Ecophysiological Responses of Calcicole Cyclobalanopsis glauca (Thunb.) Oerst. to Drought Stress and Calcium Supply
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
2.2. Leaf Relative Water Content and Water Potential
2.3. Reactive Oxygen Species (ROS) and Malondialdehyde (MDA) Content Determination
2.4. Measurement Osmoregulation Substances
2.5. Measurement of Antioxidant Enzyme Activities
2.6. Photosynthetic Parameters
2.7. Statistical Analyses
3. Results
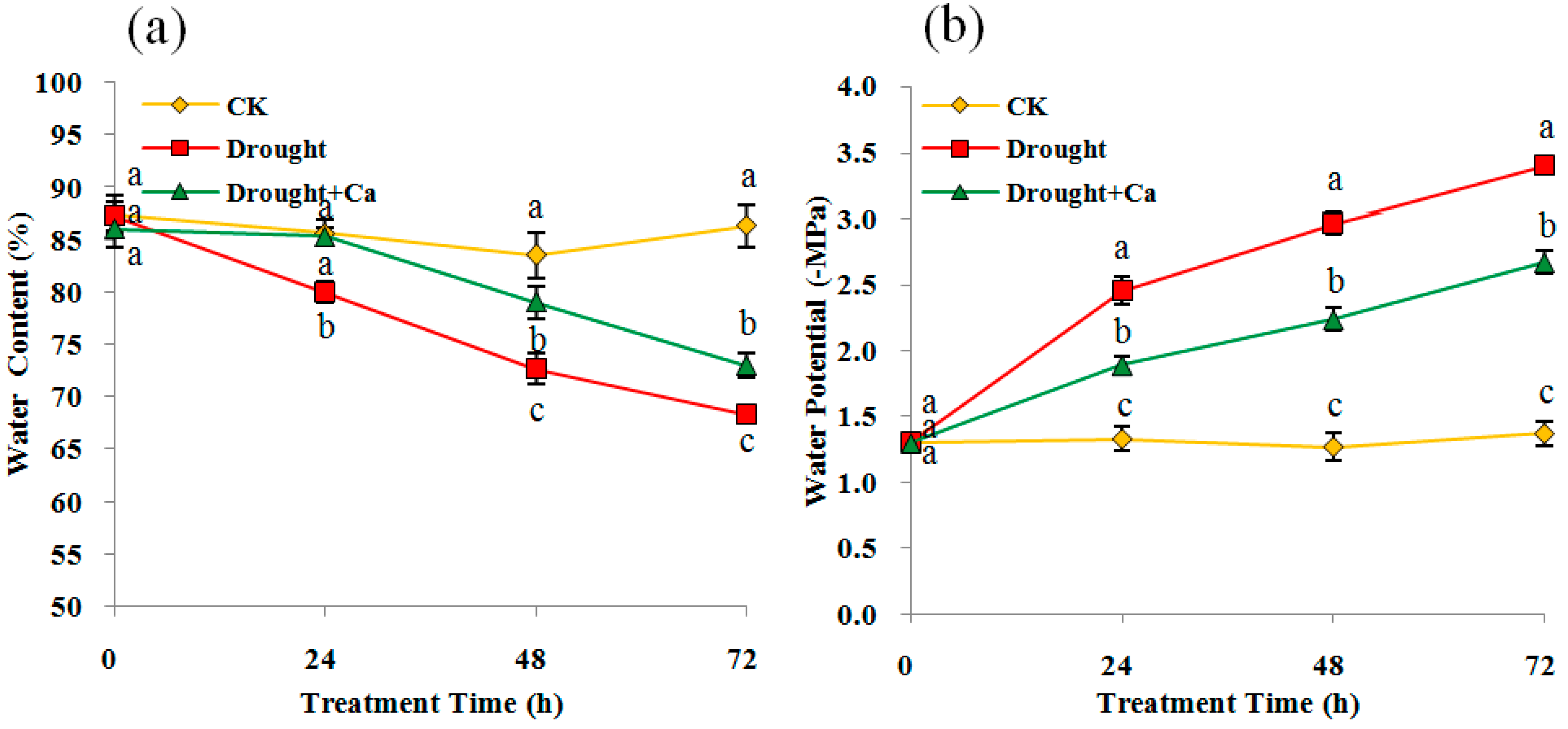
3.1. Relative Water Content (RWC) and Leaf Water Potential (WP)
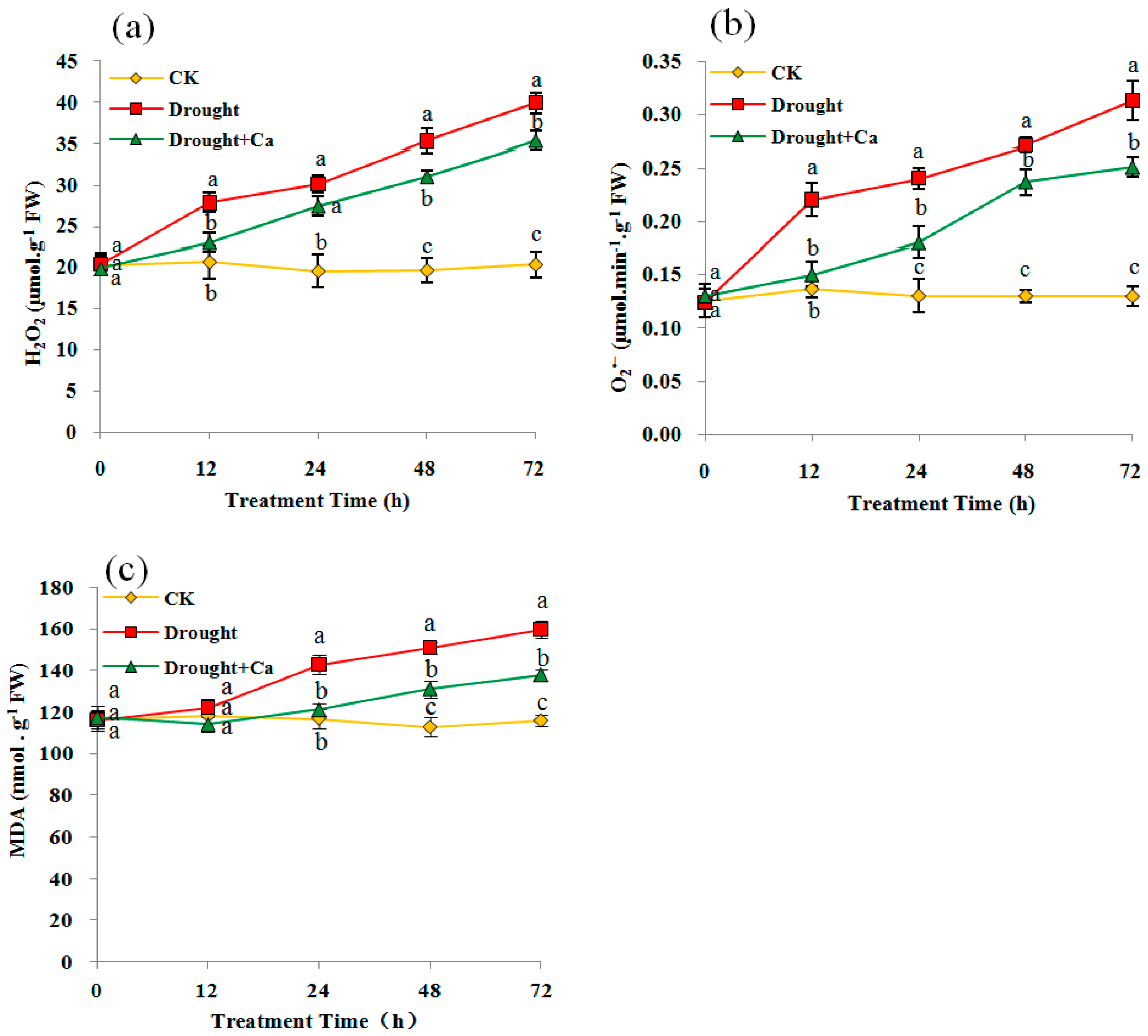
3.2. O2•− and H2O2 Generation and Lipid Peroxidation
3.3. Osmoregulation Substances
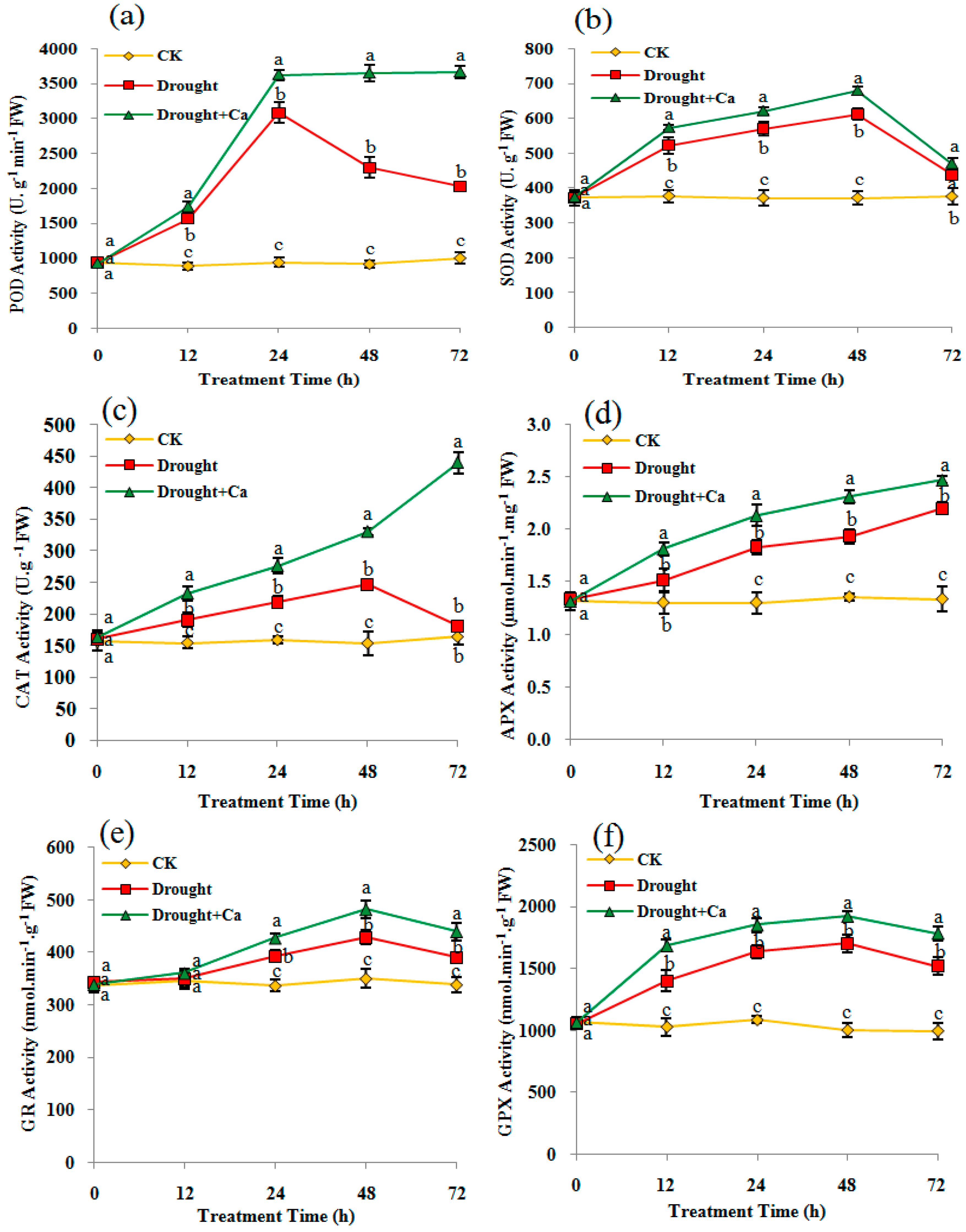
3.4. Antioxidant Enzymes
3.5. Photosynthetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Liu, Q.; Zhang, D. Karst Rocky Desertification in southwestern China: Geomorphology, landuse, impact and rehibilitation. Land Degrad. Dev. 2004, 15, 115–121. [Google Scholar] [CrossRef]
- Li, S.; Birk, S.; Xue, L.; Ren, H.; Chang, J.; Yao, X. Seasonal changes in the soil moisture distribution around bare rock outcrops within a karst rocky desertification area (Fuyuan County, Yunnan Province, China). Environ. Earth Sci. 2016, 75, 1482. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ren, H.D.; Xue, L.; Chang, J.; Yao, X.H. Influence of bare rocks on surrounding soil moisture in the karst rocky desertification regions under drought conditions. Catena 2014, 116, 157–162. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, D.; Tong, L.; Azim, M.; Yang, H.; Huang, F. An overview of Karst ecosystem in Southwest China: Current state and future management. J. Resour. Ecol. 2015, 6, 247–256. [Google Scholar]
- Zhou, Y.C.; Pan, G.X. Adaptation and adjustment of Maolan forest ecosystem to karst environment. Carsol. Sin. 2001, 20, 47–52. [Google Scholar]
- Li, A.D.; Lu, Y.F.; Wei, X.L.; Yu, L.F. Studies on the regime of soil moisture under different microhabitats in Huajiang karst valley. Carsol. Sin. 2008, 27, 56–62. [Google Scholar]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in Maize seedlings. Front. Plant Sci. 2015, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Cruz, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Parry, M.A.; Andralojc, P.J.; Khan, S.; Lea, P.J.; Keys, A.J. Rubisco activity: Effects of drought stress. Ann. Bot. 2002, 89, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Gopi, R.; Lakshmanan, G.A.; Panneerselvam, R. Triadimefon induced changes in the antioxidant metabolism and ajmalicine production in Catharanthus roseus (L.) G. Don. Plant Sci. 2011, 171, 271–276. [Google Scholar]
- Deng, X.; Liu, Y.; Xu, X.; Liu, D.; Zhu, G.; Yan, X.; Wang, Z.; Yan, Y. Comparative proteome analysis of wheat flag leaves and developing grains under water deficit. Front. Plant Sci. 2018, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Guo, K. Ecophysiological adaptations to drought stress of seedlings of four plant species with different growth forms in karst habitats. Chin. J. Plant Ecol. 2011, 35, 1070–1082. [Google Scholar]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Water deficit stress mitigation by calcium chloride in Catharanthus roseus: Effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf. B Biointerfaces 2007, 60, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, X.; Zhang, L. The Effect of Calcium Chloride on Growth, Photosynthesis, and Antioxidant Responses of Zoysia japonica under Drought Conditions. PLoS ONE 2013, 8, e68214. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q. Exogenous calcium alters activities of antioxidant enzymes in Trifolium repens L. leaves under peg-induced water deficit. J. Plant Nutr. 2010, 33, 1874–1885. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.I.; Therios, I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011, 65, 315. [Google Scholar] [CrossRef]
- Kang, J.; Zhao, W.; Zheng, Y.; Zhang, D.M.; Zhou, H.; Sun, P. Calcium chloride improves photosynthesis and water status in the C4 succulent xerophyte Haloxylon ammodendron under water deficit. Plant Growth Regul. 2017, 82, 1–12. [Google Scholar] [CrossRef]
- Li, Z.; Tan, X.F.; Lu, K.; Liu, Z.M.; Wu, L.L. The effect of CaCl2 on calcium content, photosynthesis, and chlorophyll fluorescence of tung tree seedlings under drought conditions. Photosynthetica 2017, 55, 553–560. [Google Scholar] [CrossRef]
- Marques, D.J.; Ferreira, M.M.; da Silva Lobato, A.K.; Guedes de Carvalho, J.; de Assuncao Carvalho, J.; Alves de Freitas, W.; Ribeiro Bastos, A.R.; Pereira, F.J.; Mauro de Castro, E. CaSiO3 improves water potential and gas exchange but not contribute to the production parameters of maize plants exposed to different irrigation depths. Aust. J. Crop Sci. 2015, 8, 1257–1265. [Google Scholar]
- Wang, W.H.; Chen, J.; Liu, T.W.; Chen, J.; Han, A.D.; Simon, M.; Dong, X.J.; He, J.X.; Zheng, H.L. Regulation of the calcium-sensing receptor in both stomatal movement and photosynthetic electron transport is crucial for water use efficiency and drought tolerance in Arabidopsis. J. Exp. Bot. 2014, 65, 223–234. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Raoul, R.; Alain, P. Calcium in plant defence–signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar]
- Shao, H.B.; Song, W.Y.; Chu, L.Y. Advances of calcium signals involved in plant anti-drought. C. R. Biol. 2008, 331, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.J.; Wei, F.J.; Wang, C.; Wu, J.J.; Ratnasekera, D.; Liu, W.X.; Wu, W.H. Arabidopsis calcium-dependent protein kinase CPK10 functions in ABA and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010, 154, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; You, Y.M.; Huang, Y.Q.; Li, X.K.; Zhang, J.C.; Zhang, D.N.; He, C.X. Effects of drought stress on Cyclobalanopsis glauca seedlings under simulating karst environment condition. Acta Ecol. Sin. 2012, 32, 6318–6325. [Google Scholar] [CrossRef]
- Zhang, Z.F.; You, Y.M.; Huang, Y.Q.; Li, X.K.; Zhang, J.C.; Zhang, D.N.; He, C.X. Changes of Cyclobalanopsis glauca water parameters under drought stress in karst environment. Chin. J. Ecol. 2011, 30, 664–669. [Google Scholar]
- Sergiev, I.; Alexieva, V.; Karanov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. C. R. Acad. Bulg. Sci. 1997, 51, 121–124. [Google Scholar]
- Meng, X.; Wang, J.R.; Wang, G.D.; Liang, X.Q.; Li, X.D.; Meng, Q.W. An R2R3-MYB gene, LeAN2, positively regulated the thermo-tolerance in transgenic tomato. J. Plant Physiol. 2015, 175, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Gao, J. Experimental Techniques of Plant Physiology; World Publishing Corporation: Xi’an, China, 2000. [Google Scholar]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955, 1, 764–775. [Google Scholar]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B.N. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Yuan, X.T.; Liu, W.; Xuan, Y.N.; Zhang, Y.Y.; Yan, Y.Q. Physiological effects of exogenous Ca2+ on Nitraria tangutorum under salt stress. Plant Physiol. J. 2014, 50, 88–94. [Google Scholar]
- Harsh, N. Accumulation of osmolytes and osmotic adjustment in waterstressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ. Exp. Bot. 2003, 50, 253–264. [Google Scholar]
- Wu, Y.; Liu, X.; Wang, W.; Zhang, S.; Xu, B. Calcium regulates the cell-to-cell water flow pathway in maize roots during variable water conditions. Plant Physiol. Biochem. 2012, 58, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Serraj, R.; Sinclair, T.R. Osmolyte accumulation: Can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002, 25, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Muhammad, S.N.; Rashid, A.; Muhammad, Z.I.; Muhammad, Y.A.; Yasir, H.; Shah, F. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch. Agron. Soil Sci. 2018, 64, 116–131. [Google Scholar]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Calcium signaling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997, 12, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, H.; Chen, Y. Adaptation strategies of desert riparian forest vegetation in response to drought stress. Ecohydrology 2013, 6, 956–973. [Google Scholar] [CrossRef]
- Xi, Z.M.; Sun, W.J.; Zhang, Z.W. Effect of exogenous Ca2+ on drought resistance physiological indexes of wine grape cultivar Pinot Noir under water stress. J. Northwest A&F Univ. 2007, 35, 137–140. [Google Scholar]
- Shamsul, H.; Qaiser, H.; Mohammed, N.A.; Arif, S.W.; John, P.; Aqil, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar]
- Jiang, Y.; Huang, B. Effect of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J. Exp. Bot. 2001, 52, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sairam, R.K.; Srivastava, G.C.; Tyagi, A.; Meena, R.C. Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 2005, 169, 559–570. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yi, H. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Miyao, K.; Gaber, A.; Takeda, T.; Kanaboshi, H.; Miyasaka, H.; Shigeoka, S. Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. Plant J. 2004, 37, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexa, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim Biophys Acta 2015, 1847, 933–1003. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Wu, Q.; Liu, X.; Shen, Z.J.; Chen, J.; Liu, T.W.; Chen, J.; Zhu, C.Q.; Wu, F.H.; Chen, L.; et al. Comparative proteomic analysis reveals the effects of exogenous calcium against acid rain stress in Liquidambar formosana Hance leaves. J. Proteome Res. 2015, 15, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, P.; Chen, C.; Song, G.; Tang, H. Effect of Exogenous Ca2+ on the growth, physiological characters of Sophora viciifolia seedlings in Karst mountain area under the drought stress. J. Nucl. Agric. Sci. 2017, 31, 2039–2046. [Google Scholar]
- Gu, X.; Sun, L.; Gao, B.; Sun, Q.; Liu, C.; Zhang, J.; Li, X. Effects of calcium fertilizer application on peanut growth, physiological characteristics, yield and quality under drought stress. Chin. J. Appl. Ecol. 2015, 26, 1433–1439. [Google Scholar]
- Chen, L.L.; Wang, X.F.; Liu, M.; Yang, F.J.; Shi, Q.H.; Wei, M.; Li, Q.M. Effect of calcium and ABA on photosynthesis and related enzymes activities in cucumber seedlings under drought stress. Chin. J. Appl. Ecol. 2016, 27, 3996–4002. [Google Scholar]
- Ramalho, J.C.; Rebelo, M.C.; Santos, M.E.; Antunes, M.L.; Nunes, M. Effects of calcium deficiency on Coffea arabica. Nutrient changes and correlation of calcium levels with some photosynthetic parameters. Plant Soil 1994, 172, 87–96. [Google Scholar] [CrossRef]
- Hu, W.J.; Chen, J.; Liu, T.W.; Wu, Q.; Wang, W.H.; Liu, X.; Shen, Z.J.; Simon, M.; Chen, J.; Wu, F.H.; et al. Proteome and calcium-related gene expression in Pinus massoniana needles in response to acid rain under different calcium levels. Plant Soil 2014, 2380, 285–303. [Google Scholar] [CrossRef]
- Erinle, K.O.; Jiang, Z.; Ma, B.; Li, J.; Chen, Y.; Ur-Rehman, K.; Shahla, A.; Zhang, Y. Exogenous calcium induces tolerance to atrazine stress in Pennisetum seedlings and promotes photosynthetic activity, antioxidant enzymes and psbA gene transcripts. Ecotoxicol. Environ. Saf. 2016, 132, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Y.; Wang, G.; Yang, L.; Sun, X. Ecophysiological responses of Abies fabri seedlings to drought stress and nitrogen supply. Physiol. Plantarum 2010, 139, 335–347. [Google Scholar]


| Treatment Time (h) | Treatment Type | SPAD SPAD Value | Pn (µmol m−2 s−1) | Tr (mmol m−2 s−1) | Gs (mmol m−2 s−1) | WUE (µmol CO2 mmol H2O−1) |
|---|---|---|---|---|---|---|
| 0 | CK | 50.50 ± 1.80 a | 5.63 ± 0.47 a | 3.57 ± 0.15 a | 0.23 ± 0.03 a | 1.58 ± 0.07 a |
| Drought | 51.00 ± 3.61 a | 5.77 ± 0.23 a | 3.67 ± 0.15 a | 0.23 ± 0.01 a | 1.57 ± 0.02 a | |
| Drought + Ca2+ | 50.33 ± 1.53 a | 5.83 ± 0.15 a | 3.70 ± 0.10 a | 0.23 ± 0.02 a | 1.58 ± 0.02 a | |
| 24 | CK | 50.40 ± 2.62 a | 6.00 ± 0.44 a | 3.50 ± 0.20 a | 0.22 ± 0.01 a | 1.71 ± 0.05 a |
| Drought | 45.00 ± 1.50 b | 4.40 ± 0.26 b | 2.42 ± 0.10 b | 0.16 ± 0.01 b | 1.82 ± 0.02 b | |
| Drought + Ca2+ | 47.63 ± 0.81 c | 5.01 ± 0.25 c | 2.77 ± 0.15 c | 0.20 ± 0.01 c | 1.81 ± 0.05 c | |
| 48 | CK | 49.01 ± 1.73 a | 5.60 ± 0.43 a | 3.53 ± 0.23 a | 0.23 ± 0.01 a | 1.58 ± 0.02 a |
| Drought | 42.33 ± 0.76 b | 3.70 ± 0.36 b | 2.04 ± 0.10 b | 0.12 ± 0.01 b | 1.81 ± 0.01 b | |
| Drought + Ca2+ | 46.13 ± 1.46 c | 4.38 ± 0.38 c | 2.21 ± 0.12 c | 0.15 ± 0.01 c | 1.98 ± 0.00 c | |
| 72 | CK | 49.03 ± 3.61 a | 6.07 ± 0.32 a | 3.51 ± 0.10 a | 0.22 ± 0.02 a | 1.73 ± 0.05 a |
| Drought | 40.57 ± 1.36 b | 3.13 ± 0.29 b | 1.75 ± 0.13 b | 0.10 ± 0.02 b | 1.85 ± 0.08 b | |
| Drought + Ca2+ | 43.00 ± 1.73 c | 3.73 ± 0.25 c | 1.99 ± 0.12 c | 0.13 ± 0.01 c | 1.95 ± 0.20 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Ren, H.; Long, W.; Leng, X.; Wang, J.; Yao, X.; Li, S. Ecophysiological Responses of Calcicole Cyclobalanopsis glauca (Thunb.) Oerst. to Drought Stress and Calcium Supply. Forests 2018, 9, 667. https://doi.org/10.3390/f9110667
Xue L, Ren H, Long W, Leng X, Wang J, Yao X, Li S. Ecophysiological Responses of Calcicole Cyclobalanopsis glauca (Thunb.) Oerst. to Drought Stress and Calcium Supply. Forests. 2018; 9(11):667. https://doi.org/10.3390/f9110667
Chicago/Turabian StyleXue, Liang, Huadong Ren, Wei Long, Xiuhui Leng, Jia Wang, Xiaohua Yao, and Sheng Li. 2018. "Ecophysiological Responses of Calcicole Cyclobalanopsis glauca (Thunb.) Oerst. to Drought Stress and Calcium Supply" Forests 9, no. 11: 667. https://doi.org/10.3390/f9110667




