How Reliable Are Heat Pulse Velocity Methods for Estimating Tree Transpiration?
Abstract
:1. Introduction
2. Heat Pulse Velocity Methods
3. The Accuracy of HPV Sensors
4. Sources of Error
4.1. Measurement Range
4.2. Probe Misalignment
4.3. Wounding
4.4. Thermal Diffusivity or Conductivity
4.5. Stem Moisture Content
4.6. Sapwood Radial and Azimuthal Variability
4.7. Measurement Zone of Influence and Positioning of Sensors
5. Discussion
6. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Forster, M.A. Quantifying water use in a plant-fungal interaction. Fungal Ecol. 2012, 5, 702–709. [Google Scholar] [CrossRef]
- Jara, J.; Stockle, C.O.; Kjelgaard, J. Measurement of evapotranspiration and its components in a corn (Zea mays L.) field. Agric. For. Meteorol. 1998, 92, 131–145. [Google Scholar] [CrossRef]
- Vertessy, R.; Watson, F.; O’Sullivan, S.; Davis, S.; Campbell, R.; Benyon, R.; Haydon, S. Predicting Water Yield from Mountain Ash Forest Catchments; Cooperative Research Centre for Catchment Hydrology Industry Report 98/4; Monash: Victoria, Australia, 1998. [Google Scholar]
- Kohnke, H.; Dreibelbis, F.R.; Davidson, J.M. A Survey and Discussion of Lysimeters and a Bibliography on Their Construction and Performance; Misc. Publ. No. 372; U.S. Department of Agriculture: Washington, DC, USA, 1940.
- Van Bavel, C.H.M.; Nakayama, F.S.; Ehrler, W.L. Measuring transpiration resistance of leaves. Plant Physiol. 1965, 40, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Huber, B. Beobachtung und Messung pflanzlicher Saftstr6me. Ber. Deutsch. Bot. Ges. 1932, 50, 89–109. [Google Scholar]
- Smith, D.M.; Allen, S.J. Measurement of sap flow in plant stems. J. Exp. Bot. 1996, 47, 1833–1844. [Google Scholar] [CrossRef]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants, 6th ed.; W.H. Freeman and Company: New York, NY, USA, 1999. [Google Scholar]
- Vandegehuchte, M.W.; Steppe, K. Sap-flux density measurement methods: Working principles and applicability. Funct. Plant Biol. 2013, 40, 213–223. [Google Scholar] [CrossRef]
- Swanson, R.H. An Instrument for Detecting Sap Movement in Woody Plants; Station Paper 68; USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1962.
- Marshall, D.C. Measurement of sap flow in conifers by heat transport. Plant Physiol. 1958, 33, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.H.; Bleby, T.M. An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol. 2001, 21, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Fuchs, M.; Green, G.C. Improvement of the heat pulse method for determining sap flow in trees. Plant Cell Environ. 1981, 4, 391–397. [Google Scholar] [CrossRef]
- Vandegehuchte, M.W.; Steppe, K. Sapflow+: A four-needle heat-pulse sap flow sensor enabling nonempirical sap flux density and water content measurements. New Phytol. 2012, 196, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Bleby, T.M.; Burgess, S.S.O.; Adams, M.A. A validation, comparison and error analysis of two heat-pulse methods for measuring sap flow in Eucalyptus marginata saplings. Funct. Plant Biol. 2004, 31, 645–658. [Google Scholar] [CrossRef]
- Green, S.; Clothier, B.; Jardine, B. Theory and practical application of heat pulse to measure sap flow. Agron. J. 2003, 95, 1371–1379. [Google Scholar] [CrossRef]
- Green, S.; Clothier, B.; Perie, E. A re-analysis of heat pulse theory across a wide range of sap flows. Acta Hort. 2009, 846, 95–104. [Google Scholar] [CrossRef]
- Jones, H.G.; Hamer, P.J.C.; Higgs, K.H. Evaluation of various heat-pulse methods for estimation of sap flow in orchard trees: Comparison with micrometeorological estimates of evaporation. Trees 1988, 2, 250–260. [Google Scholar] [CrossRef]
- Pearsall, K.R.; Williams, L.E.; Castorani, S.; Bleby, T.M.; McElrone, A.J. Evaluating the potential of a novel dual heat-pulse sensor to measure volumetric water use in grapevines under a range of flow conditions. Funct. Plant Biol. 2014, 41, 874–883. [Google Scholar] [CrossRef]
- Ballester, C.; Castel, J.; Sanz, F.; Yeves, A.; Intrigliolo, D.S.; Castel, J.R. Can sap flow be used for determining transpiration of citrus trees under different irrigation regimes? Acta Hort. 2011, 922, 221–228. [Google Scholar] [CrossRef]
- Dragoni, D.; Lakso, A.N.; Piccioni, R.M. Transpiration of apple trees in a humid climate using heat pulse sap flow gauges calibrated with whole-canopy gas exchange chambers. Agric. For. Meterol. 2005, 130, 85–94. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Lewis, J.D.; Medlyn, B.; Barton, C.V.M.; Duursma, R.A.; Eamus, D.; Adams, M.A.; Phillips, N.; Ellsworth, D.S.; Forster, M.A.; et al. Interactive effects of elevated CO2 and drought on nocturnal water fluxes in Eucalyptus saligna. Tree Physiol. 2011, 31, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, P.T.; Astill, M.S.; Green, S.R.; Mills, T.M.; Clothier, B.E. Water use by a kiwifruit vine: Calibration, measurement and a model. Acta Hort. 2007, 753, 535–538. [Google Scholar] [CrossRef]
- Fuchs, S.; Leuschner, C.; Link, R.; Coners, H.; Schuldt, B. Calibration and comparison of thermal dissipation, heat ratio and heat field deformation sap flow probes for diffuse-porous trees. Agric. For. Meteorol. 2017, 244–245, 151–161. [Google Scholar] [CrossRef]
- Bleby, T.M.; McElrone, A.J.; Burgess, S.S.O. Limitations of the HRM: Great at low flow rates, but no yet up to speed? In Proceedings of the 7th International Workshop on Sap Flow: Book of Abstracts, Seville, Spain, 21–24 October 2008; International Society of Horticultural Sciences: Seville, Spain, 2008. [Google Scholar]
- Forster, M.A. How significant is nocturnal sap flow? Tree Physiol. 2014, 34, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Benyon, R.G. Nighttime water use in an irrigated Eucalyptus grandis plantation. Tree Physiol. 1999, 19, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Liu, G.; Wen, M.; Horton, R.; Li, B.; Si, B. The effects of probe misalignment on sap flux density measurements and in situ probe spacing correction methods. Agric. For. Meteorol. 2017, 232, 176–185. [Google Scholar] [CrossRef]
- Barrett, D.J.; Hatton, T.J.; Ash, J.E.; Ball, M.C. Evaluation of the heat pulse velocity technique for measurement of sap flow in rainforest and eucalypt forest species of south-eastern Australia. Plant Cell Environ. 1995, 18, 463–469. [Google Scholar] [CrossRef]
- Swanson, R.H.; Whitfield, D.W.A. A numerical analysis of heat pulse velocity and theory. J. Exp. Bot. 1981, 32, 221–239. [Google Scholar] [CrossRef]
- Bleby, T.M.; McElrone, A.J.; Jackson, R.B. Water uptake and hydraulic redistribution across large woody root systems to 20 m depth. Plant Cell Environ. 2010, 33, 2132–2148. [Google Scholar] [CrossRef] [PubMed]
- Green, S.R.; Clothier, B.E. Water use of kiwifruit vines and apple trees by the heat-pulse technique. J. Exp. Bot. 1988, 39, 115–123. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Si, J.H.; Qi, F.; Yu, T.F.; Li, P.D. Comparative study of daytime and nighttime sap flow of Populus euphratica. Plant Growth Regul. 2017, 82, 353–362. [Google Scholar] [CrossRef]
- Deng, Z.; Guan, H.; Hutson, J.; Forster, M.A.; Wang, Y.; Simmons, C.T. A vegetation focused soil-plant-atmospheric continuum model to study hydrodynamic soil-plant water relations. Water Resour. Res. 2017, 53, 4965–4983. [Google Scholar] [CrossRef]
- Looker, N.; Martin, J.; Jencso, K.; Hu, J. Contribution of sapwood traits to uncertainty in conifer sap flow as estimated with the heat-ratio method. Agric. For. Meteorol. 2016, 223, 60–71. [Google Scholar] [CrossRef]
- Kluitenberg, G.J.; Ham, J.M. Improved theory for calculating sap flow with the heat pulse method. Agric. For. Meteorol. 2004, 126, 169–173. [Google Scholar] [CrossRef]
- Vandegehuchte, M.W.; Steppe, K. Improving sap flux density measurements by correctly determining thermal diffusivity, differentiating between bound and unbound water. Tree Physiol. 2012, 32, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.; Edwards, W.R.N. Corrected heat capacity of wood for sap flow calculations. Tree Physiol. 1999, 19, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Pfautsch, S.; Keitel, C.; Turnbull, T.; Braimbridge, M.J.; Wright, T.E.; Simpson, R.R.; O’Brien, J.A.; Adams, M.A. Diurnal patterns of water use in Eucalyptus victrix indicate pronounced desiccation-rehydration cycles despite unlimited water supply. Tree Physiol. 2011, 31, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- López-Bernal, A.; Testi, L.; Villalobos, F.J. Using the compensated heat pulse method to monitor trends in stem water content in standing trees. Tree Physiol. 2012, 32, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.; Hassler, S.K.; Blume, T.; Weiler, M.; Hildebrandt, A.; Guderle, M.; Schymanski, S.J.; Kleidon, A. Dominant controls of transpiration along a hillslope transect inferred from ecohydrological measurements and thermodynamic limits. Hydrol. Earth Syst. Sci. 2016, 20, 2063–2083. [Google Scholar] [CrossRef]
- Hogg, E.H.; Black, T.A.; den Hartog, G.; Neumann, H.H.; Zimmermann, R.; Hurdle, P.A.; Blanken, P.D.; Nesic, Z.; Yang, P.C.; Staebler, R.M.; et al. A comparison of sap flow and eddy fluxes of water vapor from a boreal deciduous forest. J. Geophys. Res. 1997, 102, 28929–28937. [Google Scholar] [CrossRef]
- Steppe, K.; De Pauw, D.J.W.; Doody, T.M.; Teskey, R.O. A comparison of sap flux density using thermal dissipation, heat pulse velocity and heat field deformation methods. Agric. For. Meteorol. 2010, 150, 1046–1056. [Google Scholar] [CrossRef]
- Matheny, A.M.; Bohrer, G.; Garrity, S.R.; Morin, T.H.; Howard, C.J.; Vogel, C.S. Observations of stem water storage in trees of opposing hydraulic strategies. Ecosphere 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Pfautsch, S.; Macfarlane, C.; Ebdon, N.; Meder, R. Assessing sapwood depth and wood properties in Eucalyptus and Corymbia spp. using visual methods and near infrared spectroscopy (NIR). Trees 2012, 26, 963–974. [Google Scholar] [CrossRef]
- Čermák, J.; Kučera, J.; Nadezhdina, N. Sap flow measurements with some thermodynamic methods, flow integration within trees and scaling up from sample trees to entire forest stands. Trees 2004, 18, 529–546. [Google Scholar] [CrossRef]
- Alvarado-Barrientosa, M.S.; Hernández-Santana, V.; Asbjornsen, H. Variability of the radial profile of sap velocity in Pinus patula from contrasting stands within the seasonal cloud forest zone of Veracruz, Mexico. Agric. For. Meteorol. 2013, 168, 108–119. [Google Scholar] [CrossRef]
- Dye, P.J.; Olbrich, B.W.; Poulter, A.G. The influence of growth rings in Pinus patula on heat pulse velocity and sap flow measurements. J. Exp. Bot. 1991, 42, 867–870. [Google Scholar] [CrossRef]
- Berdanier, A.B.; Miniat, C.F.; Clark, J.S. Predictive models for radial sap flux variation in coniferous, diffuse-porous and ring-porous temperate trees. Tree Physiol. 2016, 36, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E. The heat pulse velocity technique for determining water uptake of Populus deltoides. S. Afr. J. Bot. 1992, 58, 100–104. [Google Scholar] [CrossRef]
- Swanson, R.H. Velocity distribution patterns in ascending xylem sap during transpiration. In Flow—Its Measurement and Control in Science and Industry; Dowdell, R.B., Ed.; Instrument Society of America: Pittsburgh, PA, USA, 1974; pp. 1425–1430. [Google Scholar]
- Swanson, R.H. Significant historical developments in thermal methods for measuring sap flow in trees. Agric. For. Meteorol. 1994, 72, 113–132. [Google Scholar] [CrossRef]
- Phillips, N.; Oren, R.; Zimmerman, R. Radial patterns of xylem sap flow in non-, diffuse- and ring-porous tree species. Plant Cell Environ. 1996, 983, 983–990. [Google Scholar] [CrossRef]
- López-Bernal, A.; Testi, L.; Villalobos, F.J. A single-probe heat pulse method for estimating sap velocity in trees. New Phytol. 2017. [Google Scholar] [CrossRef]
- Testi, L.; Villalobos, F.J. New approach for measuring low sap velocities in trees. Agric. For. Meteorol. 2009, 149, 730–734. [Google Scholar] [CrossRef]
- Green, S.R.; Romero, R. Can we improve heat-pulse to measure low and reverse flows? Acta Hort. 2012, 951, 19–30. [Google Scholar] [CrossRef]
- Doronila, A.I.; Forster, M.A. Performance measurement via sap flow monitoring of three Eucalyptus species for mine site and dryland salinity phytoremediation. Int. J. Phytoremediat. 2015, 17, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.S.O.; Dawson, T.E. Using branch and basal trunk sap flow measurements to estimate whole-plant water capacitance: A caution. Plant Soil 2008, 305, 5–13. [Google Scholar] [CrossRef]
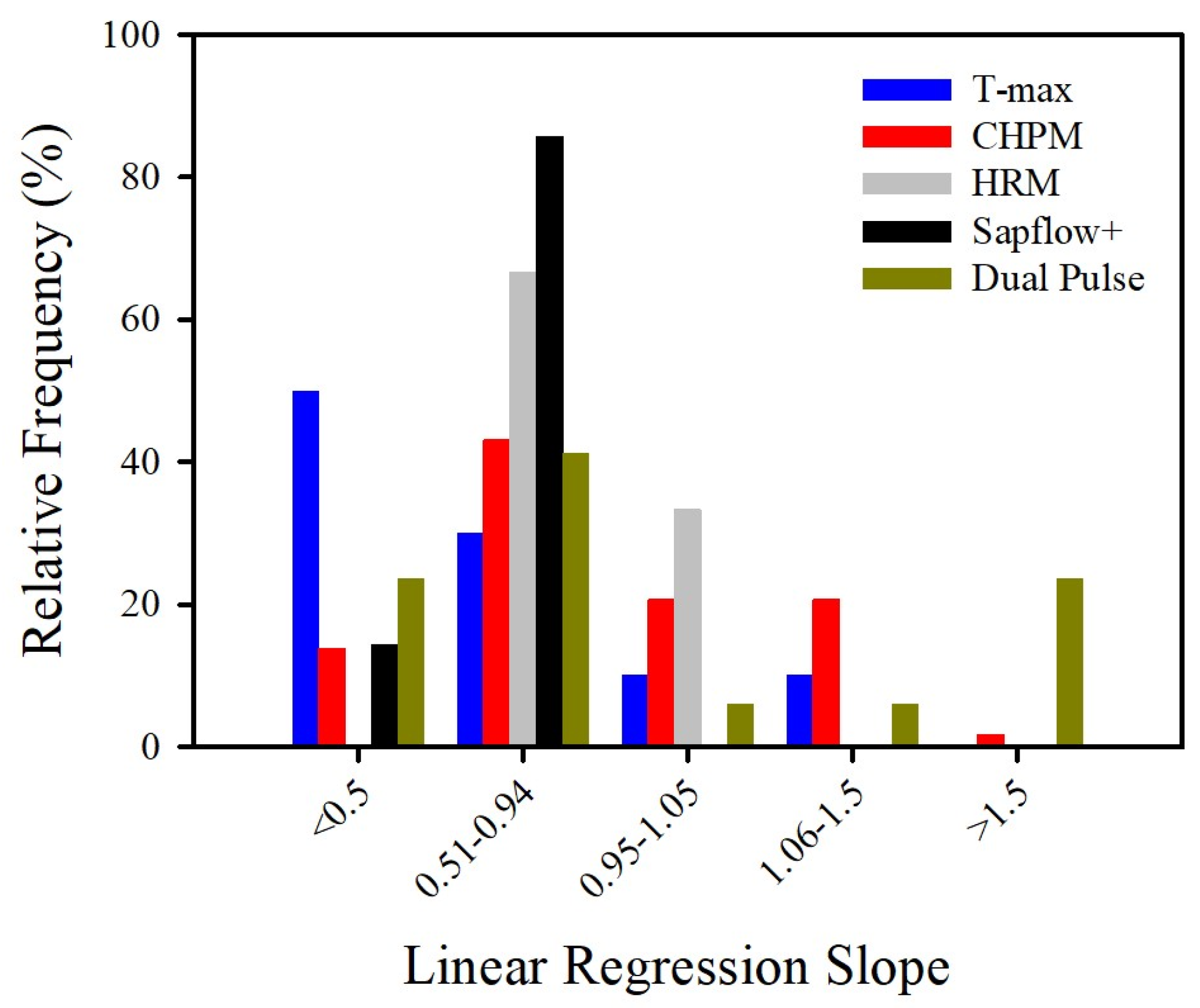
| Method | n | R2 | Slope | Deviation from Slope (%) | Minimum Range (cm/h) | Maximum Range (cm/h) |
|---|---|---|---|---|---|---|
| All methods | 104 | 0.822 | 0.860 | 34.706 | ||
| T-max | 10 | 0.859 | 0.672 | 36.560 | 5 to 10 | >200 |
| CHPM | 59 | 0.723 | 0.863 | 30.611 | 2 to 5 | >200 |
| HRM | 11 | 0.916 | 0.833 | 16.949 | −10 | 45 |
| Sapflow+ | 7 | 0.986 | 0.620 | 38.000 | −10 | >200 |
| Dual | 17 | 0.892 | 1.071 | 59.706 | −10 | >200 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forster, M.A. How Reliable Are Heat Pulse Velocity Methods for Estimating Tree Transpiration? Forests 2017, 8, 350. https://doi.org/10.3390/f8090350
Forster MA. How Reliable Are Heat Pulse Velocity Methods for Estimating Tree Transpiration? Forests. 2017; 8(9):350. https://doi.org/10.3390/f8090350
Chicago/Turabian StyleForster, Michael A. 2017. "How Reliable Are Heat Pulse Velocity Methods for Estimating Tree Transpiration?" Forests 8, no. 9: 350. https://doi.org/10.3390/f8090350
APA StyleForster, M. A. (2017). How Reliable Are Heat Pulse Velocity Methods for Estimating Tree Transpiration? Forests, 8(9), 350. https://doi.org/10.3390/f8090350





