Macronutrient Stocks in Scots Pine Stands of Different Densities
Abstract
:1. Introduction
2. Materials and Methods
3. Results
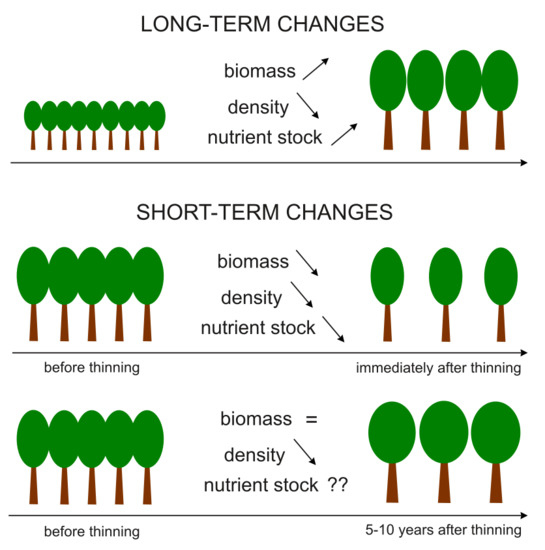
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tamm, C.O. Denitrification in forest ecosystems—Preface. For. Ecol. Manag. 1991, 44, 1–3. [Google Scholar] [CrossRef]
- Wang, J.R.; Zhong, A.L.; Simard, S.W.; Kimmins, J.P. Aboveground biomass and nutrient accumulation in an age sequence of paper birch (Betula papyrifera) in the Interior Cedar Hemlock zone, British Columbia. For. Ecol. Manag. 1996, 83, 27–38. [Google Scholar] [CrossRef]
- Nohrstedt, H.Ö. Response of coniferous forest ecosystems on mineral soils to nutrient additions: A review of swedish experiences. Scand. J. For. Res. 2001, 16, 555–573. [Google Scholar] [CrossRef]
- Prietzel, J.; Stetter, U. Long-term trends of phosphorus nutrition and topsoil phosphorus stocks in unfertilized and fertilized Scots pine (Pinus sylvestris) stands at two sites in Southern Germany. For. Ecol. Manag. 2010, 259, 1141–1150. [Google Scholar] [CrossRef]
- Modrzewska, B.; Kosiorek, M.; Wyszkowski, M. Content of some nutrients in scots pine, silver birch and Norway maple in an urbanized environment. J. Elem. 2016, 21, 149–157. [Google Scholar]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.L.; Tanner, E.V.J.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E.; et al. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paré, D.; Thiffault, E. Nutrient budgets in forests under increased biomass harvesting scenarios. Curr. For. Rep. 2016, 2, 81–91. [Google Scholar] [CrossRef]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Waldner, P.; Benham, S.; Hansen, K.; Merilä, P.; et al. Tree mineral nutrition is deteriorating in Europe. Glob. Ch. Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Mandre, M.; Lukjanova, A.; Pärn, H.; Kõresaar, K. State of Scots pine (Pinus sylvestris L.) under nutrient and water deficit on coastal dunes of the Baltic Sea. Trees Struct. Funct. 2010, 24, 1073–1085. [Google Scholar] [CrossRef]
- Joki-Heiskala, P.; Johansson, M.; Holmberg, M.; Mattsson, T.; Forsius, M.; Kortelainen, P.; Hallin, L. Long-term base cation balances of forest mineral soils in Finland. Water Air Soil Pollut. 2003, 150, 255–273. [Google Scholar] [CrossRef]
- Akselsson, C.; Westling, O.; Sverdrup, H.; Holmqvist, J.; Thelin, G.; Uggla, E.; Malm, G. Impact of harvest intensity on long-term base cation budgets in Swedish forest soils. Water Air Soil Pollut. Focus 2007, 7, 201–210. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L. Estimation of nutrient removals in stem-only and whole-tree harvesting of Scots pine, Norway spruce, and birch stands with generalized nutrient equations. Eur. J. For. Res. 2012, 131, 945–964. [Google Scholar] [CrossRef]
- Tamminen, P.; Saarsalmi, A.; Smolander, A.; Kukkola, M.; Helmisaari, H.S. Effects of logging residue harvest in thinnings on amounts of soil carbon and nutrients in Scots pine and Norway spruce stands. For. Ecol. Manag. 2012, 263, 31–38. [Google Scholar] [CrossRef]
- Ranger, J.; Turpault, M.P. Input-output nutrient budgets as a diagnostic tool for sustainable forest management. For. Ecol. Manag. 1999, 122, 139–154. [Google Scholar] [CrossRef]
- Egnell, G.; Valinger, E. Survival, growth, and growth allocation of planted Scots pine trees after different levels of biomass removal in clear-felling. For. Ecol. Manag. 2003, 177, 65–74. [Google Scholar] [CrossRef]
- Merino, A.; Balboa, M.A.; Rodríguez Soalleiro, R.; González, J.G.A. Nutrient exports under different harvesting regimes in fast-growing forest plantations in Southern Europe. For. Ecol. Manag. 2005, 207, 325–339. [Google Scholar] [CrossRef]
- Blanco, J.A.; Zavala, M.A.; Imbert, J.B.; Castillo, F.J. Sustainability of forest management practices: Evaluation through a simulation model of nutrient cycling. For. Ecol. Manag. 2005, 213, 209–228. [Google Scholar] [CrossRef]
- Węgiel, A.; Małek, S.; Bielinis, E.; Grebner, D.L.; Polowy, K.; Skonieczna, J. Determination of elements removal in different harvesting scenarios of Scots pine (Pinus sylvestris L.) stands. Scand. J. For. Res. 2018, 33, 261–270. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Hanssen, K.H.; Jacobson, S.; Kukkola, M.; Luiro, J.; Saarsalmi, A.; Tamminen, P.; Tveite, B. Logging residue removal after thinning in Nordic boreal forests: Long-term impact on tree growth. For. Ecol. Manag. 2011, 261, 1919–1927. [Google Scholar] [CrossRef]
- Wall, A. Effect of removal of logging residue on nutrient leaching and nutrient pools in the soil after clearcutting in a Norway spruce stand. For. Ecol. Manag. 2008, 256, 1372–1383. [Google Scholar] [CrossRef]
- Olsson, B.A.; Lundkvist, H.; Staaf, H. Nutrient status in needles of Norway spruce and Scots pine following harvesting of logging residues. Plant Soil 2000, 223, 161–173. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Vangansbeke, P.; De Schrijver, A.; De Frenne, P.; Verstraeten, A.; Gorissen, L.; Verheyen, K. Strong negative impacts of whole tree harvesting in pine stands on poor, sandy soils: A long-term nutrient budget modelling approach. For. Ecol. Manag. 2015, 356, 101–111. [Google Scholar] [CrossRef]
- Peltola, S.; Kilpeläinen, H.; Asikainen, A. Recovery rates of logging residue harvesting in Norway spruce (Picea abies (L.) Karsten) dominated stands. Biomass Bioenergy 2011, 35, 1545–1551. [Google Scholar] [CrossRef]
- Wall, A. Risk analysis of effects of whole-tree harvesting on site productivity. For. Ecol. Manag. 2012, 282, 175–184. [Google Scholar] [CrossRef]
- Zeide, B. Optimal stand density: A solution. Can. J. For. Res. 2004, 34, 846–854. [Google Scholar] [CrossRef]
- Kojola, S.; Ahtikoski, A.; Hökkä, H.; Penttilä, T. Profitability of alternative management regimes in Scots pine stands on drained peatlands. Eur. J. For. Res. 2012, 131, 413–426. [Google Scholar] [CrossRef]
- Tahvonen, O.; Pihlainen, S.; Niinimäki, S. On the economics of optimal timber production in boreal Scots pine stands. Can. J. For. Res. 2013, 43, 719–730. [Google Scholar] [CrossRef]
- Giuggiola, A.; Bugmann, H.; Zingg, A.; Dobbertin, M.; Rigling, A. Reduction of stand density increases drought resistance in xeric Scots pine forests. For. Ecol. Manag. 2013, 310, 827–835. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Ponette, Q.; Rapp, M. Relationships between forest tree species, stand production and stand nutrient amount. Ann. For. Sci. 2000, 57, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Effects of thinning on nutrient content pools in two Pinus sylvestris forests in the Western Pyrenees. Scand. J. For. Res. 2006, 21, 143–150. [Google Scholar] [CrossRef]
- Jacobson, S.; Kukkola, M.; Mälkönen, E.; Tveite, B. Impact of whole-tree harvesting and compensatory fertilization on growth of coniferous thinning stands. For. Ecol. Manag. 2000, 129, 41–51. [Google Scholar] [CrossRef]
- Mäkinen, H.; Isomäki, A. Thinning intensity and long-term changes in increment and stem form of Scots pine trees. For. Ecol. Manag. 2004, 203, 21–34. [Google Scholar] [CrossRef]
- Valinger, E.; Elfving, B.; Mörling, T. Twelve-year growth response of Scots pine to thinning and nitrogen fertilisation. For. Ecol. Manag. 2000, 134, 45–53. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Will, R.E.; Burkes, E.C.; Shiver, B.; Teskey, R.O. Nutrient concentrations and contents, and their relation to stem growth, of intensively managed Pinus taeda and Pinus elliottii stands of different planting densities. For. Sci. 2003, 49, 291–300. [Google Scholar]
- Noh, N.J.; Kim, C.; Bae, S.W.; Lee, W.K.; Yoon, T.K.; Muraoka, H.; Son, Y. Carbon and nitrogen dynamics in a Pinus densiflora forest with low and high stand densities. J. Plant Ecol. 2013, 6, 368–379. [Google Scholar] [CrossRef]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Nutrient return via litterfall in two contrasting Pinus sylvestris forests in the Pyrenees under different thinning intensities. For. Ecol. Manag. 2008, 256, 1840–1852. [Google Scholar] [CrossRef]
- Siipilehto, J. A comparison of two parameter prediction methods for stand structure in Finland. Silv. Fenn. 2000, 34, 331–349. [Google Scholar] [CrossRef]
- Picard, N.; Saint-André, L.; Henry, M. Manual for Building Tree Volume and Biomass Allometric Equations: From Field Measurement to Prediction; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012; p. 215. [Google Scholar]
- Augusto, L.; Meredieu, C.; Bert, D.; Trichet, P.; Porté, A.; Bosc, A.; Lagane, F.; Loustau, D.; Pellerin, S.; Danjon, F.; et al. Improving models of forest nutrient export with equations that predict the nutrient concentration of tree compartments. Ann. For. Sci. 2008, 65, 808. [Google Scholar] [CrossRef]
- Finér, L. Nutrient concentrations in Pinus sylvestris growing on an ombrotrophic pine bog, and the effects of PK and NPK fertilization. Scand. J. For. Res. 1992, 7, 205–218. [Google Scholar] [CrossRef]
- Skonieczna, J.; Małek, S.; Polowy, K.; Węgiel, A. Element content of Scots pine (Pinus sylvestris L.) stands of different densities. Drewno 2014, 57, 77–87. [Google Scholar]
- Del Río, M.; Montero, G.; Bravo, F. Analysis of diameter-density relationships and self-thinning in non-thinned even-aged Scots pine stands. For. Ecol. Manag. 2001, 142, 79–87. [Google Scholar] [CrossRef]
- Kuliešis, A.; Saladis, J.; Kuliešis, A.A. Development and productivity of young Scots pine stands by regulating density. Balt. For. 2010, 16, 235–246. [Google Scholar]
- Nilsson, U.; Agestam, E.; Ekö, P.-M.; Elfving, B.; Fahlvik, N.; Johansson, U.; Karlsson, K.; Lundmark, T.; Wallentin, C. Thinning of Scots pine and Norway spruce monocultures in Sweden. Stud. For. Suec. 2010, 219, 1–46. [Google Scholar]
- Alegria, C. Simulation of silvicultural scenarios and economic efficiency for maritime pine (Pinus pinaster Aiton) wood-oriented management in centre inland of Portugal. For. Syst. 2011, 20, 361–378. [Google Scholar]
- Dean, T.J.; Roberts, S.D.; Seymour, R.S. Toward developing a direct relation between gross volume increment and stand density. Can. J. For. Res. 2013, 43, 852–860. [Google Scholar] [CrossRef]
- Tang, X.L.; Pérez-Cruzado, C.; Vor, T.; Fehrmann, L.; Álvarez-González, J.G.; Kleinn, C. Development of stand density management diagrams for Chinese fir plantations. Forestry 2016, 89, 36–45. [Google Scholar] [CrossRef]
- Fang, X.M.; Christenson, L.M.; Wang, F.C.; Zeng, J.P.; Chen, F.S. Pine caterpillar outbreak and stand density impacts on nitrogen and phosphorus dynamics and their stoichiometry in Masson pine (Pinus massoniana) plantations in subtropical China. Can. J. For. Res. 2016, 46, 601–609. [Google Scholar] [CrossRef]
- Paré, D.; Rochon, P.; Brais, S. Assessing the geochemical balance of managed boreal forests. Ecol. Indic. 2002, 1, 293–311. [Google Scholar] [CrossRef]
- Armolaitis, K.; Varnagirytė-Kabašinskienė, I.; Stupak, I.; Kukkola, M.; Mikšys, V.; Wojcik, J. Carbon and nutrients of Scots pine stands on sandy soils in Lithuania in relation to bioenergy sustainability. Biomass Bioenergy 2013, 54, 250–259. [Google Scholar] [CrossRef]
- Bembenek, M.; Karaszewski, Z.; Kondracki, K.; Łacka, A.; Mederski, P.S.; Skorupski, M.; Strzeliński, P.; Sułkowski, S.; Węgiel, A. Value of merchantable timber in Scots pine stands of different densities. Drewno 2014, 57, 133–142. [Google Scholar]



| Sample Plot | Density, tree∙ha−1 | Mean DBH (±SD), cm | Mean Height (±SD), m | Basal Area, m2∙ha−1 | Volume, m3∙ha−1 |
|---|---|---|---|---|---|
| SP1 | 476 | 28.2 ± 4.5 | 22.9 ± 1.7 | 30.5 | 319 |
| SP2 | 590 | 25.7 ± 4.7 | 20.8 ± 1.4 | 31.5 | 302 |
| SP3 | 672 | 23.6 ± 4.3 | 19.6 ± 1.5 | 30.3 | 275 |
| SP4 | 756 | 23.9 ± 5.3 | 20.1 ± 2.0 | 35.6 | 337 |
| SP5 | 824 | 21.8 ± 4.0 | 19.3 ± 1.7 | 31.7 | 286 |
| Sample Plot | Density, tree∙ha−1 | Stem Wood | Stem Bark | Thick Branches | Thin Branches | Dead Branches | Needles | Total |
|---|---|---|---|---|---|---|---|---|
| SP1 | 476 | 115.6 | 10.1 | 14.7 | 3.0 | 5.5 | 4.8 | 153.7 |
| SP2 | 590 | 107.4 | 9.4 | 14.9 | 2.9 | 5.5 | 4.7 | 144.8 |
| SP3 | 672 | 99.2 | 8.7 | 13.5 | 2.7 | 5.0 | 4.4 | 133.5 |
| SP4 | 756 | 120.4 | 10.6 | 16.3 | 3.2 | 6.1 | 5.3 | 161.9 |
| SP5 | 824 | 107.2 | 9.5 | 12.9 | 2.8 | 4.7 | 4.7 | 141.8 |
| Element | Stem Wood | Stem Bark | Thick Branches | Thin Branches | Dead Branches | Needles |
|---|---|---|---|---|---|---|
| N | 0.83 ± 0.21 | 4.91 ± 1.06 | 2.53 ± 0.96 | 7.08 ± 1.01 | 1.89 ± 0.54 | 12.3 ± 1.19 |
| P | 0.07 ± 0.04 | 0.62 ± 0.18 | 0.33 ± 0.08 | 0.78 ± 0.10 | 0.09 ± 0.02 | 1.15 ± 0.14 |
| K | 0.27 ± 0.15 | 1.53 ± 0.52 | 1.15 ± 0.24 | 2.65 ± 0.44 | 0.18 ± 0.16 | 4.45 ± 0.61 |
| Ca | 0.65 ± 0.13 | 7.89 ± 1.85 | 2.05 ± 0.27 | 2.26 ± 0.28 | 1.49 ± 0.42 | 3.16 ± 0.38 |
| Mg | 0.27 ± 0.10 | 0.99 ± 0.31 | 0.42 ± 0.07 | 0.63 ± 0.09 | 0.17 ± 0.05 | 0.65 ± 0.11 |
| S | 0.03 ± 0.02 | 0.43 ± 0.10 | 0.25 ± 0.16 | 0.73 ± 0.12 | 0.21 ± 0.07 | 1.28 ± 0.10 |
| Tree Parts | Sample Plots—Scots Pine Stands of Different Densities | ||||
|---|---|---|---|---|---|
| SP1, 476 trees·ha−1 | SP2, 590 trees·ha−1 | SP3, 672 trees·ha−1 | SP4, 756 trees·ha−1 | SP5, 824 trees·ha−1 | |
| N (nitrogen) | |||||
| Stem wood | 85.31 | 68.17 | 89.51 | 86.54 | 126.37 |
| Stem bark | 37.90 | 51.81 | 47.11 | 54.39 | 49.14 |
| Branches | 54.03 | 65.63 | 66.52 | 75.38 | 61.48 |
| Needles | 60.84 | 57.52 | 59.75 | 56.89 | 54.78 |
| Total | 238.08 | 243.13 | 262.89 | 273.20 | 291.77 |
| P (phosphorus) | |||||
| Stem wood | 6.24 | 5.61 | 6.68 | 12.43 | 11.13 |
| Stem bark | 4.28 | 6.09 | 6.28 | 6.80 | 6.78 |
| Branches | 6.28 | 6.85 | 7.80 | 8.08 | 7.75 |
| Needles | 5.53 | 5.02 | 5.58 | 5.73 | 5.16 |
| Total | 22.33 | 23.57 | 26.34 | 33.04 | 30.82 |
| K (potassium) | |||||
| Stem wood | 28.72 | 15.54 | 29.53 | 39.11 | 44.26 |
| Stem bark | 9.74 | 15.73 | 16.68 | 18.38 | 15.18 |
| Branches | 20.39 | 24.73 | 27.21 | 25.93 | 25.27 |
| Needles | 20.28 | 21.33 | 20.90 | 25.08 | 17.83 |
| Total | 79.13 | 77.33 | 94.32 | 108.50 | 102.54 |
| Ca (calcium) | |||||
| Stem wood | 58.68 | 55.17 | 67.53 | 96.16 | 76.76 |
| Stem bark | 70.43 | 70.07 | 80.38 | 74.15 | 65.21 |
| Branches | 43.36 | 46.40 | 42.02 | 49.90 | 36.10 |
| Needles | 15.12 | 14.05 | 14.42 | 16.95 | 15.15 |
| Total | 187.59 | 185.69 | 204.35 | 237.16 | 193.22 |
| Mg (magnesium) | |||||
| Stem wood | 20.86 | 18.6 | 32.09 | 48.48 | 29.07 |
| Stem bark | 6.53 | 9.74 | 11.08 | 9.89 | 10.3 |
| Branches | 8.29 | 7.88 | 9.29 | 9.8 | 8.51 |
| Needles | 3.55 | 2.42 | 3.16 | 3.22 | 2.86 |
| Total | 39.23 | 38.64 | 55.62 | 71.39 | 50.74 |
| S (sulfur) | |||||
| Stem wood | 0.66 | 3.96 | 5.15 | 4.03 | 3.57 |
| Stem bark | 3.11 | 4.91 | 4.24 | 4.13 | 4.52 |
| Branches | 5.59 | 7.43 | 6.30 | 6.55 | 5.78 |
| Needles | 6.44 | 5.91 | 5.95 | 6.14 | 5.79 |
| Total | 15.80 | 22.21 | 21.64 | 20.85 | 19.66 |
| Variable | Tree Part | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|---|
| Nutrient stock | Stem wood | 0.683 | 0.822 | 0.733 | 0.744 | 0.637 | 0.623 |
| Stem bark | 0.689 | 0.913 * | 0.723 | −0.124 | 0.751 | 0.537 | |
| Branches | 0.567 | 0.901 * | 0.730 | -0.282 | 0.500 | −0.024 | |
| Needles | −0.841 | −0.018 | −0.015 | 0.404 | −0.263 | −0.696 | |
| Total | 0.970 ** | 0.909 * | 0.887 * | 0.497 | 0.676 | 0.475 | |
| Nutrient concentration | Stem wood | 0.670 | 0.812 | 0.659 | 0.804 | 0.655 | 0.553 |
| Stem bark | 0.463 | 0.683 | 0.439 | −0.191 | 0.620 | 0.374 | |
| Branches | 0.409 | 0.849 | 0.789 | 0.301 | 0.781 | −0.020 | |
| Needles | 0.043 | 0.591 | 0.235 | 0.769 | −0.246 | 0.186 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgiel, A.; Bielinis, E.; Polowy, K. Macronutrient Stocks in Scots Pine Stands of Different Densities. Forests 2018, 9, 593. https://doi.org/10.3390/f9100593
Węgiel A, Bielinis E, Polowy K. Macronutrient Stocks in Scots Pine Stands of Different Densities. Forests. 2018; 9(10):593. https://doi.org/10.3390/f9100593
Chicago/Turabian StyleWęgiel, Andrzej, Ernest Bielinis, and Krzysztof Polowy. 2018. "Macronutrient Stocks in Scots Pine Stands of Different Densities" Forests 9, no. 10: 593. https://doi.org/10.3390/f9100593