Remnant Trees in Enrichment Planted Gaps in Quintana Roo, Mexico: Reasons for Retention and Effects on Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bosquete Development
2.3. Sampling of Enrichment Planted Felling Gaps (i.e., Bosquetes)
2.4. Canopy Cover over Planted Seedlings
2.5. Mixed-Effects Modelling
3. Results
3.1. Standing Tree Retention in Bosquetes
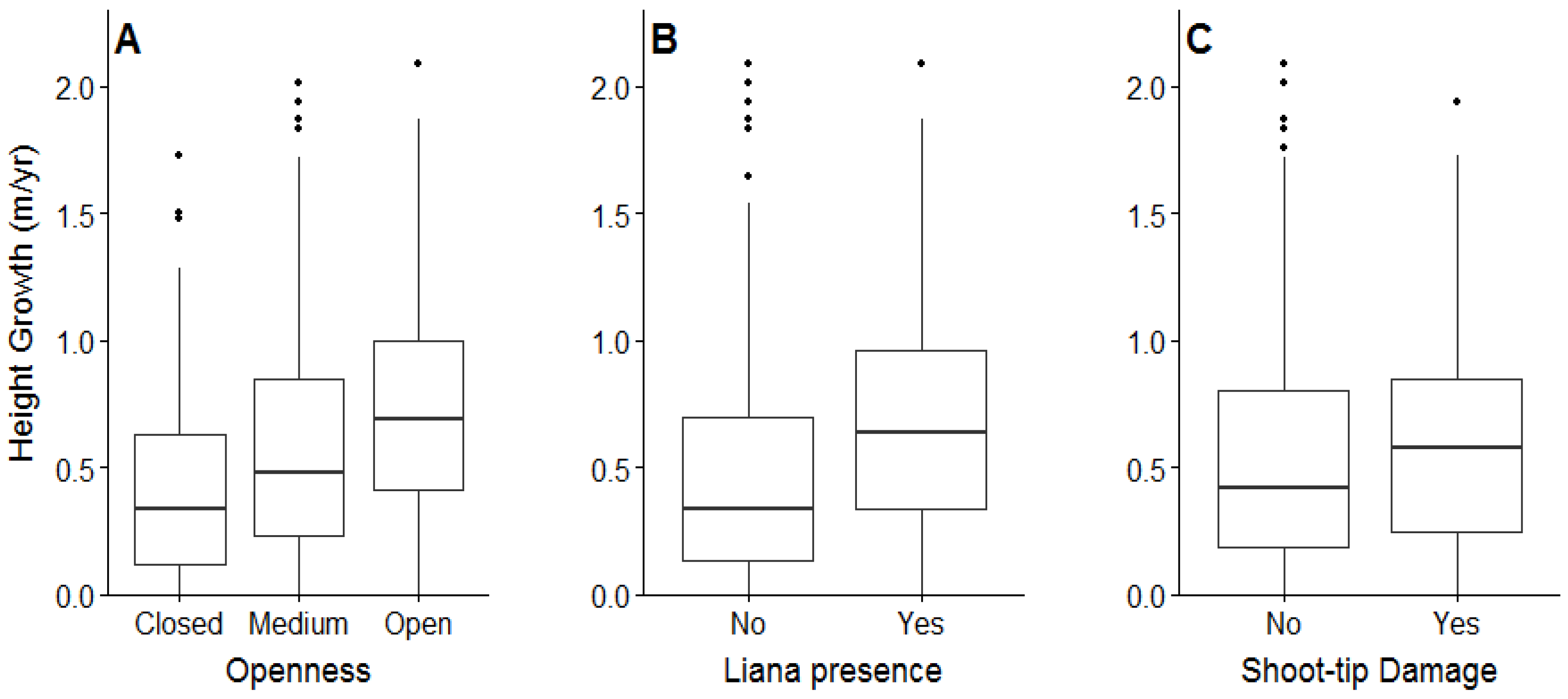
3.2. Planted Seedling Survival, Growth, Shoot Tip Damage, and Liana Infestations
4. Discussion
4.1. Tree Retention in Bosquetes
4.2. Planted Seedling Growth in Relation to Shade, Shoot Tip Damage, and Liana Infestations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peña-Claros, M.L.; Fredericksen, T.S.; Alarcon, A.; Blate, G.M.; Choque, U.; Leaño, C.; Mostacedo, B.; Pariona, W.; Villegas, Z.; Putz, F.E. Beyond reduced-impact logging: Silvicultural treatments to increase growth rates of tropical trees. For. Ecol. Manag. 2008, 256, 1458–1467. [Google Scholar] [CrossRef]
- Putz, F.E.; Romero, C. Futures of Tropical Production Forests; Occasional Paper 143; CIFOR: Bogor, Indonesia, 2015. [Google Scholar]
- Mostacedo, B.; Fredericksen, T.S. Regeneration status of important tropical forest tree species in Bolivia: Assessment and recommendations. For. Ecol. Manag. 1999, 124, 263–273. [Google Scholar] [CrossRef]
- Peña-Claros, M.L.; Boot, R.G.A.; Dorado-Lora, J.; Zonta, A. Enrichment planting of Bertholethia excelsa in secondary forest in the Bolivian Amazon: Effect of cutting line width on survival, growth and crown traits. For. Ecol. Manag. 2002, 161, 159–168. [Google Scholar] [CrossRef]
- Schulze, M. Technical and financial analysis of enrichment planting in logging gaps as a potential component of forest management in the eastern Amazon. For. Ecol. Manag. 2008, 255, 866–879. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Griffith, D.M.; Ramirez-Soria, M.J.; Pariona, W.; Golicher, D.; Palacios, G. Enrichment of big-leaf mahogany (Swietenia macrophylla King) in logging gaps in Bolivia: The effects of planting method and silvicultural treatments on long-term seedling survival and growth. For. Ecol. Manag. 2011, 232, 2271–2280. [Google Scholar] [CrossRef]
- Schwartz, G.; Lopes, J.C.A.; Mohren, G.M.J.; Peña-Claros, M. Post-harvesting silvicultural treatments in logging gaps: A comparison between enrichment planting and tending of natural regeneration. For. Ecol. Manag. 2013, 293, 57–64. [Google Scholar] [CrossRef]
- Keefe, K.; Alavalapati, J.A.A.; Pinheiro, C. Is enrichment planting worth its costs? A financial cost-benefit analysis. For. Policy Econ. 2012, 23, 10–16. [Google Scholar] [CrossRef]
- Lopes, J.C.A.; Jennings, S.B.; Matni, N.M. Planting mahogany in canopy gaps created by commercial harvesting. For. Ecol. Manag. 2008, 255, 300–307. [Google Scholar] [CrossRef]
- Negreros-Castillo, P.; Mize, C.M. Mahogany growth and mortality and the relation of growth to site characteristics in a natural forest in Quintana Roo, Mexico. For. Sci. 2014, 60, 907–913. [Google Scholar] [CrossRef]
- Watt, A.S. On the ecology of British beechwoods with special reference to their regeneration. Part II: The development and structure of beech communities on the Sussex Downs. J. Ecol. 1925, 13, 27–73. [Google Scholar] [CrossRef]
- Aubreville, A. Forêt Coloniale. Les Forêts de l′Afrique Occidentale Française; Annals Academie des Sciences Coloniales: Paris, France, 1938; p. 244. [Google Scholar]
- Denslow, J.S. Tropical rain-forest gaps and tree species-diversity. Annu. Rev. Ecol. Syst. 1987, 18, 431–451. [Google Scholar] [CrossRef]
- Schliemann, S.A.; Bochheim, J.G. Methods for studying treefall gaps: A review. For. Ecol. Manag. 2011, 261, 1143–1151. [Google Scholar] [CrossRef]
- Brokaw, N. The definition of treefall gap and its effect on measures of forest dynamics. Biotropica 1982, 14, 158–160. [Google Scholar] [CrossRef]
- Lieberman, M.; Lieberman, D.; Peralta, R. Forests are not just Swiss cheese: Canopy stereogeometry of non-gaps in tropical forests. Ecology 1989, 70, 550–552. [Google Scholar] [CrossRef]
- Stevenson, N.S. Silvicultural treatment of mahogany forests in British Honduras. Emp. For. J. 1927, 6, 219–227. [Google Scholar]
- Lamb, F.B. Mahogany of Tropical America; University of Michigan Press: Ann Arbor, MI, USA, 1966. [Google Scholar]
- Snook, L.K. Stand Dynamics of Mahogany (Swietenia macrophylla King) and Associated Species after Fire and Hurricane in the Tropical Forests of the Yucatan Peninsula, Mexico. Ph.D. Thesis, Yale University, New Haven, CT, USA, 1993. [Google Scholar]
- Negreros-Castillo, P.; Mize, C.M. Regeneration of mahogany and Spanish cedar in gaps created by railroad tie extraction in Quintana Roo, Mexico. For. Ecol. Manag. 2008, 255, 308–312. [Google Scholar] [CrossRef]
- Snook, L.K. Catastrophic disturbance, logging and the ecology of mahogany (Swietenia macrophylla King): Grounds for listing a major tropical timber species in CITES. Bot. J. Linn. Soc. 1996, 122, 35–46. [Google Scholar] [CrossRef]
- Ellis, E.A.; Kainer, K.; Sierra Huelsz, A.; Negreros-Castillo, P.; Rodriguez-Ward, D.; DiGiano, M. Endurance and adaptation of community forest management in Quintana Roo, México. Forests 2015, 6, 4295–4327. [Google Scholar] [CrossRef]
- Registro Agrario Nacional (RAN). Plano Definitivo de Tierras de uso Común del Ejido Noh Bec Esc 1:50,000; Ejido Noh Bec: Noh Bec, Quintana Roo, Mexico, 2011. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen, 4th ed.; UNAM: Mexico City, Mexico, 1987; p. 217. [Google Scholar]
- Miranda, F. Vegetación de la Península Yucateca; Colegio de Postgraduados: Chapingo, Mexico, 1978; p. 271. [Google Scholar]
- Sánchez-Sánchez, O.; Islebe, G.A. Tropical forest communities in southeastern Mexico. Plant Ecol. 2002, 158, 183–200. [Google Scholar] [CrossRef]
- Vester, H.F.M.; Navarro-Martínez, M.A.; López, C.Y.; Canul, U.V.E.; Wetering, M.; Schonck, S. Informe del Proyecto uso y Monitoreo de los Recursos Naturales en el Corredor Biológico Mesoamericano (Áreas Focales Xpujil-Zoh Laguna y Carrillo Puerto); ECOSUR-CONABIO: Chetumal, Mexico, 2005; p. 64. [Google Scholar]
- Sierra-Huelsz, J.A.; Kainer, K.A.; Keys, E.; Colli-Balam, S.S. Three stories under the same hut: Market preferences and forest governance drive the evolution of tourism construction materials. For. Policy Econ. 2017, 78, 151–161. [Google Scholar] [CrossRef]
- Argüelles, L.A.; Synnott, T.; Gutiérrez, S.; del Ángel, B. Regeneración y silvícultura de la caoba en la Selva Maya Mexicana, ejido Noh Bec. Recur. Nat. Ambient. 2005, 44, 45–52. [Google Scholar]
- Secretaria de Medio Ambiente y Recursos Naturales (SEMARNAT). Norma Oficial Mexicana NOM-059-SEMARNAT-2010; Diario Oficial de la Federación (DOF): Mexico D.F., Mexico, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Keefe, K.; Schulze, M.D.; Pinheiro, C.; Zweede, J.C.; Zarin, D. Enrichment planting as a silvicultural in the eastern Amazon: A case study of Fazenda Cauaxi. For. Ecol. Manag. 2009, 258, 1950–1959. [Google Scholar] [CrossRef]
- Arevalo, B.; Valladarez, J.; Muschamp, S.; Kay, E.; Finkral, A.; Roopsind, A.; Putz, F.E. Effects of reduced-impact selective logging on palm regeneration in Belize. For. Ecol. Manag. 2016, 369, 155–160. [Google Scholar] [CrossRef]
- Dickinson, M.B.; Whigham, D.F. Regeneration of mahogany (Swietenia macrophylla) in the Yucatan. Int. For. Rev. 1999, 1, 35–39. [Google Scholar]
- Snook, L.K.; Negreros-Castillo, P. Regenerating mahogany (Swietenia macrophylla King) on clearings in Mexico’s Maya forest: The effects of clearing method and cleaning on seedling survival and growth. For. Ecol. Manag. 2004, 189, 143–160. [Google Scholar] [CrossRef]
- Clark, D.; Clark, D. Distribution and effects on tree growth of lianas and woody hemiepiphytes in a Costa Rican tropical wet forest. J. Trop. Ecol. 1990, 6, 321–331. [Google Scholar] [CrossRef]
- Gerwing, J. Testing liana cutting and controlled burning as silvicultural treatments for a logged forest in the eastern Amazon. J. Appl. Ecol. 2001, 38, 1264–1276. [Google Scholar] [CrossRef]
- Grauel, W.; Putz, F. Effects of lianas on growth and regeneration of Prioria copaifera in Darien, Panama. For. Ecol. Manag. 2004, 190, 99–108. [Google Scholar] [CrossRef]
- Grogan, J.E. Bigleaf Mahogany (Swietenia macrophylla King) in Southeast Para, Brazil: A Life History Study with Management Guidelines for Sustained Production from Natural Forest. Ph.D. Thesis, Yale University, New Haven, CT, USA, 2001. [Google Scholar]
- Lowe, R.; Walker, P. Classification of canopy, stem, crown status, and climber infestation in natural tropical forest in Nigeria. J. Appl. Ecol. 1977, 14, 897–903. [Google Scholar] [CrossRef]
- Kainer, K.A.; Wadt, L.H.O.; Gomes-Silva, D.A.P.; Capanu, M. Liana loads and their association with Bertholletia excelsa fruit and nut production, diameter growth and crown attributes. J. Trop. Ecol. 2006, 22, 147–154. [Google Scholar] [CrossRef]
- Dawkins, H.C. The Management of Natural Tropical High-Forest with Special Reference to Uganda; Commonwealth Forestry Institute Paper 34; Oxford University: Oxford, UK, 1958; p. 155. [Google Scholar]
- Petrokofsky, G.; Sist, P.; Blanc, L.; Doucet, J.L.; Finegan, B.; Gourlet-Fleury, S.; Healey, J.R.; Livoreil, B.; Nasi, R.; Peña-Claros, M.; et al. Comparative effectiveness of silvicultural interventions for increasing timber production and sustaining conservation values in natural tropical production forests. A systematic review protocol. Environ. Evid. 2015, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]

| Area (m2) | Seedlings (n) | Remnant Trees (n) | Remnants per Hectare | Canopy Cover (% of Gap Area) | Remnant Tree Mean DBH (cm) | Max DBH (cm) |
|---|---|---|---|---|---|---|
| 440 | 117 | 14 | 322 | 43.5 | 18.7 | 35 |
| 560 | 117 | 23 | 413 | 54.3 | 25.1 | 90 |
| 780 | 178 | 16 | 204 | 37.7 | 22.2 | 85 |
| 800 | 220 | 33 | 415 | 27.5 | 15.6 | 37 |
| 920 | 148 | 17 | 184 | 22.6 | 20.2 | 47 |
| 950 | 145 | 17 | 178 | 25.7 | 24.9 | 69 |
| 1030 | 110 | 11 | 107 | 10.1 | 23.4 | 59 |
| 1040 | 324 | 27 | 260 | 30.3 | 17.7 | 64 |
| 1160 | 330 | 31 | 267 | 20.1 | 19.6 | 86 |
| 1170 | 180 | 32 | 275 | 29.2 | 17.9 | 46 |
| 1250 | 162 | 11 | 88 | 13.4 | 22.9 | 47 |
| 1390 | 270 | 25 | 179 | 31.4 | 20.6 | 40 |
| 1710 | 315 | 35 | 204 | 29.0 | 24.8 | 55 |
| 1780 | 266 | 49 | 275 | 30.6 | 19.1 | 54 |
| 1070 * | 206 * | 24.3 * | 241 * | 29.0 * | 20.9 * | 58 * |
| Species | n | Reasons for Retention | ||||
|---|---|---|---|---|---|---|
| Timber | Cultural | Posts | Regulation | Fauna | ||
| Pouteria reticulata | 80 | ● | ||||
| Brosimum alicastrum | 35 | ● | ● | |||
| Sabal japa (palm) | 29 | ● | ||||
| Manilkara zapota | 28 | ● | ● | |||
| Pouteria campechiana | 11 | ● | ● | |||
| Bursera simaruba | 10 | ● | ● | |||
| Cryosophila argentea (palm) | 10 | ● | ||||
| Diospyros cuneata | 10 | ● | ● | |||
| Simira salvadorensis | 10 | ● | ||||
| Trichilia minutiflora | 10 | ● | ● | |||
| Guettarda elliptica | 9 | ● | ||||
| Simarouba amara | 9 | ● | ||||
| Gymnanthes lucida | 8 | ● | ||||
| Alseis yucatanensis | 7 | ● | ||||
| Dendropanax arboreus | 7 | ● | ||||
| Metopium brownei | 7 | ● | ||||
| Vitex gaumeri | 7 | ● | ● | |||
| Coccoloba cozumelensis | 6 | ● | ● | |||
| Sideroxylon gaumeri | 6 | ● | ||||
| Protium copal | 4 | ● | ● | |||
| Motivation for Retention | % Cover in Planted Gaps Mean | Range |
|---|---|---|
| Timber | 11% | 3–27% |
| Cultural | 10% | 1–22% |
| Posts | 7% | 2–11% |
| Regulation | 1% | 0–2% |
| ANY | 29% | 10–54% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Martínez, A.; Palmas, S.; Ellis, E.A.; Blanco-Reyes, P.; Vargas-Godínez, C.; Iuit-Jiménez, A.C.; Hernández-Gómez, I.U.; Ellis, P.; Álvarez-Ugalde, A.; Carrera-Quirino, Y.G.; et al. Remnant Trees in Enrichment Planted Gaps in Quintana Roo, Mexico: Reasons for Retention and Effects on Seedlings. Forests 2017, 8, 272. https://doi.org/10.3390/f8080272
Navarro-Martínez A, Palmas S, Ellis EA, Blanco-Reyes P, Vargas-Godínez C, Iuit-Jiménez AC, Hernández-Gómez IU, Ellis P, Álvarez-Ugalde A, Carrera-Quirino YG, et al. Remnant Trees in Enrichment Planted Gaps in Quintana Roo, Mexico: Reasons for Retention and Effects on Seedlings. Forests. 2017; 8(8):272. https://doi.org/10.3390/f8080272
Chicago/Turabian StyleNavarro-Martínez, Angélica, Sebastian Palmas, Edward A. Ellis, Pascual Blanco-Reyes, Carolina Vargas-Godínez, Ana Cecilia Iuit-Jiménez, Irving Uriel Hernández-Gómez, Peter Ellis, Alfredo Álvarez-Ugalde, Yavé Guadalupe Carrera-Quirino, and et al. 2017. "Remnant Trees in Enrichment Planted Gaps in Quintana Roo, Mexico: Reasons for Retention and Effects on Seedlings" Forests 8, no. 8: 272. https://doi.org/10.3390/f8080272
APA StyleNavarro-Martínez, A., Palmas, S., Ellis, E. A., Blanco-Reyes, P., Vargas-Godínez, C., Iuit-Jiménez, A. C., Hernández-Gómez, I. U., Ellis, P., Álvarez-Ugalde, A., Carrera-Quirino, Y. G., Armenta-Montero, S., & Putz, F. E. (2017). Remnant Trees in Enrichment Planted Gaps in Quintana Roo, Mexico: Reasons for Retention and Effects on Seedlings. Forests, 8(8), 272. https://doi.org/10.3390/f8080272








