Climate Sensitivity of Tropical Trees Along an Elevation Gradient in Rwanda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites, Climate, Species, and Measurement Campaigns
2.2. Leaf Gas Exchange
2.3. Leaf Temperature
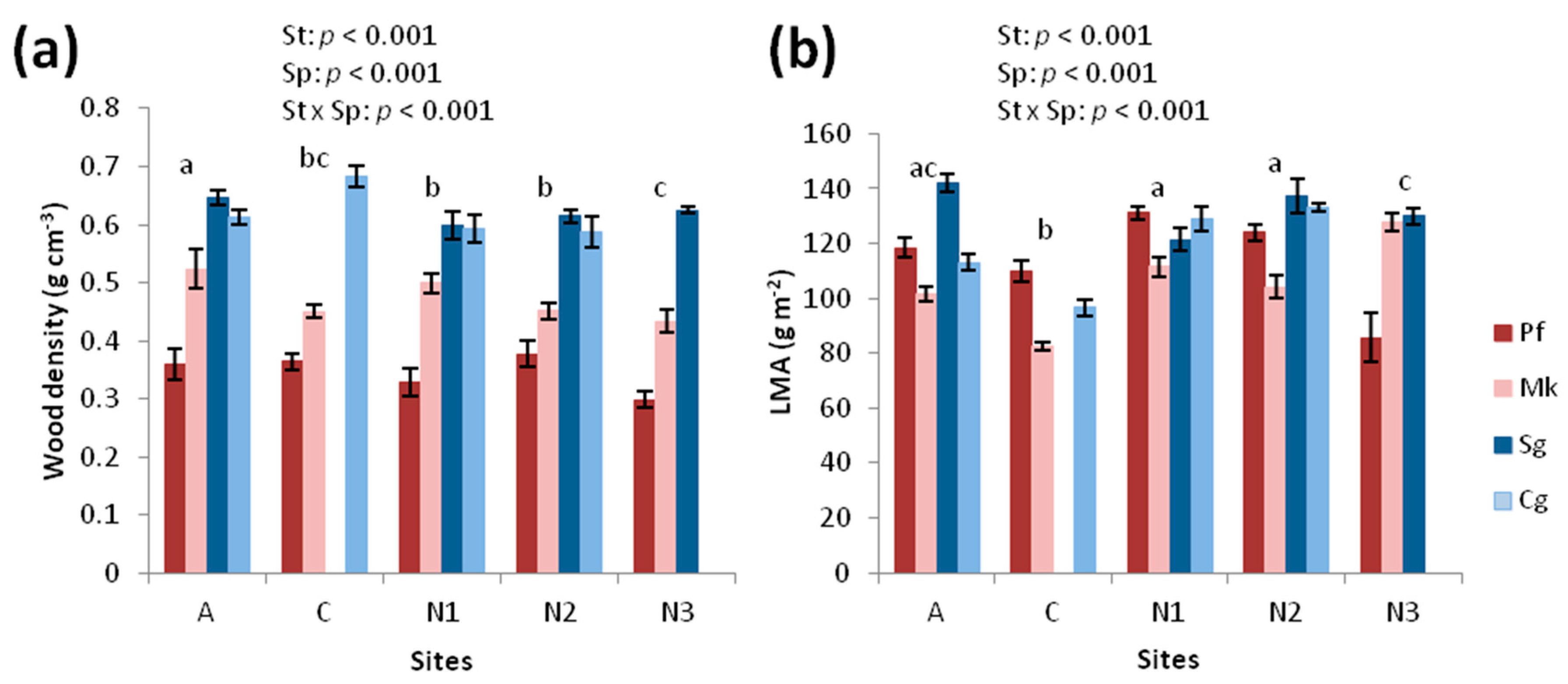
2.4. Tree and Leaf Structural Traits
2.5. Chemical Traits
2.6. Statistical Analyses
3. Results
3.1. Physiological Traits
3.1.1. Leaf Gas Exchange
3.1.2. iWUE from Stable Carbon Isotopes
3.1.3. Leaf Temperature
3.2. Structural Traits
3.3. Chemical Traits
4. Discussion
4.1. Elevation Patterns in Gas Exchange
4.2. Leaf Temperature
4.3. Nutrient Limitations
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature response of parameters of a biochemically based model of photosynthesis. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef]
- Kattge, J.; Knorr, W.; Farquhar, T. Temperature acclimation in a biochemical model of photosynthesis: A reanalysis of data from 36 species. Plant Cell Environ. 2007, 30, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.A.; Hara, K.H.O.; Campion, C.M. Thermal plasticity of photosynthesis: The role of acclimation in forest responses to a warming climate. Glob. Chang. Biol. 2010, 16, 2272–2286. [Google Scholar] [CrossRef]
- Lewis, S.L. Tropical forests and the changing earth system. Philos. Trans. R. Soc. B 2006, 361, 195–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhi, Y.; Silman, M.; Salinas, N.; Bush, M.; Meir, P.; Saatchi, S. Introduction: Elevation gradients in the tropics: Laboratories for ecosystem ecology and global change research. Glob. Chang. Biol. 2010, 16, 3171–3175. [Google Scholar] [CrossRef]
- Corlett, R.T. Impacts of warming on tropical lowland rainforests. Trends Ecol. Evol. 2011, 26, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Feeley, K.E.J.; Davies, S.T.J.; Perez, R.; Hubbell, S.P.; Foster, R.B. Directional changes in the species composition of a tropical forest. Ecology 2011, 92, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janzen, D.H. Why mountain passes are higher in the tropics. Am. Nat. 1967, 101, 233–249. [Google Scholar] [CrossRef]
- Wright, S.J.; Muller-Landau, H.C.; Schipper, J. The future of tropical species on a warmer planet. Conserv. Biol. 2009, 23, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Doughty, C.E.; Goulden, M.L. Are tropical forests near a high temperature threshold? J. Geophys. Res. 2008, 113, 1–12. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R.A.M. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Slot, M.; Winter, K. The Effects of Rising Temperature on the Ecophysiology of Tropical Forest Trees. In Tropical Tree Physiology; Springer: Cham, Switzerland, 2016; pp. 385–412. [Google Scholar]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Stevenson, P.R.; Feeley, K.J. Thermophilization of adult and juvenile tree communities in the northern tropical Andes. Proc. Natl. Acad. Sci. USA 2015, 112, 10744–10749. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Bonal, D.; Barigah, T.S.; Granier, A.; Guehl, J.M. Late-stage canopy tree species with extremely low d 13 C and high stomatal sensitivity to seasonal soil drought in the tropical rainforest of French Guiana. Plant Physiol. 2000, 23, 445–459. [Google Scholar]
- Doughty, C.E. An In Situ Leaf and Branch Warming Experiment in the Amazon. Biotropica 2011, 43, 658–665. [Google Scholar] [CrossRef]
- Zhao, J.; Hartmann, H.; Trumbore, S.; Ziegler, W.; Zhang, Y. High temperature causes negative whole-plant carbon balance under mild drought. New Phytol. 2013, 200, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheesman, A.W.; Winter, K. Growth response and acclimation of CO2 exchange characteristics to elevated temperatures in tropical tree seedlings. J. Exp. Bot. 2013, 64, 3817–3828. [Google Scholar] [CrossRef] [PubMed]
- Slot, M.; Garcia, M.N.; Winter, K. Temperature response of CO2 exchange in three tropical tree species. Funct. Plant Biol. 2016, 43, 468–478. [Google Scholar] [CrossRef]
- Vårhammar, A.; Wallin, G.; McLean, C.M.; Dusenge, M.E.; Medlyn, B.E.; Hasper, T.B.; Nsabimana, D.; Uddling, J.A. Photosynthetic temperature responses of tree species in Rwanda: Evidence of pronounced negative effects of high temperature in montane rainforest climax species. New Phytol. 2015, 206, 1000–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, K. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar]
- Motzer, T.; Munz, N.; Küppers, M.; Schmitt, D.; Anhuf, D. Stomatal conductance, transpiration and sap flow of tropical montane rain forest trees in the southern Ecuadorian Andes. Tree Physiol. 2017, 25, 1283–1293. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Crausbay, S.D.; Giambelluca, T.W.; Weintraub, A.E.; Longman, R.J.; Asbjornsen, H.; Hotchkiss, S.C.; Dawson, T.E. Water relations and microclimate around the upper limit of a cloud forest in Maui, Hawai. Tree Physiol. 2014, 34, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Gotsch, S.G.; Asbjornsen, H.; Holwerda, F.; Goldsmith, G.R.; Weintraub, A.E.; Dawson, T.E. Foggy days and dry nights determine crown-level water balance in a seasonal tropical montane cloud forest. Plant Cell Environ. 2014, 37, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Cordell, S.; Goldstein, G.; Meinzer, F.C.; Handley, L.L. Allocation of nitrogen and carbon in leaves of Metrosideros polymorpha regulates carboxylation capacity and δ13C along an altitudinal gradient. Funct. Ecol. 1999, 13, 811–818. [Google Scholar] [CrossRef]
- Wittich, B.; Horna, V.; Homeier, J.; Leuschner, C. Altitudinal Change in the Photosynthetic Capacity of Tropical Trees: A Case Study from Ecuador and a Pantropical Literature Analysis. Ecosystems 2012, 15, 958–973. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhou, Z.; Hu, H.; Xu, K.; Yan, N.; Li, S. Photosynthetic performances of Quercus pannosa vary with altitude in the Hengduan Mountains, southwest China. For. Ecol. Manag. 2005, 212, 291–301. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Chang, K.-W. Altitudinal trends in photosynthetic rate and leaf characteristics of Miscanthus populations from central Taiwan. Aust. J. Bot. 2001, 49, 509–514. [Google Scholar] [CrossRef]
- Friend, A.A.D.; Woodward, F.I.; Switsur, V.R. Field Measurements of Photosynthesis, Stomatal Conductance, Leaf Nitrogen and δ13C Along Altitudinal Gradients in Scotland. Funct. Ecol. 1989, 3, 117–122. [Google Scholar] [CrossRef]
- Premoli, A.C.; Brewer, C.A. Environmental v. genetically driven variation in ecophysiological traits of Nothofagus pumilio from contrasting elevations. Aust. J. Bot. 2007, 55, 585–591. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Wallin, G.; Gårdesten, J.; Niyonzima, F.; Adolfsson, L.; Nsabimana, D.; Uddling, J. Photosynthetic capacity of tropical montane tree species in relation to leaf nutrients, successional strategy and growth temperature. Oecologia 2015, 177, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.P.; Martin, R.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Carranza-Jiménez, L.; Martinez, P. Amazonian functional diversity from forest canopy chemical assembly. Proc. Natl. Acad. Sci. USA 2014, 111, 5604–5609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitousek, P.M.; Matson, P.A.; Turner, D.R. Elevational and age gradients in hawaiian montane rainforest: Foliar and soil nutrients. Oecologia 1988, 77, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Korner, C.H.; Farquar, G.D.; Roksandic, Z. A global survey of carbon isotope discrimination in plants from high altitude. Oecologia 1988, 74, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M. Litterfall, Nutrient Cycling, and Nutrient Limitation in Tropical Forests. Ecology 1984, 65, 285–298. [Google Scholar] [CrossRef]
- Tanner, E.V.J.; Vitousek, P.M.; Cuevas, E. Experimental Investigation of Nutrient Limitation of Forest Growth on Wet Tropical Mountains. Ecology 1998, 79, 10–22. [Google Scholar] [CrossRef]
- Dalling, J.W.; Heineman, K.; Lopez, O.R.; Wright, S.J.; Turner, B.L. Nutrient availability in tropical rain forests: The paradigm of phosphorus limitation. In Tropical Tree Physiology; Springer International Publishing: Berlin, Germany, 2016; pp. 261–273. [Google Scholar]
- Fisher, J.B.; Malhi, Y.; Daniel, T. Nutrient limitation in rainforests and cloud forests along a 3000-m elevation gradient in the Peruvian Andes. Oecologia 2013, 172, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Soethe, N.; Lehmann, J.; Engels, C. Nutrient availability at different altitudes in a tropical montane forest in Ecuador. J. Trop. Ecol. 2008, 24, 397–406. [Google Scholar] [CrossRef]
- Van de Weg, M.J.; Meira, P.; Gracea, J.; Atkinb, O.K. Altitudinal variation in leaf mass per unit area, leaf tissue density and foliar nitrogen and phosphorus content along an Amazon-Andes gradient in Peru. Plant Ecol. Divers. 2009, 2, 243–254. [Google Scholar] [CrossRef]
- Kitayama, K.; Aiba, S. Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus on Mount Kinabalu, Borneo. J. Ecol. 2002, 90, 37–51. [Google Scholar] [CrossRef]
- Nyirambangutse, B.; Zibera, E.; Dusenge, M.E.; Nsabimana, D.; Pleijel, H.; Uddling, J.; Wallin, G. Canopy nutrient cycling in Afromontane tropical forests at different successional stages. Tree Physiol. 2017. submitted for publication. [Google Scholar]
- Nsabimana, D.; Klemedtson, L.; Kaplin, B.A.; Wallin, G. Soil CO2 flux in six monospecific forest plantations in Southern Rwanda. Soil Biol. Biochem. 2009, 41, 396–402. [Google Scholar] [CrossRef]
- Gross-Camp, N.D.; Martin, A.; Mcguire, S.; Bereket Kebe, D.E.; Munyarukaza, J. Payments for ecosystem services in an African protected area: Exploring issues of legitimacy, fairness, equity and effectiveness. Fauna Flora Int. Oryx 2012, 46, 24–33. [Google Scholar] [CrossRef]
- Plumptre, A.J.; Davenport, T.R.B.; Behangana, M.; Kityo, R.; Eilu, G.; Ssegawa, P.; Ewango, C.; Meirte, D.; Kahindo, C.; Herremans, M.; et al. The biodiversity of the Albertine Rift. Biol. Conserv. 2007, 134, 178–194. [Google Scholar] [CrossRef]
- Plumptre, A.J.; Masozera, M.; Fashing, P.J.; McNeilage, A.; Ewango, C.; Kaplin, B.A.; Liengola, I. Biodiversity Surveys of the Nyungwe Forest Reserve in S.W.; Working Paper; Wildlife Conservation Society: New York, NY, USA, 2002. [Google Scholar]
- Kindt, R.; van Breugel, P.; Lillesø, J.-P.B.; Minani, V.; Ruffo, C.K.; Gapusi, J.; Jamnadass, R.; Graudal, L. Atlas and tree species composition for Rwanda. In Potential Natural Vegetation of Eastern Africa 2014; Department of Geosciences and Natural Resource Management, University of Copenhagen: Copenhagen, Denmark, 2014. [Google Scholar]
- Nyirambangutse, B.; Zibera, E.; Uwizeye, F.K.; Nsabimana, D.; Bizuru, E.; Pleijel, H.; Uddling, J.; Wallin, G. Carbon stocks and dynamics at different successional stages in an Afromontane tropical forest. Biogeosciences 2017, 14, 1285–1303. [Google Scholar] [CrossRef]
- Bloesch, U.; Troupin, G.; Derungs, N. Les Plantes Ligneuses du Rwanda: Flore, Écologie et Usages; Shaker Verlag: Aachen, Germany, 2009. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Ericsson, T.; Kähr, M. Growth and nutrition of birch seedlings in relation to potassium supply rate. Trees 1993, 7, 78–85. [Google Scholar] [CrossRef]
- Ericsson, T.; Kähr, M. Growth and nutrition of birch seedlings at varied relative addition rates of magnesium. Tree Physiol. 1995, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Göransson, A. Growth and nutrition of small Betula pendula plants at different relative addition rates of iron. Trees 1993, 8, 31–38. [Google Scholar] [CrossRef]
- Göransson, A. Growth and nutrition of small Betula pendula plants at different relative addition rates of manganese. Tree Physiol. 1994, 14, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Göransson, A. Steady-state nutrition and growth responses of Betula pendula plants at different relative supply rates of copper. Plant Cell Environ. 1998, 21, 937–944. [Google Scholar] [CrossRef]
- Göransson, A. Growth and nutrition of Betula pendula at different relative supply rates of zinc. Tree physiol. 1999, 19, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Knecht, M.F.; Göransson, A. Terrestrial plants require nutrients in similar proportions. Tree Physiol. 2004, 24, 447–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linder, S. Foliar Analysis for Detecting and Correcting Nutrient Imbalances in Norway Spruce. Ecol. Bull. 1995, 44, 178–190. [Google Scholar]
- Kumagai, T.; Saitoh, T.M.; Sato, Y.; Takahashi, H. Annual water balance and seasonality of evapotranspiration in a Bornean tropical rainforest. Agric. For. Meteorol. 2005, 128, 81–92. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Goldstein, G.; Jackson, P.; Holbrook, N.M.; Gutiérrez, M.V.; Cavelier, J. International Association for Ecology Environmental and Physiological Regulation of Transpiration in Tropical Forest Gap Species: The Influence of Boundary Layer and Hydraulic Properties. Oecologia 1995, 101, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Pegoraro, E.; Nobre, A.D.; Pereira, M.G.P.; Grace, J.; Culf, A.D.; Clement, R. Energy and water dynamics of a central Amazonian rain forest. J. Geophys. Res. 2002, 107, 8061. [Google Scholar] [CrossRef]
- Malhi, Y.; Girardin, C.A.J.; Goldsmith, G.R.; Doughty, C.E.; Salinas, N.; Metcalfe, D.B.; Huasco, W.H.; Silva-Espejo, J.E.; Aguilla-Pasquell, J.D.; Amézquita, F.F.; et al. The variation of productivity and its allocation along a tropical elevation gradient: A whole carbon budget perspective. New Phytol. 2017, 214, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.; Martinez, C.A.; Ferreira, L.L.; Prado, C. Photosynthesis and water use efficiency in twenty tropical tree species of differing succession status in a Brazilian reforestation. Photosynthetica 2004, 42, 351–356. [Google Scholar] [CrossRef]
- Haggag, M.; Kalisa, J.C.; Abdeldayem, A.W. Projections of precipitation, air temperature and potential evapotranspiration in Rwanda under changing climate conditions. Afr. J. Environ. Sci. Technol. 2016, 10, 18–33. [Google Scholar]
- Rey-sánchez, A.C.; Slot, M.; Posada, J.M.; Kitajima, K. Spatial and seasonal variation in leaf temperature within the canopy of a tropical forest. Clim. Res. 2016, 71, 75–89. [Google Scholar] [CrossRef]
- Tan, Z.; Zeng, J.; Zhang, Y.; Slot, M.; Gamo, M.; Hirano, T. Optimum air temperature for tropical forest photosynthesis: Mechanisms involved and implications for climate warming. Environ. Res. Lett. 2017, 12, 1–12. [Google Scholar] [CrossRef]
- Pšidová, E.; Živčák, M.; Stojnić, S.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarová, L.; Střelcová, K.; Brestič, M.; Kalaji, H.M. Altitude of origin influences the responses of PSII photochemistry to heat waves in European beech (Fagus sylvatica L.). Environ. Exp. Bot. 2018, 152, 97–106. [Google Scholar] [CrossRef]
- Rütting, T.; Ntaboba, L.C.; Roobroeck, D.; Bauters, M.; Huygens, D.; Boeckx, P. Leaky nitrogen cycle in pristine African montane rainforest soil. Glob. Biogeochem. Cycles 2015, 29, 1754–1762. [Google Scholar] [CrossRef] [Green Version]






| Parameter | Arboretum (A) | Cyamudongo (C) | Nyungwe West (N1) | Nyungwe East (N2) | Bigugu Mountain (N3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elevation (m a.s.l.) | 1700 | 1850 | 1950 | 2500 | 2700 | ||||||||||
| MAT (°C) | 19.5 | ± | 0.2 | 17.9 | 16.2 | ± | 0.4 | 14.3 | ± | 0.2 | 13.3 | ||||
| T day (°C) | 21.1 | ± | 0.1 | 19.2 | 18.2 | ± | 0.5 | 15.6 | ± | 0.2 | 14.7 | ||||
| T 1%ile (°C) | 16.0 | ± | 0.5 | 13.3 | ± | 0.4 | 11.7 | ± | 0.3 | ||||||
| T 99%ile (°C) | 24.1 | ± | 0.2 | 20.4 | ± | 0.3 | 18.1 | ± | 0.2 | ||||||
| MAP (mm) | 979 | ± | 206 | 3016 | ± | 63 | 1657 | ± | 163 | ||||||
| VPD-day (kPa) | 1.02 | ± | 0.04 | 0.44 | ± | 0.06 | 0.39 | ± | 0.09 | ||||||
| PPFD day (μmol m−2 s−1) | 733 | ± | 25 | 572 | ± | 17 | 633 | ± | 32 | ||||||
| Experiment-dry | |||||||||||||||
| T 10–16 (°C) | 23.3 | 18.6 | 17.0 | ||||||||||||
| VPD 10–16 (kPa) | 1.48 | 0.27 | 0.40 | ||||||||||||
| P 3 month (mm) | 135 | 309 | |||||||||||||
| Experiment-wet | |||||||||||||||
| T 10–16 (°C) | 22.6 | ± | 0.8 | 17.8 | ± | 0.4 | 16.9 | ± | 0.3 | ||||||
| VPD 10–16 (kPa) | 1.27 | ± | 0.09 | 0.11 | ± | 0.01 | 0.27 | ± | 0.01 | ||||||
| P 3 month (mm) | 360 | ± | 185 | 499 | ± | 125 | |||||||||
| Trait | A | C | N1 | N2 | N3 | Optimal Ratios a |
|---|---|---|---|---|---|---|
| Pa (g m−2) | 0.156 ± 0.011 | 0.144 ± 0.026 | 0.173 ± 0.010 | 0.163 ± 0.017 | 0.140 ± 0.002 | |
| Na (g m−2) | 2.45 ± 0.102 | 2.13 ± 0.196 | 2.64 ± 0.203 | 2.65 ± 0.280 | 2.42 ± 0.146 | |
| Pm (%) | 0.129 ± 0.010 | 0.142 ± 0.011 | 0.137 ± 0.006 | 0.135 ± 0.007 | 0.126 ± 0.016 | |
| Nm (%) | 2.04 ± 0.135 | 2.09 ± 0.128 | 2.07 ± 0.033 | 2.21 ± 0.193 | 2.19 ± 0.218 | |
| N/P (g g−1) | 16.0 ± 0.493 | 15.6 ± 1.37 | 15.2 ± 0.948 | 17.2 ± 0.989 | 17.7 ± 0.619 | |
| P/N (%) | 6.34 ± 0.198 | 6.72 ± 0.622 | 6.72 ± 0.447 | 6.29 ± 0.449 | 5.83 ± 0.243 | 8–10 |
| K/N (%) | 29.6 ± 5.21 | 47.8 ± 10.0 | 31.6 ± 4.71 | 39.2 ± 3.26 | 27.8 ± 3.31 | 30–35 |
| S/N (%) | 12.6 ± 1.50 | 8.53 ± 1.92 | 10.7 ± 1.84 | 7.88 ± 1.57 | 6.99 ± 0.938 | 4 |
| Cu/N (%) | 0.033 ± 0.003 | 0.035 ± 0.003 | 0.053 ± 0.005 | 0.039 ± 0.005 | 0.034 ± 0.003 | 0.01–0.03 |
| Zn/N (%) | 0.094 ± 0.026 | 0.123 ± 0.029 | 0.128 ± 0.047 | 0.080 ± 0.013 | 0.060 ± 0.008 | 0.04–0.05 |
| Mn/N (%) | 3.47 ± 1.48 | 2.50 ± 0.807 | 2.82 ± 1.27 | 0.926 ± 0.167 | 2.02 ± 0.696 | 0.03–0.05 |
| Fe/N (%) | 2.99 ± 0.591 | 2.71 ± 0.499 | 2.52 ± 0.376 | 2.92 ± 0.895 | 3.42 ± 0.553 | 0.13–0.2 |
| Ca/N (%) | 47.8 ± 4.69 | 37.4 ± 3.84 | 35.6 ± 4.43 | 20.1 ± 2.56 | 28.8 ± 7.41 | 2–2.5 |
| B/N (%) | 0.190 ± 0.063 | 0.117 ± 0.004 | 0.090 ± 0.015 | 0.083 ± 0.014 | 0.082 ± 0.006 | 0.05 |
| Mg/N (%) | 22.0 ± 2.61 | 11.8 ± 1.03 | 14.9 ± 1.36 | 10.3 ± 0.945 | 12.8 ± 3.62 | 3.3–5 |
| SPAD | 55.0 ± 3.60 | 52.9 ± 5.18 | 57.7 ± 5.83 | 56.2 ± 6.92 | 47.9 ± 0.745 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujawamariya, M.; Manishimwe, A.; Ntirugulirwa, B.; Zibera, E.; Ganszky, D.; Ntawuhiganayo Bahati, E.; Nyirambangutse, B.; Nsabimana, D.; Wallin, G.; Uddling, J. Climate Sensitivity of Tropical Trees Along an Elevation Gradient in Rwanda. Forests 2018, 9, 647. https://doi.org/10.3390/f9100647
Mujawamariya M, Manishimwe A, Ntirugulirwa B, Zibera E, Ganszky D, Ntawuhiganayo Bahati E, Nyirambangutse B, Nsabimana D, Wallin G, Uddling J. Climate Sensitivity of Tropical Trees Along an Elevation Gradient in Rwanda. Forests. 2018; 9(10):647. https://doi.org/10.3390/f9100647
Chicago/Turabian StyleMujawamariya, Myriam, Aloysie Manishimwe, Bonaventure Ntirugulirwa, Etienne Zibera, Daniel Ganszky, Elisée Ntawuhiganayo Bahati, Brigitte Nyirambangutse, Donat Nsabimana, Göran Wallin, and Johan Uddling. 2018. "Climate Sensitivity of Tropical Trees Along an Elevation Gradient in Rwanda" Forests 9, no. 10: 647. https://doi.org/10.3390/f9100647





