Lianas Abundance is Positively Related with the Avian Acoustic Community in Tropical Dry Forests
Abstract
:1. Introduction
2. Materials and Methods
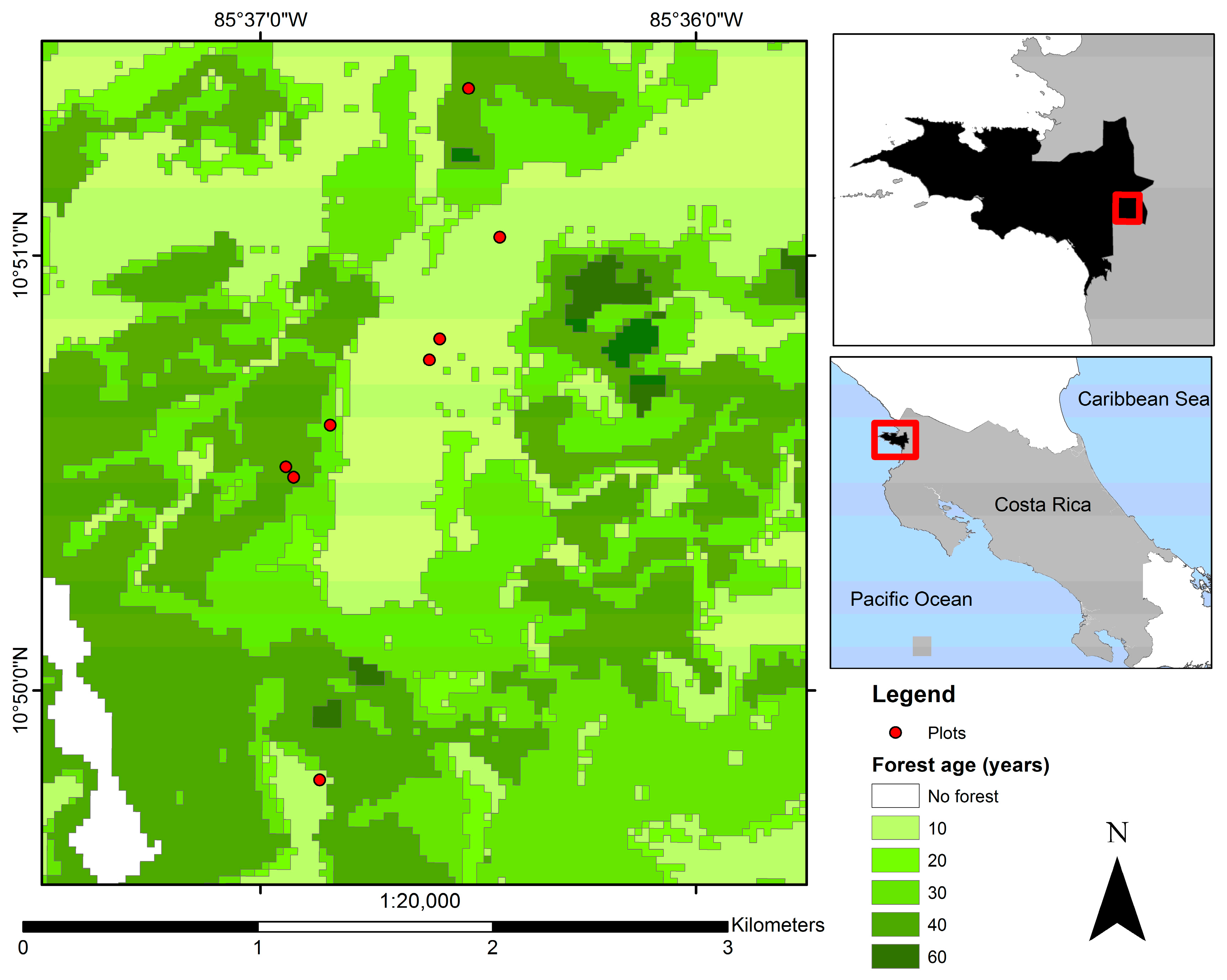
2.1. Study Site and Experimental Design
2.2. Forest Biophysical Properties
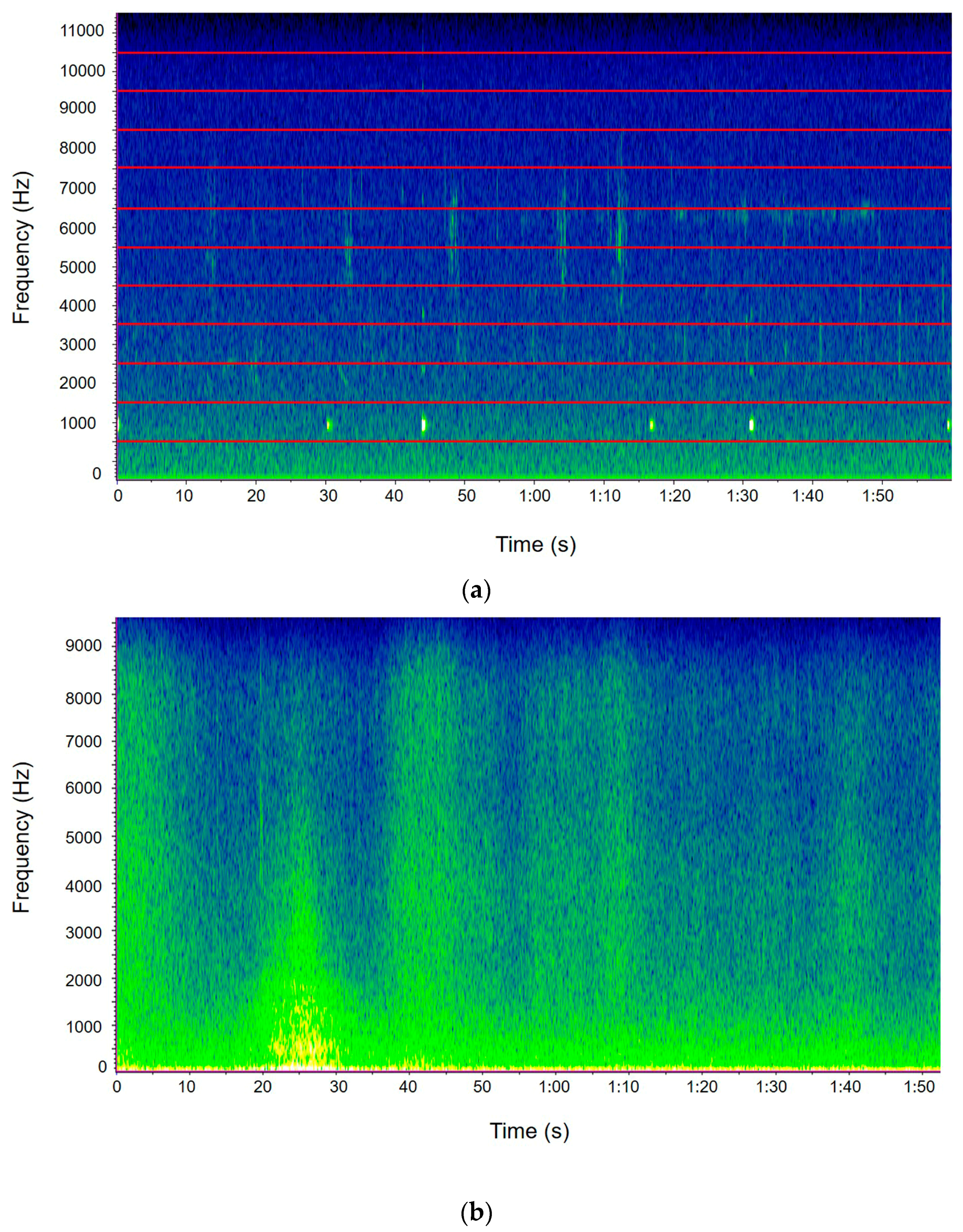
2.3. The Avian Acoustic Community
2.4. Statistical Analysis
3. Results
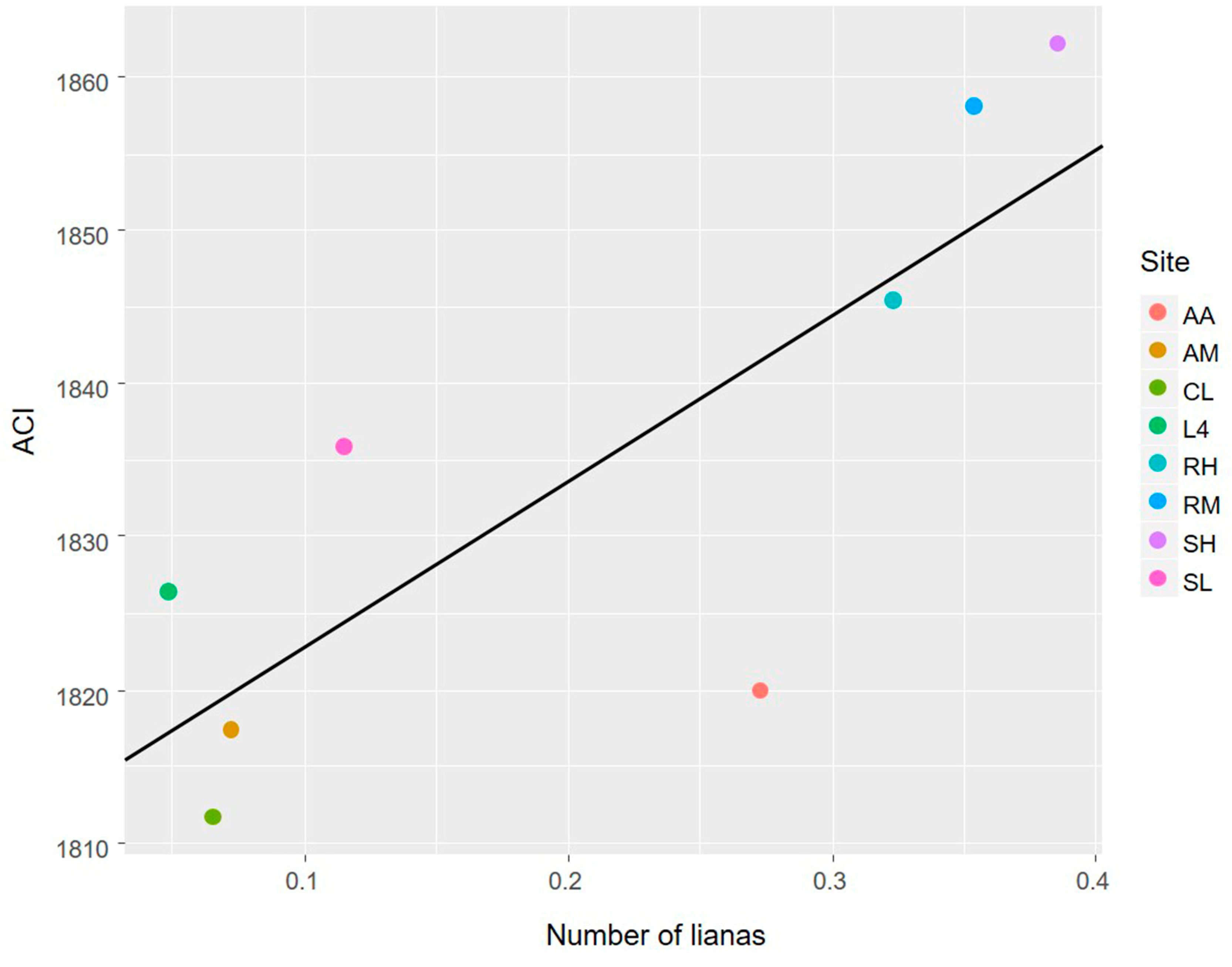
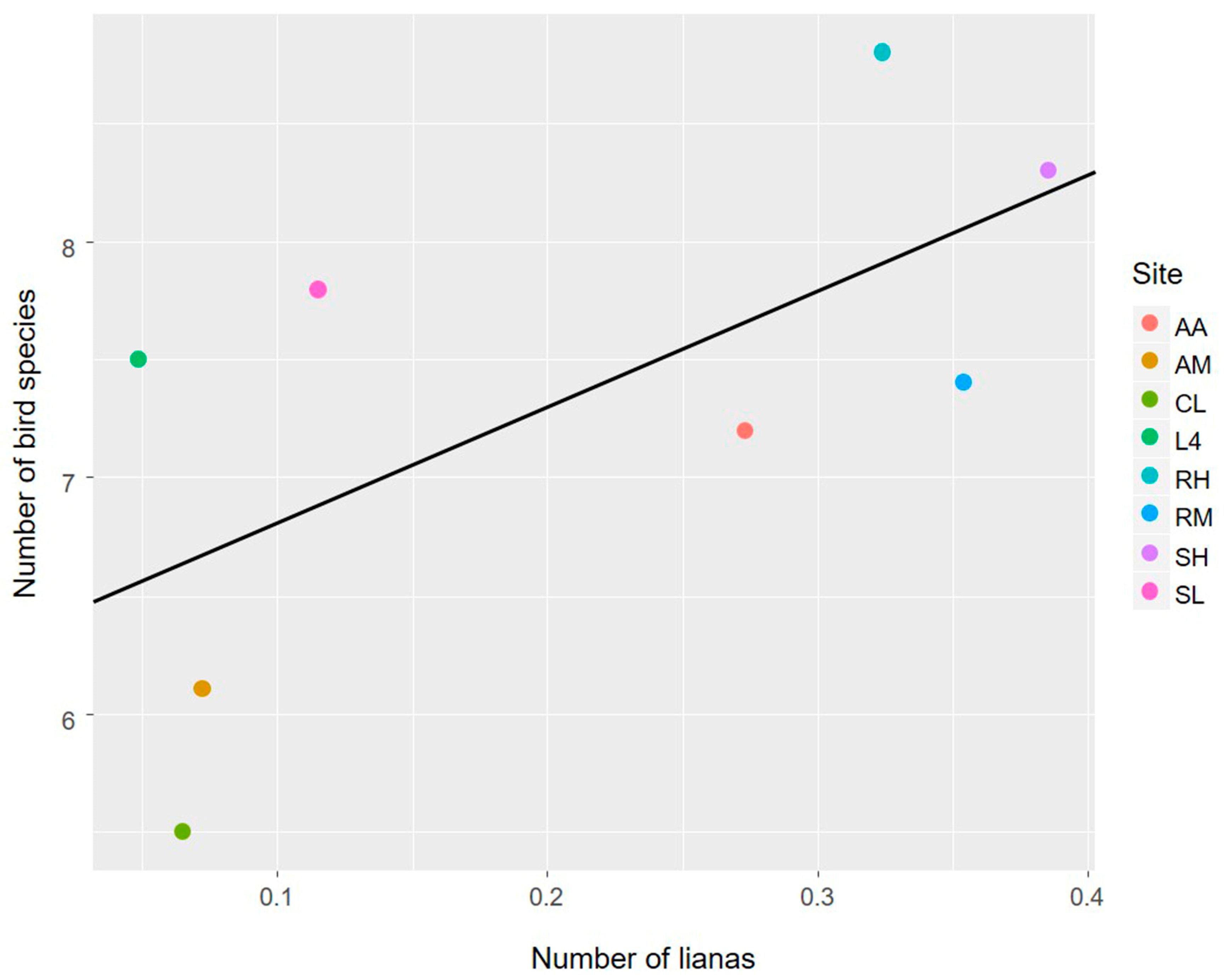
3.1. The Avian Acoustic Community Relationship with Liana Abundance and Forest Biophysical Properties
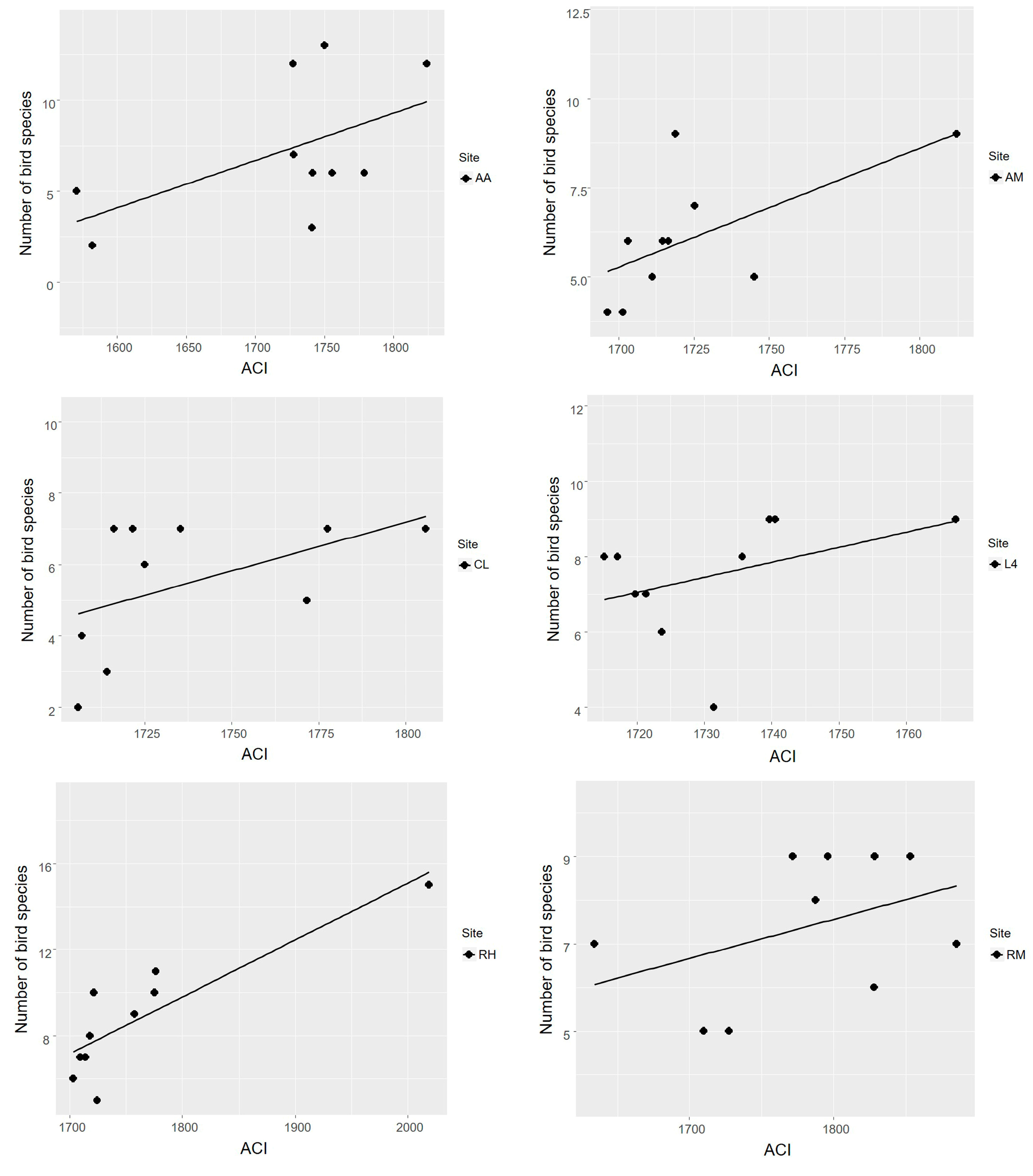
3.2. Acoustic Complexity Index as an Estimator of Bird Species Richness
4. Discussion and Conclusions
4.1. The Avian Acoustic Community Relationship with Liana Abundance and Forest Biophysical Properties
4.2. Acoustic Complexity Index as an Estimator of Bird Species Richness
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gillespie, T.W.; Grijalva, A.; Farris, C.N. Diversity, composition, and structure of tropical dry forests in Central America. Plant Ecol. 2000, 147, 37–47. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Castro-Esau, K. Canopy observations on the hyperspectral properties of a community of tropical dry forest lianas and their host trees. Int. J. Remote Sens. 2006, 27, 2101–2109. [Google Scholar] [CrossRef]
- Gerwing, J.J. The influence of reproductive traits on liana abundance 10 years after conventional and reduced-impacts logging in the eastern Brazilian Amazon. For. Ecol. Manag. 2006, 221, 83–90. [Google Scholar] [CrossRef]
- Schnitzer, S.A. A mechanistic explanation for global patterns of liana abundance and distribution. Am. Nat. 2005, 166, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Michel, N.L.; Robinson, W.D.; Sherry, T.W. Liana-bird relationships: A review. In Ecology of Lianas; Schnitzer, S., Bongers, F., Burnham, R.J., Putz, F.E., Eds.; John Wiley & Sons: Chichester, UK, 2014; pp. 362–397. [Google Scholar]
- Muller-Landau, H.C.; Hardesty, B.D. Seed dispersal of woody plants in tropical forests: Concepts, examples and future directions. In Biotic Interactions in the Tropics: Their Role in the Maintenance of Species Diversity; Burslem, D., Pinard, M., Hartley, S., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 267–309. [Google Scholar]
- Fleming, T.H.; Muchhala, N.; Ornelas, J.F. New World nectar-feeding vertebrates: Community patterns and processes. In Contribuciones Mastozoológicas en Homenaje a Bernardo Villa; Sánchez-Cordero, V., Medellín, R.A., Eds.; Institutos de Biología y Ecología, UNAM, and CONABIO: Mexico City, Mexico, 2005; pp. 163–186. [Google Scholar]
- Ansell, F.A.; Edwards, D.P.; Hamer, K.C. Rehabilitation of Logged Rain Forests: Avifaunal Composition, Habitat Structure, and Implications for Biodiversity-Friendly REDD+. Biotropica 2011, 43, 504–511. [Google Scholar] [CrossRef]
- Felton, A.; Wood, J.T.; Felton, A.M.; Lindenmayer, D.B.; Hennessey, B.A. A comparison of bird communities in the anthropogenic and natural-tree fall gaps of a reduced-impact logged subtropical forest in Bolivia. Bird Conserv. Int. 2008, 18, 129–143. [Google Scholar] [CrossRef]
- Robinson, W.D.; Robinson, W.D. Changes in abundance of birds in a Neotropical forest fragment over 25 years: A review. Anim. Biodivers. Conserv. 2001, 24, 51–65. [Google Scholar]
- Sueur, J.; Pavoine, S.; Hamerlynck, O.; Duvail, S. Rapid acoustic survey for biodiversity appraisal. PLoS ONE 2008, 3, e4065. [Google Scholar] [CrossRef] [PubMed]
- Aide, T.M.; Corrada-Bravo, C.; Campos-Cerqueira, M.; Milan, C.; Vega, G.; Alvarez, R. Real-time bioacoustics monitoring and automated species identification. PeerJ 2013, e103. [Google Scholar] [CrossRef] [PubMed]
- Sueur, J.; Farina, A.; Gasc, A.; Pieretti, N.; Pavoine, S. Acoustic indices for biodiversity assessment and landscape investigation. Acta Acust. Acust. 2014, 100, 772–781. [Google Scholar] [CrossRef]
- Towsey, M.; Wimmer, J.; Williamson, I.; Roe, P. The use of acoustic indices to determine avian species richness in audio-recordings of the environment. Ecol. Inform. 2014, 21, 110–119. [Google Scholar] [CrossRef]
- Farina, A.; Pieretti, N.; Piccioli, L. The soundscape methodology for long-term bird monitoring: A Mediterranean Europe case-study. Ecol. Inform. 2011, 6, 354–363. [Google Scholar] [CrossRef]
- Pieretti, N.; Farina, A.; Morri, D. A new methodology to infer the singing activity of an avian community: The Acoustic Complexity Index (ACI). Ecol. Indic. 2011, 11, 868–873. [Google Scholar] [CrossRef]
- Depraetere, M.; Pavoine, S.; Jiguet, F.; Gasc, A.; Duvail, S.; Sueur, J. Author’s personal copy Monitoring animal diversity using acoustic indices: Implementation in a temperate woodland. Ecol. Indic. 2012, 13, 46–54. [Google Scholar] [CrossRef]
- Pieretti, N.; Farina, A. Application of a recently introduced index for acoustic complexity to an avian soundscape with traffic noise. J. Acoust. Soc. Am. 2013, 134, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Gasc, A.; Sueur, J.; Jiguet, F.; Devictor, V.; Grandcolas, P.; Burrow, C.; Depraetere, M.; Pavoine, S. Assessing biodiversity with sound: Do acoustic diversity indices reflect phylogenetic and functional diversities of bird communities? Ecol. Indic. 2013, 25, 279–287. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gasc, A.; Pavoine, S.; Grandcolas, P.; Gaucher, P.; Sueur, J. Temporal and spatial variability of animal sound within a neotropical forest. Ecol. Inform. 2014, 21, 133–143. [Google Scholar] [CrossRef]
- Buxton, R.T.; Brown, E.; Sharman, L.; Gabriele, C.M.; McKenna, M.F. Using bioacoustics to examine shifts in songbird phenology. Ecol. Evol. 2016, 6, 4697–4710. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yu, Q.; Sanchez-Azofeifa, A.; Feng, J.; Rivard, B.; Gu, Z. Mapping tropical dry forest succession using multiple criteria spectral mixture analysis. ISPRS J. Photogramm. Remote Sens. 2015, 109, 17–29. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Quesada, M.; Rodríguez, J.P.; Nassar, J.M.; Stoner, K.E.; Castillo, A.; Garvin, T.; Zent, E.L.; Calvo-Alvarado, J.C.; Kalacska, M.E.R.; et al. Research priorities for neotropical dry forests. Biotropica 2005, 37, 474. [Google Scholar] [CrossRef]
- Quesada, M.; Stoner, K.E. Threats to the conservation of the tropical dry forest in Costa Rica. In Biodiversity Conservation in Costa Rica: Learning the Lessons in a Seasonal Dry Forest; Frankie, G.W., Mata, A., Vinson, S.B., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 266–280. [Google Scholar]
- Arroyo-Mora, J.P.; Sánchez-Azofeifa, G.A.; Rivard, B.; Calvo, J.C.; Janzen, D.H. Dynamics in landscape structure and composition for the Chorotega region, Costa Rica from 1960 to 2000. Agric. Ecosyst. Environ. 2005, 106, 27–39. [Google Scholar] [CrossRef]
- Kalácska, M.; Sánchez-Azofeifa, G.; Calvo-Alvarado, J.; Quesada, M.; Rivard, B.; Janzen, D. Species composition, similarity and diversity in three successional stages of a seasonally dry tropical forest. For. Ecol. Manag. 2004, 200, 227–247. [Google Scholar] [CrossRef]
- Janzen, D.H. Plants. Species accounts. In Costa Rican Natural History; Janzen, D.H., Ed.; University of Chicago Press: Chicago, IL, USA, 1983; pp. 148–350. [Google Scholar]
- Hilje, B.; Calvo-Alvarado, J.; Jiménez-Rodríguez, C.; Sánchez-Azofeifa, A. Tree species composition, breeding systems, and pollination and dispersal syndromes in three forest successional stages in a tropical dry forest in Mesoamerica. Trop. Conserv. Sci. 2015, 8, 76–94. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Guzmán-Quesada, J.A.; Vega-Araya, M.; Campos-Vargas, C.; Durán, S.M.; D’Souza, N.; Gianoli, T.; Portillo-Quintero, C.; Sharp, I. Can terrestrial laser scanners (TLSs) and hemispherical photographs predict tropical dry forest succession with liana abundance? Biogeosciences 2017, 14, 977–988. [Google Scholar] [CrossRef]
- Kalácska, M.E.R.; Sánchez-Azofeifa, G.A.; Calvo-Alvarado, J.C.; Rivard, B.; Quesada, M. Effects of season and successional stage on leaf area index and spectral vegetation indices in three mesoamerican tropical dry forests. Biotropica 2005, 37, 486–496. [Google Scholar] [CrossRef]
- Delta-T Devices, Ltd. Hemispherical Photograph Analyser User Guide; The University of Kansas Centre for Research, Inc.: Kansas City, KS, USA, 1999. [Google Scholar]
- Chen, J.M.; Black, T.A.; Adams, R.S. Evaluation of hemispherical photography for determining plant area index and geometry of a forest stand. Agric. For. Meteorol. 1991, 56, 129–143. [Google Scholar] [CrossRef]
- Wildlife Acoustics Inc. Songscope: Bioacoustics Software, Version 4.0; Wildlife Acoustics Inc.: Maynard, MA, USA, 2011. [Google Scholar]
- Villanueva-Rivera, L.J.; Pijanowski, B.C.; Villanueva-Rivera, M.L.J. Soundscape Ecology; Package “Soundecology” Version 1.3.2; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Farina, A.; Pieretti, N. Sonic environment and vegetation structure: A methodological approach for a soundscape analysis of a Mediterranean maqui. Ecol. Inform. 2014, 21, 120–132. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2003. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Soc. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Sánchez, N.V.; Vargas-Castro, L.E.; Avalos, G.; Paniagua, F. Effect of prey availability on the abundance of White-breasted Wood-Wrens, insectivorous birds of tropical lowland forests. J. Field Ornithol. 2014, 85, 347–354. [Google Scholar] [CrossRef]
- Skutch, A.F. Life histories of Central American birds. II: Families Vireonidae, Sylviidae, Turdidae, Troglodytidae, Paridae, Corvidae, Hirundinidae and Tyrannidae. Pac. Coast Avifauna 1960, 34, 116–210. [Google Scholar]
- Yanoviak, S.P. Effects of Lianas on Canopy Arthropod Community Structure. Ecology of Lianas; John Wiley & Sons Ltd.: Chichester, UK, 2015. [Google Scholar]
- Ødegaard, F. How many species of arthropods? Erwin’s estimate revised. Biol. J. Linnean Soc. 2000, 71, 583–597. [Google Scholar]
- Wolda, H. Abundance and diversity of Homoptera in the canopy of a tropical forest. Ecol. Entomol. 1979, 4, 181–190. [Google Scholar] [CrossRef]
- Janzen, D.H. Insect diversity of a Costa Rican dry forest: Why keep it, and how? Biol. J. Linnean Soc. 1987, 30, 343–356. [Google Scholar] [CrossRef]
- García León, M.M.; Martínez Izquierdo, L.; Mello, F.N.A.; Powers, J.S.; Schnitzer, S.A. Lianas reduce community-level canopy tree reproduction in a Panamanian forest. J. Ecol. 2017, 1–9. [Google Scholar] [CrossRef]
- Nassar, J.; Stoner, K.E.; Ávila-Cabadilla, L.; Do Espírito-Santo, M.M.; Aranguren, C.I.; González-Carcacía, J.A.; Lobato-García, J.M.; Olívio-Leite, L.; Álvarez-Añorve, M.; De Matos Brandão, H.N.; et al. Fruit-Eating Bats and Birds of Three Seasonal Tropical Dry Forests in the Americas. In Tropical Dry Forests in the Americas: Ecology, Conservation and Management; Sánchez-Azofeifa, A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2013; pp. 173–220. [Google Scholar]
- Bertucci, F.; Parmentier, E.; Lecellier, G.; Hawkins, A.D.; Lecchini, D. Acoustic indices provide information on the status of coral reefs: An example from Moorea Island in the South Pacific. Sci. Rep. 2016, 6, 33326. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H. Tropical dry forests. In Biodiversity; Wilson, E.O., Peter, F.M., Eds.; National Academy Press: Washington, DC, USA, 1988; pp. 130–137. [Google Scholar]
- Farina, A.; James, P. The acoustic communities: Definition, description and ecological role. Biosystems 2016, 147, 11–20. [Google Scholar] [CrossRef] [PubMed]


| Model Rank | AICc | ΔAICc | AICcw | K |
|---|---|---|---|---|
| number of lianas | 75.82 | 0 | 0.6352 | 2 |
| CH + number of lianas | 79.70 | 3.889 | 0.0908 | 3 |
| number of lianas + PAI | 79.77 | 3.954 | 0.0879 | 3 |
| BA + number of lianas | 79.80 | 3.984 | 0.0866 | 3 |
| NULL | 81.62 | 5.804 | 0.0348 | 1 |
| BA + CH + number of lianas | 83.64 | 7.822 | 0.0127 | 4 |
| CH + number of lianas + PAI | 83.68 | 7.864 | 0.0124 | 4 |
| BA + number of lianas + PAI | 83.77 | 7.953 | 0.0119 | 4 |
| PAI | 84.11 | 8.291 | 0.0100 | 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilje, B.; Stack, S.; Sánchez-Azofeifa, A. Lianas Abundance is Positively Related with the Avian Acoustic Community in Tropical Dry Forests. Forests 2017, 8, 311. https://doi.org/10.3390/f8090311
Hilje B, Stack S, Sánchez-Azofeifa A. Lianas Abundance is Positively Related with the Avian Acoustic Community in Tropical Dry Forests. Forests. 2017; 8(9):311. https://doi.org/10.3390/f8090311
Chicago/Turabian StyleHilje, Branko, Shauna Stack, and Arturo Sánchez-Azofeifa. 2017. "Lianas Abundance is Positively Related with the Avian Acoustic Community in Tropical Dry Forests" Forests 8, no. 9: 311. https://doi.org/10.3390/f8090311






