scholar] [CrossRef]
- Mesen, F.; Newton, A.C.; Leakey, R.R.B. Vegetative propagation of Cordia allidora (Ruiz and Pavon) Oken: The effects of IBA concentration, propagation medium and cutting origin. For. Ecol. Manag. 1997, 92, 45–54. [Google Scholar] [CrossRef]
- Palanisamy, K.; Ansari, S.A.; Pramod, K.; Gupta, B.N. Adventitious rooting in shoot cuttings of Azardirachta indica and Pongamia pinnata. New For. 1998, 16, 81–88. [Google Scholar] [CrossRef]
- Puri, S.; Shamet, G.S. Rooting of stem cuttings of some social forestry trees. Int. Tree Crop J. 1988, 5, 63–69. [Google Scholar] [CrossRef]
- Tiwari, R.K.S.; Das, K. Effect of stem cuttings and hormonal pre-treatment on propagation of Embelia tsjeriam and Caesalpinia bonduc, two important medicinal plant species. J. Med. Plants Res. 2010, 4, 1577–1583. [Google Scholar]
- Tworkoski, T.; Takeda, F. Rooting response of shoot cuttings from three peach growth habits. Sci. Hortic. 2007, 115, 98–100. [Google Scholar] [CrossRef]
- Yan, Y.H.; Li, J.L.; Zhang, X.Q.; Yang, W.Y.; Wan, Y.; Ma, Y.M.; Zhu, Y.Q.; Peng, Y.; Huang, L.K. Effect of Naphthalene Acetic Acid on Adventitious Root Development and Associated Physiological Changes in Stem Cutting of Hemarthria compressa. PLoS ONE 2014, 9, e90700. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.J.; Hanecakova, J. Ethylene and rooting of mung bean cuttings. The role of auxin induced ethylene synthesis and phase-dependent effects. Plant Growth Regul. 2008, 56, 203–209. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U.; et al. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, K. The influence of benzyladenine and naphthalene-1-acetic acid on rooting and growth of Fuchsia hybrida cuttings. Acta Sci. Polhortoru. 2013, 12, 101–113. [Google Scholar]
- De Klerk, G.J.; Keppel, M.; Ter Brugge, J.; Meekes, H. Timing of the phases in adventitious root formation apple microcuttings. J. Exp. Bot. 1995, 46, 965–972. [Google Scholar] [CrossRef]
- Mendes, A.F.S.; Cidade, L.C.; Otoni, W.C.; Soares-Filho, W.S.; Costa, M.G.C. Role of auxins, polyamines and ethylene in root formation and growth in sweet orange. Biol. Plant 2011, 55, 375–378. [Google Scholar] [CrossRef]
- Schwambach, J.; Ruedell, C.M.; de Almeida, M.R.; Penchel, R.M.; de Araujo, E.F.; Fett-Neto, A.G. Adventitious rooting of Eucalyptus globulus x maidennii mini-cuttings derived from mini-stumps grown in sand bed and intermittent flooding trays: A comparative study. New For. 2008, 36, 261–271. [Google Scholar] [CrossRef]
- Amanda, J.K.; Helen, M.W.; David, A.W.; Matthew, F.A.; Stephen, J.T. Improved Root Formation in Eucalypt Cuttings Following Combined Auxin and Anti-ethylene Treatments. J. Plant Sci. 2012, 7, 138–153. [Google Scholar]
- Tsipouridis, C.; Thomidis, T.; Bladenopoulou, S. Rhizogenesis of GF677, Early Crest May Crest and Arm King stem cuttings during the year in relation to carbohydrate and natural hormone content. Sci. Hortic. 2006, 108, 200–204. [Google Scholar] [CrossRef]
- Malá, J.; Gaudinová, A.; Dobrev, P.; Eder, J.; Cvirková, M. Role of phytohormones in organogenic ability of elm multiplicated shoots. Biol. Plant 2005, 50, 8–14. [Google Scholar] [CrossRef]
- Trifunović-Momčilov, M.; Motyka, V.; DragićevićIvana, I.Č.; Petric, M.; Jevremović, S.; Malbeck, J.; Holík, J.; Dobrev, P.I.; Subotić, A. Endogenous phytohormones in spontaneously regenerated Centaurium erythraea Rafn. Plants Grown In Vitro. J. Plant Growth Regul. 2016, 35, 543–552. [Google Scholar] [CrossRef]
- Vazquez, A.; Mato, M.C. Effects of hydroxybenzaldehydes on rooting and indole-3-acetic acid-oxidase activity in bean cuttings. Physiol. Plant. 1991, 83, 597–600. [Google Scholar] [CrossRef]
- Liu, Z.H.; Hsiao, I.C.; Pan, Y.W. Effect of NAA on indoleacetic acid in hypocotyl cuttings of soybean during root formation. Bot. Bull. Acad. Sin. 1996, 37, 247–253. [Google Scholar]
- Hoffmann-Benning, S.; Kende, H. On the role of Abscisic acid and Gibberellin in the regulation of growth in rice. Plant Physiol. 1992, 99, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.; Thorpe, T. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell. Dev. Bio.-Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Liu, G.F.; Yang, C.P.; Qu, G.Z.; You, X.L. Dynamic changes of four endogenous hormones in the larch hybrid during cutting rooting. J. Northeast For. Univ. 2001, 29, 1–3, (In Chinese with an English abstract). [Google Scholar]
- Huang, Y.; Ji, K.S.; Zhai, J.R. Relationship between rooting ability and endogenous phytohormone changes in successive continuous generation cuttings of Buxus sinica var. parvifolia, an endangered woody species in China. For. Stud. China 2007, 9, 189–197. [Google Scholar] [CrossRef]
- Feng, D.L.; Huang, X.H.; Liu, Y.; Martin Willison, J.H. Growth and changes of endogenous hormones of mulberry roots in a simulated rocky desertification area. Environ. Sci. Pollut. Res. 2016, 23, 11171–11180. [Google Scholar] [CrossRef] [PubMed]
- Hisano, H.; Matsuura, T.; Mori, I.C.; Sato, K. Endogenous hormone levels affect the regeneration ability of callus derived from different organs in barley. Plant Physiol. Biochem. 2016, 99, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Fu, X.L.; Zang, D.K.; Ma, Y. Effect of auxin treatments, cuttings’ collection date and initial characteristics on Paeonia ‘Yang Fei Chu Yu’ cutting propagation. Sci. Hortic. 2009, 119, 177–181. [Google Scholar] [CrossRef]
- Rout, G.R. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regul. 2006, 48, 111–117. [Google Scholar] [CrossRef]
- Bernards, M.A.; Fleming, W.D.; Llewellyn, D.B.; Priefer, R.; Yang, X.; Sabatino, A.; Plourde, G.L. Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol. 1999, 121, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barcelo, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; de Forchetti, S.M.; Tigier, H.; Valpuesta, V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 2000, 122, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Moncousin, C.; Favre, J.M.; Gaspar, T. Changes in peroxidase activity and endogenous IAA leves during adventitious rooting in vine cuttings. In Physiology and Biochemistry of Auxins in Plants; Kutacek, M., Bandurski, R.S., Krekule, J., Eds.; Academic Publishing: Prague, Czech Republic, 1988; pp. 331–337. [Google Scholar]
- Lagrimini, L.M.; Vaughn, J.; Finer, J.; Klotz, K.; Rubaihayo, P. Expression of a Chimeric Tobacco Peroxidase Gene in Transgenic Tomato Plants. J. Am. Soc. Hort. Sci. 1992, 117, 1012–1016. [Google Scholar]
- Aeschbacher, R.A.; Schiefelbein, J.W.; Benfey, P.N. The genetic and molecular basis of root development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 25–45. [Google Scholar] [CrossRef]
- Chen, L.M.; Cheng, J.T.; Chen, E.L.; Yiu, T.J.; Liu, Z.H. Naphthaleneacetic acid suppresses peroxidase activity during the induction of adventitious roots in soybean hypocotyls. J. Plant Physiol. 2002, 159, 1349–1354. [Google Scholar] [CrossRef]
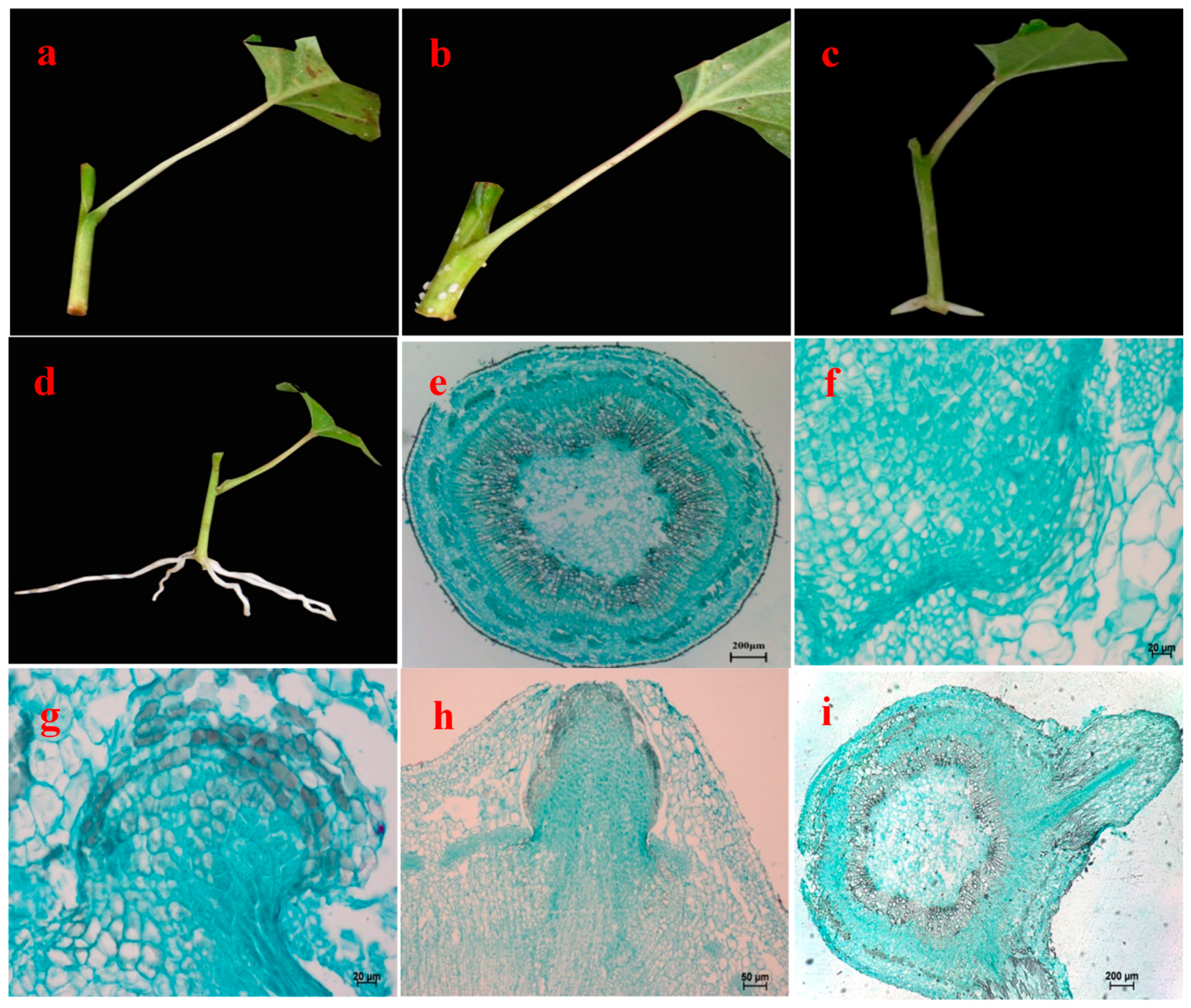
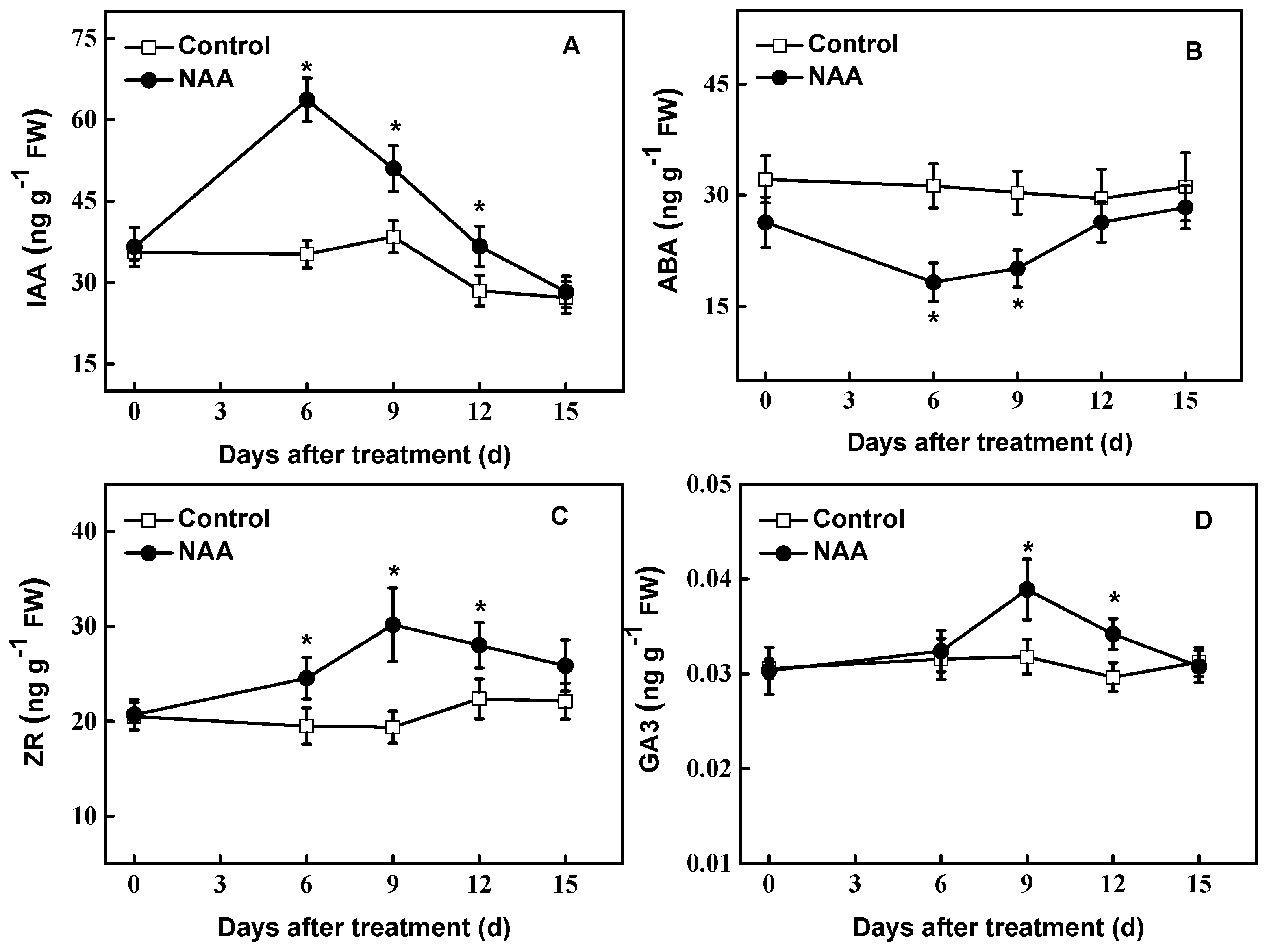
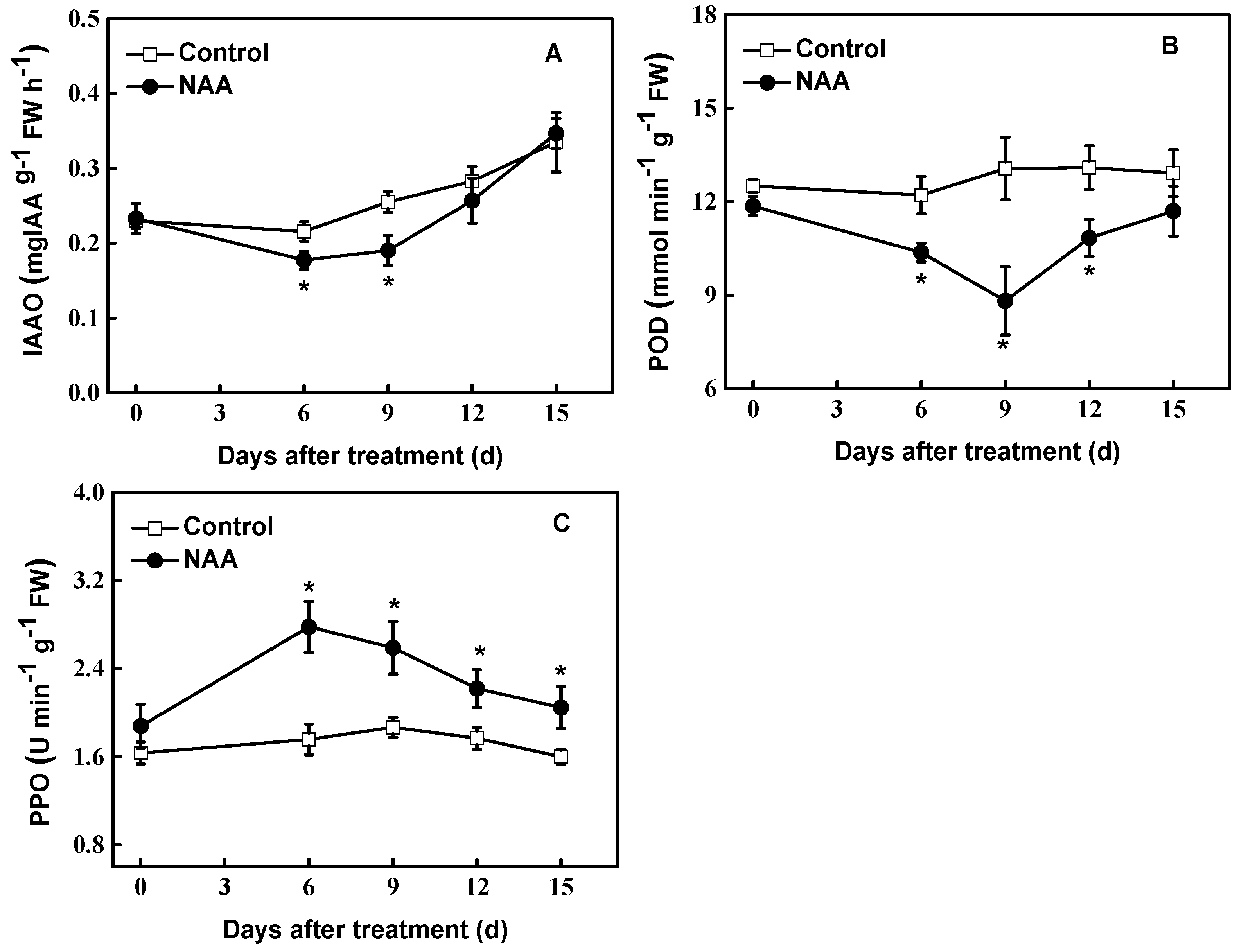
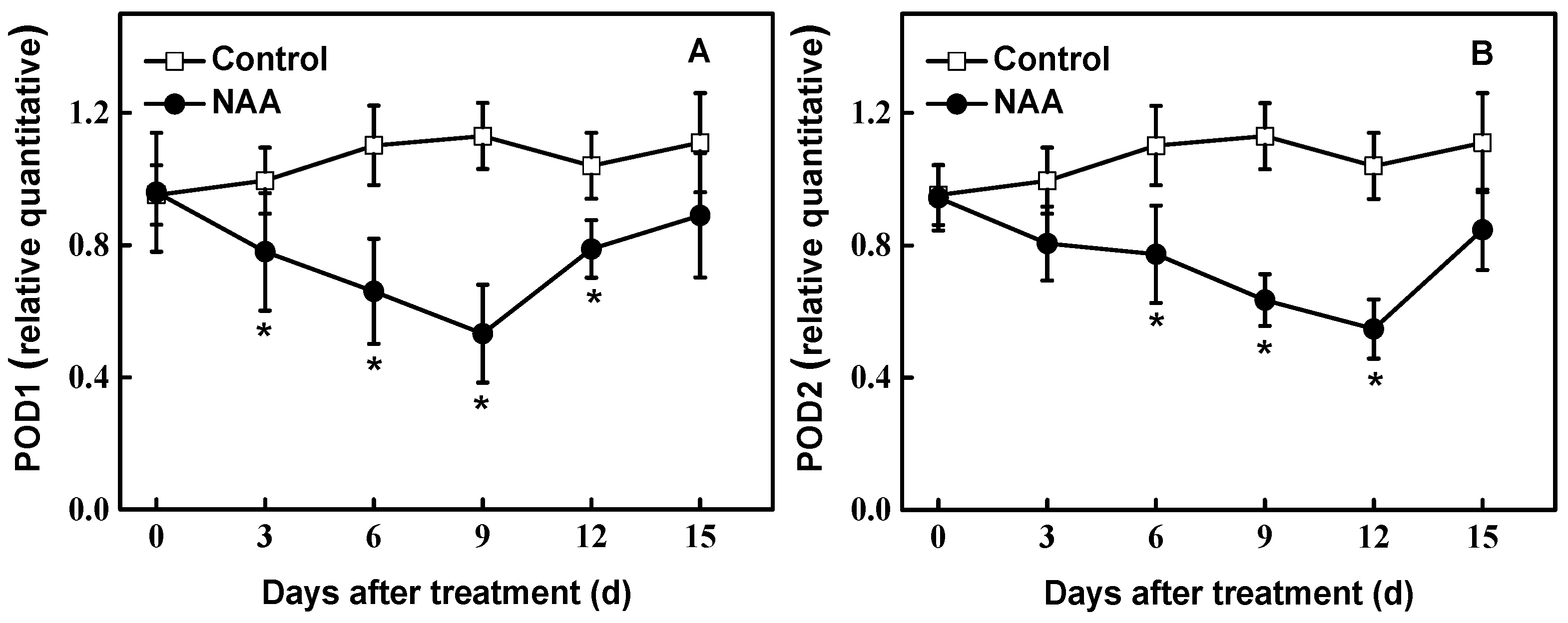
| Different Treatments | % of Rooting (Mean ± SE) | No. of Roots/Cutting (Mean ± SE) | Av. Root Length (cm) (Mean ± SE) |
|---|---|---|---|
| Control (H2O) | 5.80 ± 0.45 i | 1.40 ± 0.05 e | 1.43 ± 0.04 f |
| IAA 0.57 mM | 35.48 ± 0.63 e | 2.72 ± 0.23 c,d | 2.54 ± 0.14 c |
| IAA 1.71 mM | 19.13 ± 0.38 g | 2.87 ± 0.11 c | 2.38 ± 0.14 c |
| IAA 2.85 mM | 15.11 ± 0.78 h | 2.53 ± 0.10 d | 2.01 ± 0.13 d |
| IBA 0.49 mM | 42.10 ± 0.67 d | 3.00 ± 0.06 b,c | 3.54 ± 0.08 a |
| IBA 1.47 mM | 34.34 ± 1.38 e | 3.16 ± 0.10 b | 2.92 ± 0.10 b |
| IBA 2.45 mM | 24.16 ± 0.78 f | 2.72 ± 0.05 c,d | 2.45 ± 0.04 c |
| NAA 0.54 mM | 79.39 ± 0.87 a | 4.08 ± 0.28 a | 1.74 ± 0.11 e |
| NAA 1.62 mM | 63.73 ± 1.09 b | 3.90 ± 0.13 a | 1.53 ± 0.13 e,f |
| NAA 2.70 mM | 52.86 ± 0.24 c | 3.90 ± 0.26 a | 1.50 ± 0.28 e,f |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.P.; Yang, R.H.; Wang, F.; Sun, L.N.; Song, X.S. Effect of Auxins and Associated Metabolic Changes on Cuttings of Hybrid Aspen. Forests 2017, 8, 117. https://doi.org/10.3390/f8040117
Yan SP, Yang RH, Wang F, Sun LN, Song XS. Effect of Auxins and Associated Metabolic Changes on Cuttings of Hybrid Aspen. Forests. 2017; 8(4):117. https://doi.org/10.3390/f8040117
Chicago/Turabian StyleYan, Shao Peng, Rui Hua Yang, Fang Wang, Li Na Sun, and Xing Shun Song. 2017. "Effect of Auxins and Associated Metabolic Changes on Cuttings of Hybrid Aspen" Forests 8, no. 4: 117. https://doi.org/10.3390/f8040117




