Short-Term Vegetation Responses to Invasive Shrub Control Techniques for Amur Honeysuckle (Lonicera maackii [Rupr.] Herder)
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Experimental Design
2.3. Vegetation Sampling
2.4. Soil Temperature
2.5. Mulching Head Treatment Intensity
2.6. Data Preparation
2.7. Data Analysis
3. Results
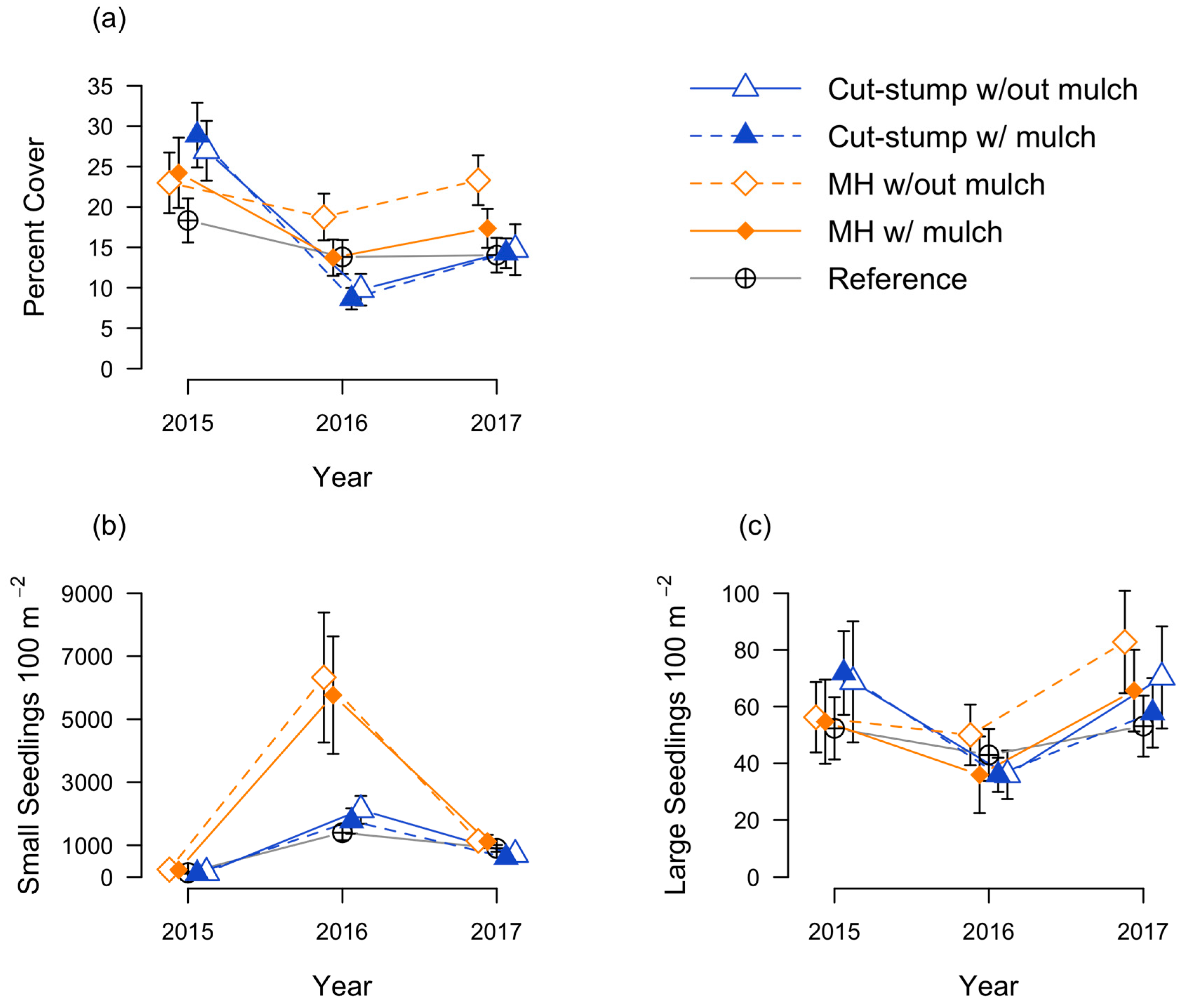
3.1. Honeysuckle Responses
3.2. Native Seedling Responses
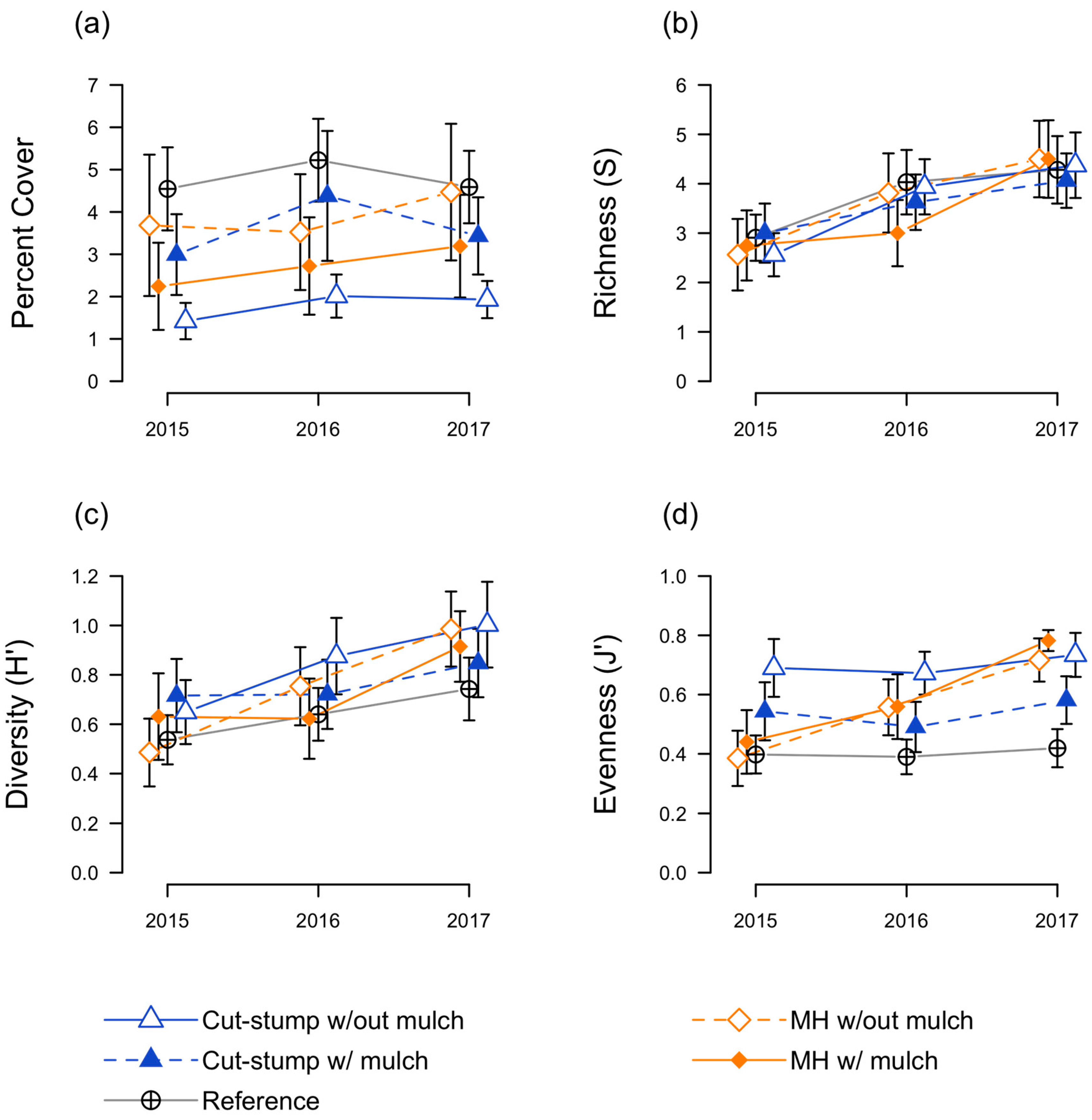
3.3. Herbaceous Plant Responses
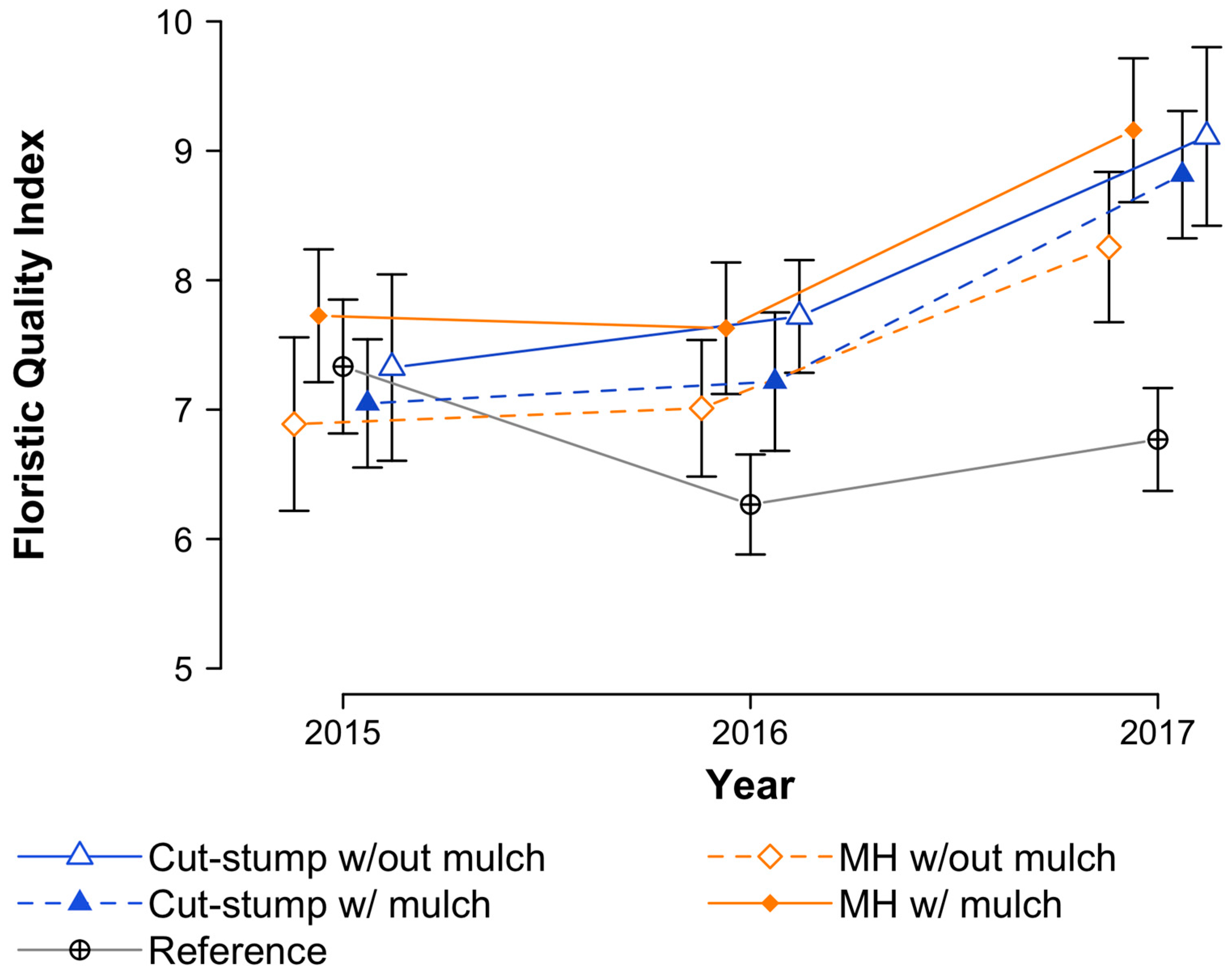
3.4. Floristic Quality
3.5. Soil Temperature
4. Discussion
4.1. Treatment Effectiveness
4.2. Responses of Exotic Flora
4.3. Native Plant Community Recovery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webster, C.R.; Jenkins, M.A.; Jose, S. Woody invaders and the challenges they pose to forest ecosystems in the eastern United States. J. For. 2006, 104, 366–374. [Google Scholar] [CrossRef]
- Mascaro, J.; Schnitzer, S.A. Rhamnus cathartica L. (common buckthorn) as an ecosystem dominant in southern Wisconsin forests. Northeast. Nat. 2007, 14, 387–402. [Google Scholar] [CrossRef]
- Hartman, K.M.; McCarthy, B.C. Restoration of a forest understory after the removal of an invasive shrub, Amur honeysuckle (Lonicera maackii). Restor. Ecol. 2004, 12, 154–165. [Google Scholar] [CrossRef]
- Schulte, L.A.; Mottl, E.C.; Palik, B.J. The association of two invasive shrubs, common buckthorn (Rhamnus cathartica) and Tartarian honeysuckle (Lonicera tatarica), with oak communities in the midwestern United States. Can. J. For. Res. 2011, 41, 1981–1992. [Google Scholar] [CrossRef]
- Collier, M.H.; Vankat, J.L.; Hughes, M.R. Diminished plant richness and abundance below Lonicera maackii, an invasive shrub. Am. Midl. Nat. 2002, 147, 60–71. [Google Scholar] [CrossRef]
- Meiners, S.J. Apparent competition: An impact of exotic shrub invasion on tree regeneration. Biol. Invasions 2007, 9, 849–855. [Google Scholar] [CrossRef]
- Schmidt, K.A.; Whelan, C.J. Effects of exotic Lonicera and Rhamnus on songbird nest predation. Conserv. Biol. 1999, 13, 1502–1506. [Google Scholar] [CrossRef]
- Watling, J.I.; Hickman, C.R.; Orrock, J.L. Invasive shrub alters native forest amphibian communities. Biol. Conserv. 2011, 144, 2597–2601. [Google Scholar] [CrossRef]
- Luken, J.O.; Mattimiro, D.T. Habitat-specific resilience of the invasive shrub Amur honeysuckle (Lonicera maackii) during repeated clipping. Ecol. Appl. 1991, 1, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.G.; Saunders, M.R.; Lowe, Z.E. A cost comparison of five midstory removal methods. In Proceedings of the 17th Central Hardwood Forest Conference, Lexington, KY, USA, 5–7 April 2010; pp. 535–543. [Google Scholar]
- Ducrey, M.; Turrel, M. Influence of cutting methods and dates on stump sprouting in Holm oak (Quercus ilex L) coppice. Ann. For. Sci. 1992, 49, 449–464. [Google Scholar] [CrossRef]
- Ward, J.S.; Williams, S.C.; Worthley, T.E. Comparing effectiveness and impacts of Japanese barberry (Berberis thunbergii) control treatments and herbivory on plant communities. Invasive Plant Sci. Manag. 2013, 6, 459–469. [Google Scholar] [CrossRef]
- Hanula, J.L.; Horn, S.; Taylor, J.W. Chinese privet (Ligustrum sinense) removal and its effect on native plant communities of riparian forests. Invasive Plant Sci. Manag. 2009, 2, 292–300. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Invasive plant removal method determines native plant community responses. J. Appl. Ecol. 2009, 46, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Boyce, R.L. Recovery of native plant communities in southwest Ohio after Lonicera maackii removal. J. Torrey Bot. Soc. 2015, 142, 193–204. [Google Scholar] [CrossRef]
- Hudson, J.R.; Hanula, J.L.; Horn, S. Impacts of removing Chinese privet from riparian forests on plant communities and tree growth five years later. For. Ecol. Manag. 2014, 324, 101–108. [Google Scholar] [CrossRef]
- Shields, J.M.; Saunders, M.R.; Gibson, K.D.; Zollner, P.A.; Dunning, J.B.; Jenkins, M.A. Short-term response of native flora to the removal of non-native shrubs in mixed-hardwood forests of Indiana, USA. Forests 2015, 6, 1878–1896. [Google Scholar] [CrossRef]
- Luken, J.O.; Kuddes, L.M.; Tholemeier, T.C. Response of understory species to gap formation and soil disturbance in Lonicera maackii thickets. Restor. Ecol. 1997, 5, 229–235. [Google Scholar] [CrossRef]
- Miller, E.M.; Seastedt, T.R. Impacts of woodchip amendments and soil nutrient availability on understory vegetation establishment following thinning of a ponderosa pine forest. For. Ecol. Manag. 2009, 258, 263–272. [Google Scholar] [CrossRef]
- Bartuszevige, A.M.; Hrenko, R.L.; Gorchov, D.L. Effects of leaf litter on establishment, growth and survival of invasive plant seedlings in a deciduous forest. Am. Midl. Nat. 2007, 158, 472–477. [Google Scholar] [CrossRef]
- Cipollini, K.; Ames, E.; Cipollini, D. Amur honeysuckle (Lonicera maackii) management method impacts restoration of understory plants in the presence of white-tailed deer. Invasive Plant Sci. Manag. 2009, 2, 45–54. [Google Scholar] [CrossRef]
- Wilson, H.N.; Arthur, M.A.; Schörgendorfer, A.; Paratley, R.D.; Lee, B.D.; McEwan, R.W. Site characteristics as predictors of Lonicera maackii in second-growth forests of central Kentucky, USA. Nat. Areas J. 2013, 33, 189–198. [Google Scholar] [CrossRef]
- Luken, J.O. Population structure and biomass allocation of the naturalized shrub Lonicera maackii (Rupr.) Maxim. in forest and open habitats. Am. Midl. Nat. 1988, 119, 258–267. [Google Scholar] [CrossRef]
- Bartuszevige, A.M.; Gorchov, D.L. Avian seed dispersal of an invasive shrub. Biol. Invasions 2006, 8, 1013–1022. [Google Scholar] [CrossRef]
- Castellano, S.M.; Gorchov, D.L. White-tailed deer (Odocoileus virginianus) disperse seeds of the invasive shrub, amur honeysuckle (Lonicera maackii). Nat. Areas J. 2013, 33, 78–80. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Invasive shrub distribution varies with distance to roads and stand age in eastern deciduous forests in Indiana, USA. Plant Ecol. 2006, 184, 131–141. [Google Scholar] [CrossRef]
- Shields, J.M.; Jenkins, M.A.; Saunders, M.R.; Zhang, H.; Jenkins, L.H.; Parks, A.M. Age distribution and spatial patterning of an invasive shrub in secondary hardwood forests. For. Sci. 2014, 60, 830–840. [Google Scholar] [CrossRef]
- Fridley, J.D. Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 2012, 485, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Heberling, J.M.; Fridley, J.D. Functional traits and resource-use strategies of native and invasive plants in Eastern North American forests. New Phytol. 2013, 200, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Web Soil Survey. Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Available online: https://websoilsurvey.sc.egov.usda.gov (accessed on 15 October 2015).
- Yatskievych, K. Field Guide to Indiana Wildflowers; Indiana University Press: Bloomington, IN, USA, 2000; ISBN 0-253-21420-3. [Google Scholar]
- Peet, R.K.; Wentworth, T.R.; White, P.S. A flexible, multipurpose method for recording vegetation composition and structure. Castanea 1998, 63, 262–274. [Google Scholar]
- Rothrock, P.E. Floristic Quality Assessment in Indiana: The Concept, Use, and Development of Coefficients of Conservatism; Final Report; ARN A: Indianapolis, IN, USA, 2004. [Google Scholar]
- Rothrock, P.E.; Homoya, M.A. An evaluation of Indiana’s floristic quality assessment. Proc. Indiana Acad. Sci. 2005, 114, 9–18. [Google Scholar]
- Love, J.P.; Anderson, J.T. Seasonal effects of four control methods on the invasive morrow’s honeysuckle (Lonicera morrowii) and initial responses of understory plants in a southwestern Pennsylvania old field. Restor. Ecol. 2009, 17, 549–559. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Sugiura, N. Further analysts of the data by akaike’s information criterion and the finite corrections. Commun. Stat. Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.15.6. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 10 January 2016).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 13. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Polgar, C.; Gallinat, A.; Primack, R.B. Drivers of leaf-out phenology and their implications for species invasions: Insights from Thoreau’s Concord. New Phytol. 2014, 202, 106–115. [Google Scholar] [CrossRef] [PubMed]
- De Villalobos, A.E.; Vázquez, D.P.; Martin, J.L. Soil disturbance, vegetation cover and the establishment of the exotic shrub Pyracantha coccinea in southern France. Biol. Invasions 2010, 12, 1023–1029. [Google Scholar] [CrossRef]
- Kalisz, S.; Spigler, R.B.; Horvitz, C.C. In a long-term experimental demography study, excluding ungulates reversed invader’s explosive population growth rate and restored natives. Proc. Natl. Acad. Sci. USA 2014, 111, 4501–4506. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.R.; Wagner, H.H. A temporal dimension to the stress gradient hypothesis for intraspecific interactions. Oikos 2014, 123, 1323–1330. [Google Scholar] [CrossRef]
- Packer, A.; Clay, K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 2000, 404, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Orrock, J.L.; Christopher, C.C.; Dutra, H.P. Seed bank survival of an invasive species, but not of two native species, declines with invasion. Oecologia 2012, 168, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Kuebbing, S.E.; Classen, A.T.; Call, J.J.; Henning, J.A.; Simberloff, D. Plant-soil interactions promote co-occurrence of three nonnative woody shrubs. Ecology 2015, 96, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- DeWalt, S.J.; Denslow, J.S.; Ickes, K. Natural-enemy release facilitates habitat expansion of the invasive tropical shrub Clidemia hirta. Ecology 2004, 85, 471–483. [Google Scholar] [CrossRef]
- Dorrough, J.; Scroggie, M.P. Plant responses to agricultural intensification. J. Appl. Ecol. 2008, 45, 1274–1283. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.E.; Gorchov, D.L. The invasive shrub, Lonicera maackii, reduces growth and fecundity of perennial forest herbs. Oecologia 2004, 139, 359–375. [Google Scholar] [CrossRef] [PubMed]




| Species | Common Name | Basal Area | |||
|---|---|---|---|---|---|
| LF1 | LF2 | LF3 | PWA | ||
| Acer saccharinum L. | silver maple | -- | -- | -- | 1.53 |
| Acer saccharum Marshall | sugar maple | 2.30 | 1.15 | 3.83 | -- |
| Carya spp. Nutt. | hickory species | 0.77 | 5.36 | 5.74 | 8.04 |
| Celtis occidentalis L. | common hackberry | 0.77 | -- | 2.30 | 0.38 |
| Cornus florida L. | flowering dogwood | 0.38 | -- | -- | -- |
| Crataegus spp. L. | hawthorn species | 0.38 | 0.77 | -- | -- |
| Fraxinus americana L. | white ash | 1.91 | 2.30 | 3.44 | -- |
| Juglans nigra L. | black walnut | 2.30 | 3.06 | 1.53 | -- |
| Liriodendron tulipifera L. | tulip tree | 0.77 | -- | -- | -- |
| Morus alba L. | white mulberry | -- | -- | -- | 0.77 |
| Picea rubens Sarg. | red spruce | -- | -- | 2.30 | -- |
| Platanus occidentalis L. | American sycamore | -- | 0.38 | -- | -- |
| Prunus serotina Ehrh. | black cherry | 3.44 | 0.77 | 1.91 | 6.12 |
| Quercus alba L. | white oak | -- | 3.06 | -- | 2.30 |
| Quercus imbricaria Michx. | shingle oak | -- | 1.91 | 0.38 | 1.15 |
| Quercus rubra L. | northern red oak | 4.21 | -- | 0.38 | 1.53 |
| Quercus velutina Lam. | black oak | 0.38 | 1.53 | -- | 1.91 |
| Robinia pseudoacacia L. | black locust | -- | 0.77 | 0.38 | -- |
| Sassafras albidum (Nutt.) Nees | sassafras | 3.06 | -- | -- | 2.68 |
| Tilia americana L. | American basswood | 0.38 | -- | 0.38 | -- |
| Ulmus americana L. | American elm | 0.77 | 0.38 | 0.77 | -- |
| Ulmus rubra Muhl. | slippery elm | 0.77 | -- | 0.77 | -- |
| Total Site Basal Area | 22.58 | 21.43 | 24.11 | 26.40 | |
| Cut-Stump | Mulching Head | Model Predictors | |||||
|---|---|---|---|---|---|---|---|
| No Mulch | With Mulch | No Mulch | With Mulch | Test Statistic | p-Value | ||
| Resprouting stump density 100 m−2 | 80 ± 23 | 89 ± 24 | 95 ± 24 | 95 ± 25 | Treatment | F3,51 = 0.77 | 0.52 |
| Shrubs(lg) | F1,50 = 27.54 | <0.01 | |||||
| Shrubs(sm) | F1,51 = 0.60 | 0.44 | |||||
| Seedlings(sm) | F1,53 = 2.80 | 0.10 | |||||
| Treatment × Shrubs(sm) | F3,51 = 3.75 | 0.02 | |||||
| Height of resprouting stumps (cm) | 31 ± 2 | 26 ± 3 | 25 ± 2 | 25 ± 2 | Treatment | F3,56 = 2.70 | 0.054 |
| Seedlings(lg) | F1,56 = 2.42 | 0.13 | |||||
| Treatment × Seedlings(lg) | F3,56 = 2.85 | 0.046 | |||||
| Stems per resprouting stump | 11 ± 1 | 8 ± 1 | 9 ± 1 | 10 ± 1 | Treatment | F3,54 = 3.85 | 0.01 |
| Shrubs(lg) | F1,4 = 8.61 | 0.04 | |||||
| Shrubs(sm) | F1,53 = 4.81 | 0.03 | |||||
| Treatment × Shrubs(lg) | F3,54 = 3.43 | 0.02 | |||||
| Ref. | Cut-Stump | Mulching Head | ||||||
|---|---|---|---|---|---|---|---|---|
| -- | No Mulch | With Mulch | No Mulch | With Mulch | F Value [df] | p-Value | ||
| Canopy cover (%) | 2015 (Pretreatment) | 95 ± 0.3 | 94 ± 0.6 | 94 ± 0.6 | 94 ± 0.4 | 94 ± 0.4 | 1.26 [4,88] | 0.29 |
| 2015–2016 Change | −2 ± 0.3 | −4 ± 0.9 | −3 ± 0.9 | −5 ± 0.9 | −4 ± 0.9 | 4.55 [4,88] | <0.01 | |
| 2015–2017 Change | 1 ± 0.4 | −2 ± 0.8 | −3 ± 0.9 | −3 ± 0.7 | −3 ± 0.8 | 8.13 [4,88] | <0.01 | |
| Midstory (1–5 m) L. maackii cover (%) | 2015 (Pretreatment) | 68 ± 4.5 | 55 ± 6.7 | 60 ± 6.6 | 76 ± 6.1 | 54 ± 8.7 | 2.01 [4,91] | 0.10 |
| 2015–2016 Change | 9 ± 2.5 | −54 ± 6.8 | −60 ± 6.6 | −76 ± 6.1 | −54 ± 8.7 | 44.12 [4,90] | <0.01 | |
| 2015–2017 Change | 4 ± 3.4 | −52 ± 7.5 | −59 ± 6.6 | −75 ± 6.2 | −52 ± 8.6 | 33.93 [4,91] | <0.01 | |
| Herb-layer (0–1 m) L. maackii cover (%) | 2015 (Pretreatment) | 18 ± 2.7 | 27 ± 3.7 | 29 ± 4.0 | 23 ± 3.8 | 24 ± 4.4 | 3.50 [4,88] | 0.01 |
| 2015–2016 Change | −5 ± 1.3 | −17 ± 3.2 | −20 ± 3.9 | −4 ± 4.3 | −11 ± 4.5 | 7.86 [4,88] | <0.01 | |
| 2015–2017 Change | −4 ± 1.7 | −12 ± 3.1 | −15 ± 4.2 | 0 ± 3.8 | −7 ± 3.5 | 4.50 [4,88] | <0.01 | |
| Treatment | Treatment × Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA | ANOVA | Planned Comparisons (z Values) | ||||||||
| df | F | df | F | R vs. 0 | R vs. C[−] | R vs. M[+] | C[−] vs. M[+] | C vs. M | [−] vs. [+] | |
| L. maackii | ||||||||||
| Herb-Layer Cover (<1 m) | 4, 81 | 2.25 · | 4, 91 | 3.91 ** | −1.51 | −2.37 · | −0.23 | 1.85 | 3.46 ** | −0.84 |
| Seedlings <0.5 m | 4, 69 | 2.40 · | 4, 132 | 0.63 | -- | -- | -- | -- | -- | -- |
| Seedlings 0.5–1.37 m | 4, 105 | 0.44 | 4, 187 | 1.30 | -- | -- | -- | -- | -- | -- |
| Herb-Layer Native Plants †† | ||||||||||
| Cover | 4, 71 | 0.31 | 4, 91 | 14.37 ** | −1.80 | 5.84 ** | 4.91 ** | −0.81 | −0.10 | −1.05 |
| FQI | 4, 89 | 0.43 | 4, 91 | 2.91 ** | 0.71 | 2.64 * | 2.15 | −0.43 | −0.64 | 0.04 |
| S | 4, 98 | 1.77 | 4, 145 | 29.06 ** | 0.36 | 7.73 ** | 8.10 ** | −2.03 | 2.48 · | 0.32 |
| H′ | 4, 80 | 4.06 | 4, 91 | 7.36 ** | 1.70 | 3.45 ** | 4.02 ** | 0.50 | 2.26 | −1.55 |
| J′ | 4, 80 | 2.11 · | 4, 85 | 1.49 | -- | -- | -- | -- | -- | -- |
| Spring Perennials | ||||||||||
| Cover | 4, 87 | 2.43 · | 4, 91 | 1.57 | -- | -- | -- | -- | -- | -- |
| S | 4, 88 | 9.41 | 4, 91 | 0.39 | -- | -- | -- | -- | -- | -- |
| H′ | 4, 88 | 0.78 | 4, 91 | 2.17 · | 2.71 * | 1.25 | 1.01 | −0.21 | 1.63 | −1.93 |
| J′ | 4, 73 | 2.67 * | 4, 82 | 3.66 ** | 0.48 | −0.31 | 2.50 · | 2.37 · | 3.31 ** | 0.16 |
| Summer Herbs | ||||||||||
| Cover | 4, 76 | 0.45 | 4, 91 | 21.52 | −1.01 | 5.97 ** | 6.59 ** | 0.54 | 1.83 | −1.06 |
| S | 4, 114 | 1.65 | 4, 147 | 18.69 ** | 0.88 | 6.10 ** | 6.34 ** | 0.21 | 2.13 | −1.83 |
| H′ | 4, 82 | 1.57 | 4, 91 | 9.88 ** | 0.93 | 4.22 ** | 4.98 ** | 0.66 | 2.11 | −1.19 |
| J′ ‡ | 4, 67 | 1.70 | 4, 68 | 0.73 | -- | -- | -- | -- | -- | -- |
| Annual Natives | 4, 127 | 0.86 | 4, 99 | 2.20 · | −0.01 | 0.69 | 0.98 | −0.33 | −0.49 | −0.59 |
| Biennial Natives | 4, 87 | 0.69 | 4, 91 | 2.92 * | 0.25 | 0.64 | 0.80 | 0.38 | −0.05 | 0.24 |
| Perennial Natives | 4, 86 | 0.20 | 4, 91 | 14.33 ** | 0.16 | 1.97 | 2.74 * | −1.97 | 1.62 | −0.25 |
| Native Vines | 4, 85 | 1.43 | 4, 91 | 8.13 ** | −2.25 | 3.10 ** | 3.14 ** | 0.27 | 0.81 | 0.77 |
| Native Graminoids | 4, 96 | 0.60 | 4, 176 | 5.87 ** | 0.25 | 0.14 | 1.73 | −0.87 | 2.37 · | 1.06 |
| Exotics | 4, 172 | 15.90 | 4, 169 | 2.18 · | −0.28 | 0.19 | 0.03 | −1.10 | −0.06 | −0.82 |
| Native Seedlings | ||||||||||
| Cover | 4, 84 | 0.63 | 4, 182 | 5.03 ** | −2.51 · | 3.89 ** | 2.18 | −1.49 | −0.56 | −1.54 |
| Density | 4, 56 | 0.78 | 4, 91 | 2.44 · | −2.17 | 2.85 * | 1.15 | −1.48 | −1.93 | −0.16 |
| S | 4, 85 | 1.55 | 4, 91 | 9.79 ** | −1.36 | 4.48 ** | 5.04 ** | 0.49 | 0.62 | 0.07 |
| H′ | 4, 87 | 1.69 | 4, 91 | 8.30 ** | −1.27 | 4.25 ** | 4.41 ** | 0.14 | 0.69 | −0.49 |
| J′ | 4, 77 | 1.28 | 4, 75 | 3.08 * | −1.54 | 2.27 | 2.31 · | 0.01 | 1.28 | −1.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, G.S.; Saunders, M.R.; Jenkins, M.A. Short-Term Vegetation Responses to Invasive Shrub Control Techniques for Amur Honeysuckle (Lonicera maackii [Rupr.] Herder). Forests 2018, 9, 607. https://doi.org/10.3390/f9100607
Frank GS, Saunders MR, Jenkins MA. Short-Term Vegetation Responses to Invasive Shrub Control Techniques for Amur Honeysuckle (Lonicera maackii [Rupr.] Herder). Forests. 2018; 9(10):607. https://doi.org/10.3390/f9100607
Chicago/Turabian StyleFrank, Graham S., Michael R. Saunders, and Michael A. Jenkins. 2018. "Short-Term Vegetation Responses to Invasive Shrub Control Techniques for Amur Honeysuckle (Lonicera maackii [Rupr.] Herder)" Forests 9, no. 10: 607. https://doi.org/10.3390/f9100607





