Carbon in Mature Native Forests in Australia: The Role of Direct Weighing in the Derivation of Allometric Equations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Tree Measurements
2.3. Weight Determinations in the Field
2.4. Biomass Estimation
2.5. Selected Allometric Equations for Biomass Estimates
2.6. Statistical Methods
3. Results
3.1. Forest Structure
3.2. Standing Above-Ground Biomass (AGB)—Tree Component and Total Biomass
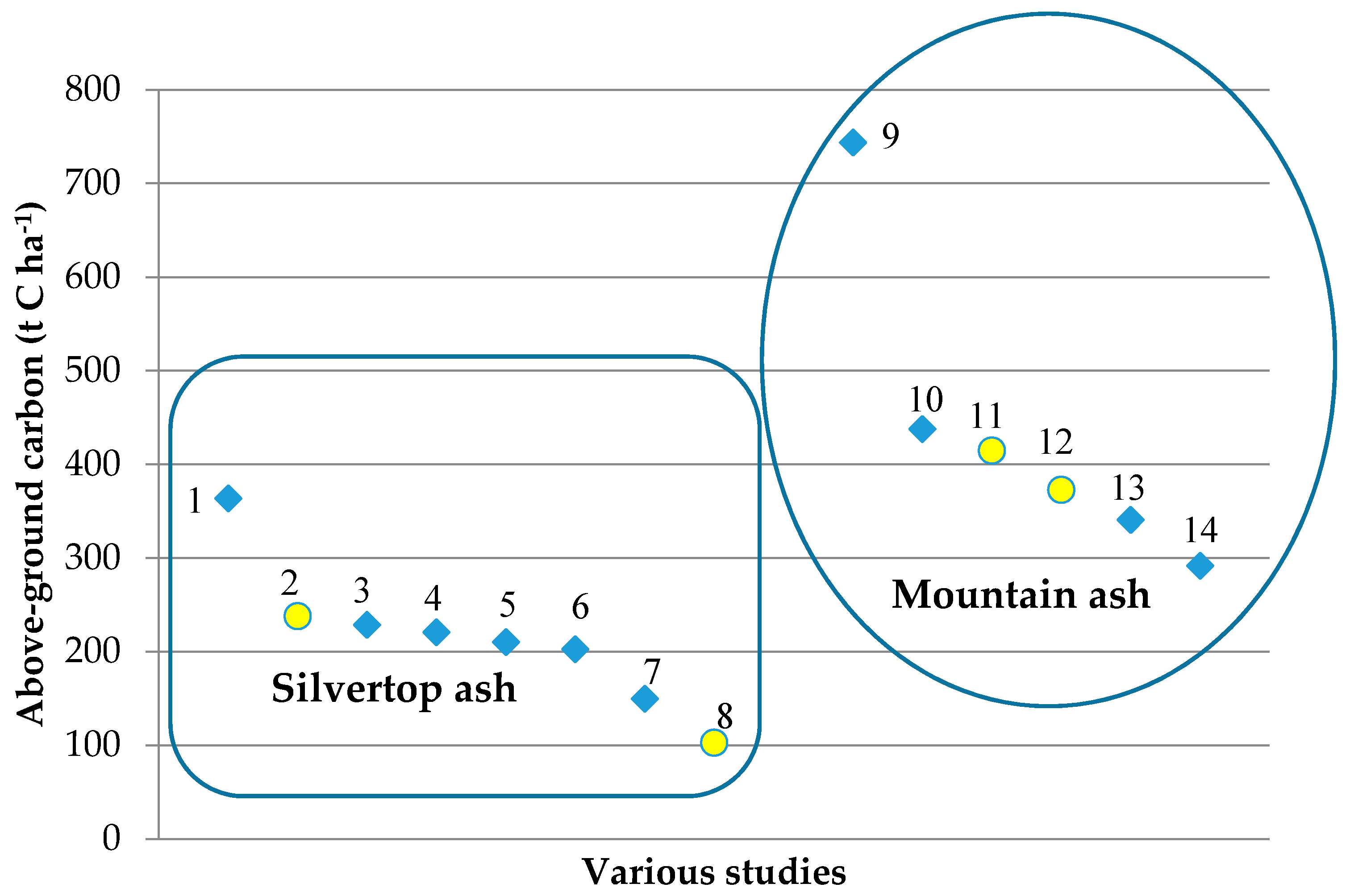
3.3. Allometric Equations for Key Species in the Study Sites: Development and Comparison with Existing Equations
3.4. Evaluation of the Performance of Existing Equations in Estimating Biomass for Key Species in the Study Sites
4. Discussion
5. Recommendations for Further Research
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Site Details
Appendix A.1. NSW South Coast—Eden—Silvertop Ash
Appendix A.1.1. Silvertop Ash Production Site

Appendix A.1.2. Silvertop Ash Conservation Site

Appendix A.2. NSW North Coast—Wauchope—Blackbutt
Appendix A.2.1. Blackbutt Production Site
Appendix A.2.2. Blackbutt Conservation Site
Appendix A.3. VIC Central Highlands—Mountain Ash
Appendix A.3.1. Mountain Ash Production Site
Appendix A.3.2. Mountain Ash Conservation Site
Appendix B. Moisture and Density of Tree Components and AGB by DBH Class
Appendix B.1. Method for Determining Moisture Content and Basic Density of Biomass Components
| Tree Component | Species | |||
|---|---|---|---|---|
| Blackbutt | Silvertop Ash | Mountain Ash | Total | |
| Stem (lower part–stump) | 38 | 50 | 25 | 113 |
| Stem (middle part) | 24 | 28 | 42 | 94 |
| Stem (upper part) | 40 | 37 | 20 | 97 |
| Bark | 83 | 70 | 51 | 204 |
| Branches | 13 | 16 | 13 | 39 |
Appendix B.2. Moisture Content and Basic Density of Biomass Samples
| Site | Status | Stump | Stem | Crown 1 | Bark | ||||
|---|---|---|---|---|---|---|---|---|---|
| MC (%) | BD (kg/m3) | MC (%) | BD (kg/m3) | MC (%) | BD (kg/m3) | MC (%) | BD (kg/m3) | ||
| Blackbutt | Regrowth | 43 (5) | 660 (61) | 41 (3) | 663 (52) | 39 (4) | 661 (67) | 56 (6) | 377 (56) |
| Blackbutt | Mature | 38 (3) | 766 (44) | 37 (3) | 767 (58) | 35 (4) | 783 (61) | 57 (4) | 416 (45) |
| Mountain ash | Regrowth | 57 (3) | 474 (43) | 49 (4) | 533 (45) | 44 (7) | 567 (55) | 60 (5) | 380 (39) |
| Silvertop ash | Regrowth | 46 (4) | 633 (72) | 41 (3) | 671 (34) | 40 (4) | 678 (57) | 46 (7) | 541 (68) |
| Silvertop ash | Mature | 46 (5) | 635 (85) | 39 (2) | 688 (43) | 38 (6) | 679 (71) | 46 (8) | 523 (121) |
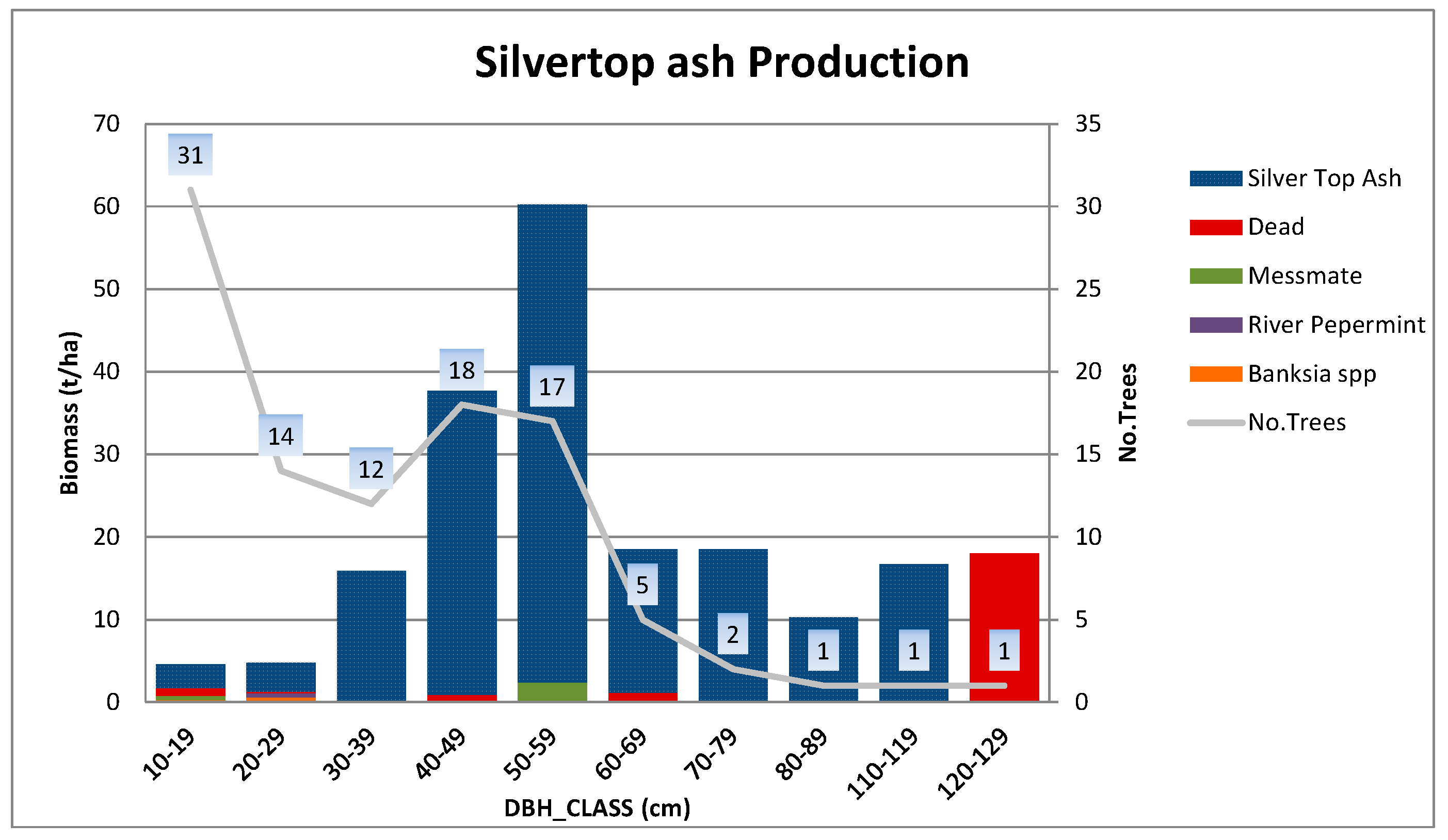
Appendix B.3. Standing Above-Ground Biomass (AGB)—Contribution by DBH Class

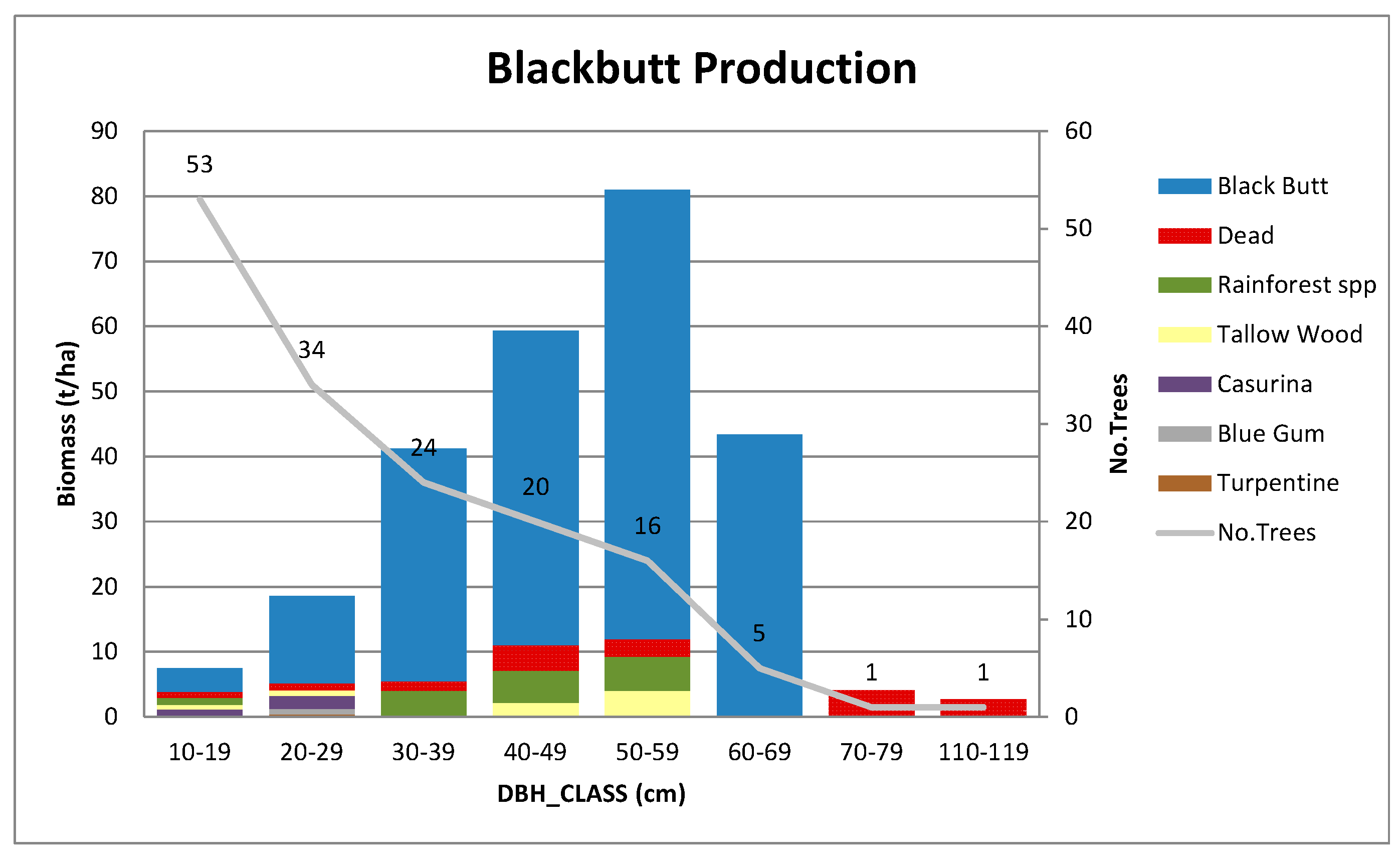
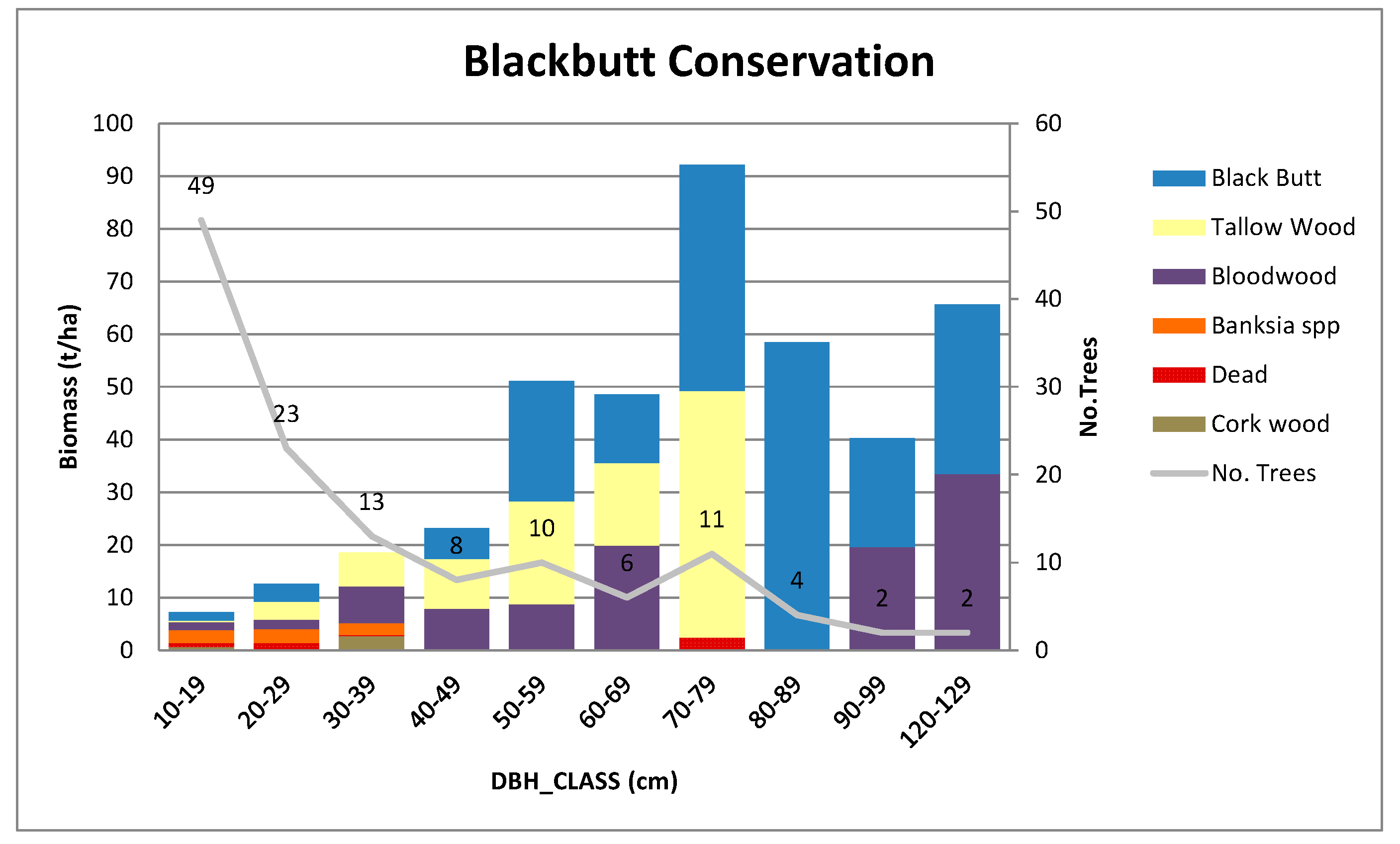
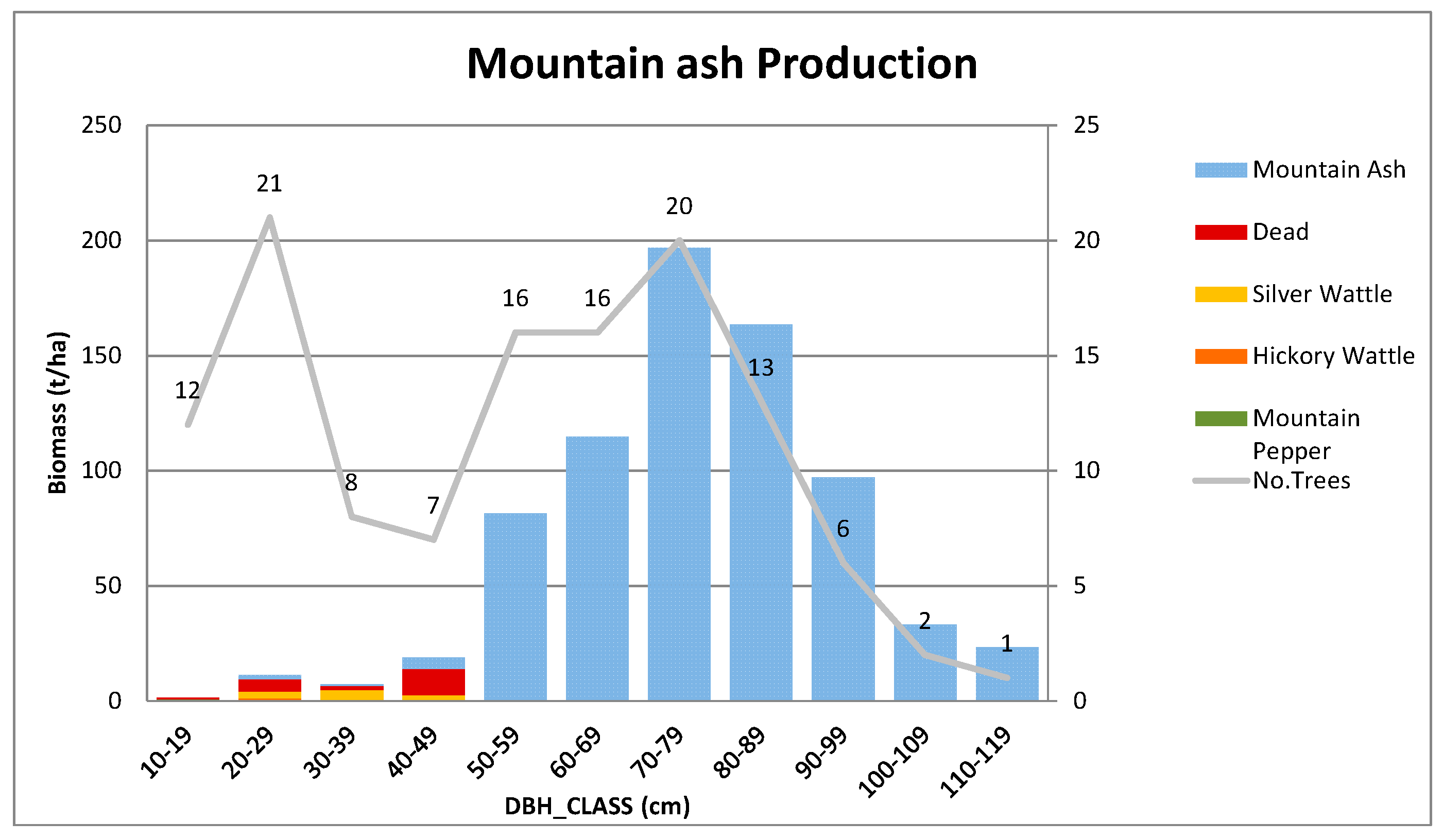

Appendix C. Further Details on Allometric Equations Used in This Study
| Source | Description of Equation Development |
|---|---|
| Montagu et al. [23] | Montague et al. [23] derived a number of equations specifically for blackbutt across seven study sites (five in the central and north coast of NSW and two at Fraser Island, Queensland), including plantations and native forests—we used the general DBH equation which was fitted across all study sites (DBH range 5–129 cm) without a correction factor. The biomass estimates were based on a mix of direct measurements of the fresh mass of the entire tree and subsampling techniques to estimate the biomass of tree components. |
| Mackowski [24] | Mackowski [24] proposed a number of equations for blackbutt with specific DBH ranges, derived for blackbutt-dominated forests 30–40 km south east of Grafton, NSW, based on measurements of ninety trees with DBH up to 189.6 cm—we used the equation for a DBH range of 45–135 cm [22]. Stem and branch volume were estimated using a log volume formula, with biomass estimated using published density values |
| Applegate [25] | Applegate [25] derived a number of equations based on blackbutt biomass data collected from Fraser Island, Queensland—we used the regeneration old growth equation which covered a DBH range of 13–129 cm [22]. Twenty-nine large trees ranging from 12.2 to 128.9 cm were felled; however the DBH class distribution was limited with only one tree with DBH greater than 60 cm (128.9 cm). The branches and foliage of each tree were weighed in the field for all but the large tree and samples taken for dry weight determinations. Biomass for the stem was calculated for logs based on the volume of the sapwood, heartwood and bark and density determinations. |
| Ashton [26] | Equation developed for silvertop ash and mountain ash (both study sites in Victoria). It was based on five 27-year old silvertop ash trees which were felled, with branches and leaves weighed in the field and stem biomass estimated from volume and physical parameters derived from stem discs. |
| Feller [27] | DBH and height equation developed specifically for mountain ash located at the Maroondah catchment area (State of Victoria), based on destructive sampling of six trees of varying size (four trees ranging from 15 to 30 cm DBH, one around 50 cm and the largest one around 70 cm DBH). The biomass of the crown was derived by a combination of determining the moisture content of a sub-sample of smaller diameter branches, and the use of published density values for the measured larger diameter branches. The biomass of the stem was also calculated based on published density values and stem measurements. |
| Stewart et al. [29] | Additive equation developed specifically for silvertop ash, in the State of Victoria. They sampled ten silvertop ash with DBH ranging between 28 and 89 cm, with the larger trees being more than 100-year old. The diameter of the stem and branches from every tree was measured, and biomass estimated based on destructive sampling of a sub-set of stem discs and branch samples of varying sizes. |
| Silett et al. [28] (derived) | Equation derived using their published biomass data for 22 trees with a DBH range of 80–312 cm, located at Kinglake National Park, VIC [40]. The biomass estimates were based on detailed field measurements (especially of the crown component), with subsampling techniques used to estimate the biomass of tree components. |
| Keith et al. [22] | Generic equation for native sclerophyll forests (including for the key species in this study), based on 25 records and 135 data points, with biomass of individual trees calculated for trees with DBHs ranging from 10 to 100 cm DBH (10 cm increments). |
References
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Chapter 3, Section 3.2 Forest Land. In Good Practice Guidance for LULUCF; Nabuurs, G., Ravindranath, N.H., Paustian, K., Freibauer, A., Hohenstein, W., Makundi, W., Eds.; IPCC National Greenhouse Gas Inventories Programme: Hayama, Japan, 2003; Available online: http://www.ipcc-nggip.iges.or.jp/public/gpglulucf/gpglulucf_files/Chp3/Chp3_2_Forest_Land.pdf (accessed on 02 March 2017).
- Intergovernmental Panel on Climate Change (IPCC). Revised Supplementary Methods and Good Practice Guidance Arising from the Kyoto Protocol; Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., Troxler, T.G., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Available online: https://www.montrealprocess.org/documents/publications/techreports/MontrealProcessSeptember2015.pdf (accessed on 13 December 2017).
- Bellassen, V.; Luyssaert, S. Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Krankina, O.N.; Harmon, M.E.; Schnekenburger, F.; Sierra, C.A. Carbon balance on federal forest lands of Western Oregon and Washington: The impact of the Northwest Forest Plan. For. Ecol. Manag. 2012, 286, 171–182. [Google Scholar] [CrossRef]
- Colombo, S.J.; Chen, J.; Ter-Mikaelian, M.T.; McKechnie, J.; Elkie, P.C.; MacLean, H.L.; Heath, L.S. Forest protection and forest harvest as strategies for ecological sustainability and climate change mitigation. For. Ecol. Manag. 2012, 281, 140–151. [Google Scholar] [CrossRef]
- Hennigar, C.R.; MacLean, D.A.; Amos-Binks, L.J. A novel approach to optimize management strategies for carbon stored in both forests and wood products. For. Ecol. Manag. 2008, 256, 786–797. [Google Scholar] [CrossRef]
- Klein, D.; Hollerl, S.; Blaschke, M.; Schulz, C. The contribution of managed and unmanaged forests to climate change mitigation—A model approach at stand level for the main tree species in Bavaria. Forests 2013, 4, 43–69. [Google Scholar] [CrossRef]
- Keith, H.; Lindenmayer, D.; Macintosh, A.; Mackey, B. Under what circumstances do wood products from native forests benefit climate change mitigation? PLoS ONE 2015, 10, e0139640. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, F.; George, B.H.; Cowie, A.; Williams, J.; Kelly, G. Greenhouse gas balance of native forests in New South Wales, Australia. Forests 2012, 3, 653–683. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Cohen, W.B.; Parker, G.G.; Harding, D.J. LiDAR remote sensing for ecosystem studies. BioScience 2002, 52, 19–30. [Google Scholar] [CrossRef]
- Brown, S. Measuring carbon in forests: Current status and future challenges. Environ. Pollut. 2002, 116, 363–372. [Google Scholar] [CrossRef]
- Clark, D.B.; Kellner, J.R. Tropical forest biomass estimation and the fallacy of misplaced concreteness. J. Veg. Sci. 2012, 23, 1191–1196. [Google Scholar] [CrossRef]
- Chave, J.; Condit, R.; Aguilar, S.; Hernandez, A.; Lao, S.; Perez, R. Error propagation and scaling for tropical forest biomass estimates. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004, 359, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Van Breugel, M.; Ransijn, J.; Craven, D.; Bongers, F.; Hall, J.S. Estimating carbon stock in secondary forests: Decisions and uncertainties associated with allometric biomass models. For. Ecol. Manag. 2011, 262, 1648–1657. [Google Scholar] [CrossRef]
- Picard, N.; Laurent, S.-A.; Henry, M. Manual for Building Tree Volume and Biomass Allometric Equations: From Field Measurement to Prediction; Food and Agricultural Organization of the United Nations, Rome and Centre de Cooperation Internationale en recherche Agronomique pur le Developpement: Montpellier, France, 2012. [Google Scholar]
- Bragg, D.C. Modeling loblolly pine aboveground live biomass in a mature pine-hardwood stand: A cautionary tale. J. Ark. Acad. Sci. 2011, 65, 31–38. [Google Scholar]
- Keith, H.; Mackey, B.; Lindenmayer, D. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl. Acad. Sci. USA 2009, 106, 11635–11640. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, F.; Roxburgh, S.; Cameron, N.; Coburn, R.; Bi, H. Carbon Stocks and Flows in Native Forests and Harvested Wood Products in SE Australia. Report Prepared for Forest and Wood Products Australia. 2016. Available online: http://www.fwpa.com.au/images/resources/Amended_Final_report_C_native_forests_PNC285-1112.pdf (accessed on 03 May 2017).
- Ximenes, F.A.; Gardner, W.D.; Kathuria, A. Proportion of above-ground biomass in commercial logs and residues following the harvest of five commercial forest species in Australia. For. Ecol. Manag. 2008, 256, 335–346. [Google Scholar] [CrossRef]
- Keith, H.; Barrett, D.; Keenan, R. Review of Allometric Relationships for Woody Biomass for NSW, ACT, VIC, TAS, SA; National C Accounting Technical Report No. 5b; Australian Greenhouse Office: Canberra, Austrilia, 2000. [Google Scholar]
- Montagu, K.D.; Duttmer, K.; Barton, C.V.M.; Cowie, A.L. Developing general allometric relationships for regional estimates of carbon sequestration—An example using Eucalyptus pilularis from seven contrasting sites. For. Ecol. Manag. 2005, 204, 113–127. [Google Scholar] [CrossRef]
- Mackowski, C.M. Wildlife Hollows and Timber Management in Blackbutt Forest. Master’s Thesis, University of New England, Armidale, Australia, 1987. [Google Scholar]
- Applegate, G.B. Biomass of Blackbutt (Eucalyptus pilularis Sm.) Forests on Fraser Island. Master’s Thesis, University of New England, Armidale, Australia, 1982. [Google Scholar]
- Ashton, D.H. Phosphorus in forest ecosystems at Beenak, Victoria. J. Ecol. 1976, 64, 171–186. [Google Scholar] [CrossRef]
- Feller, M.C. Biomass and nutrient distribution in two Eucalypt forest ecosystems. Aust. J. Ecol. 1980, 5, 309–333. [Google Scholar] [CrossRef]
- Sillett, S.C.; van Pelt, R.; Kramer, R.D.; Carroll, A.L.; Koch, G.W. Biomass and growth potential of Eucalyptus regnans up to 100 m tall. For. Ecol. Manag. 2015, 348, 78–91. [Google Scholar] [CrossRef]
- Stewart, H.T.L.; Flinn, D.W.; Aeberli, B.C. Aboveground biomass of a mixed Eucalypt forest in eastern Victoria. Aust. J. Bot. 1979, 27, 725–740. [Google Scholar] [CrossRef]
- Tukey, J.W. We need both exploratory and confirmatory. Am. Stat. Assoc. 1980, 34, 23–25. [Google Scholar]
- Jolliffe, I.T.; Stephenson, D.B. Forecast Verification: A Practitioner’s Guide in Atmospheric Science; Wiley & Sons: New York, NY, USA, 2003; 240p. [Google Scholar]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models Part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 05 May 2017).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Zambrano-Bigiarini, M. hydroGOF: Goodness-of-Fit Functions for Comparison of Simulated and Observed Hydrological Time Seriesr Package Version 0.3-10. 2017. Available online: http://hzambran.github.io/hydroGOF/ (accessed on 05 May 2017). [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75968-5. [Google Scholar]
- Roxburgh, S.H.; Wood, S.W.; Mackey, B.G.; Woldendorp, G.; Gibbons, P. Assessing the carbon sequestration potential of managed forests: A case study from temperate Australia. J. Appl. Ecol. 2006, 43, 1149–1159. [Google Scholar] [CrossRef]
- Turner, J.; Lambert, M.J. Effects of forest harvesting nutrient removals on soil nutrient reserves. Oecologia 1986, 70, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, F.A.; Gardner, W.D.; Richards, G.P. Total above-ground biomass and biomass in commercial logs following the harvest of spotted gum (Corymbia maculata) forests of SE NSW. Aust. For. 2006, 69, 213–222. [Google Scholar] [CrossRef]
- Keith, H.; Lindenmayer, D.; Mackey, B.; Blair, D.; Carter, L.; McBurney, L.; Okada, S.; Konishi-Nagano, T. Managing temperate forests for carbon storage: Impacts of logging versus forest protection on C stocks. Ecosphere 2014, 5, 1–34. [Google Scholar] [CrossRef]
- Fedrigo, M.; Kasel, S.; Bennett, L.T.; Roxburgh, S.H.; Nitschke, C.R. Carbon stocks in temperate forests of south-eastern Australia reflect large tree distribution and edaphic conditions. For. Ecol. Manag. 2014, 334, 129–143. [Google Scholar] [CrossRef]
- Sillett, S.C.; van Pelt, R.; Koch, G.W.; Ambrose, A.R.; Carroll, A.L.; Antoine, M.E.; Mifsud, B.M. Increasing wood production through old age in tall trees. For. Ecol. Manag. 2010, 259, 976–994. [Google Scholar] [CrossRef]
- Loehle, C. Tree life history strategies: The role of defenses. Can. J. For. Res. 1988, 18, 209–222. [Google Scholar] [CrossRef]
- Fujimori, T.; Kawanabe, S.; Saito, H.; Grier, C.C.; Shidei, T. Biomass and primary production in forests of three major vegetation zones of the Northwestern United States. J. Jpn. For. Sci. 1976, 58, 360–373. [Google Scholar]
- Means, J.E.; MacMillan, P.C.; Cromack, K. Biomass and nutrient content of Douglas-fir logs and other detrital pools in an old-growth forest, Oregon, USA. Can. J. For. Res. 1992, 22, 1536–1546. [Google Scholar] [CrossRef]
- Busing, R.T. Composition, structure and diversity of cove forest stands in the Great Smokey Mountains: A patch dynamics perspective. J. Veg. Sci. 1998, 9, 881–890. [Google Scholar] [CrossRef]
- Vann, D.R.; Joshi, A.; Pérez, C.; Johnson, A.H.; Frizano, J.; Zarin, D.J.; Armesto, J.J. Distribution and cycling of C, N, Ca, Mg, K and P in three pristine, old-growth forests in the Cordillera de Piuchué, Chile. Biogeochemistry 2002, 60, 25–47. [Google Scholar] [CrossRef]
- Schlegel, B.C.; Donoso, P.J. Effects of forest type and stand structure on coarse woody debris in old-growth rainforests in the Valdivian Andes, south-central Chile. For. Ecol. Manag. 2008, 255, 1906–1914. [Google Scholar] [CrossRef]
- Ash, J.; Helman, C. Floristics and vegetation biomass of a forest catchment, Kioloa, south coastal New South Wales. Cunninghamia 1990, 2, 167–182. [Google Scholar]
- Luyssaert, S.; Schulze, E.-D.; Borner, A.; Knohl, A.; Hessenmoller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Attiwill, P. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
- Roxburgh, S.H.; Paul, K.I.; Clifford, D.; England, J.R.; Raison, R.J. Guidelines for constructing allometric models for the prediction of woody biomass: How many individuals to harvest? Ecosphere 2015, 6, 38. [Google Scholar] [CrossRef]
- Hoover, C.M.; Smith, J.E. Evaluating revised biomass equations: Are some forest types more equivalent than others? Carbon Balance Manag. 2016, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, L.; Rourke, O.; Dubayah, R. Small sample sizes yield biased allometric equations in temperate forests. Sci. Rep. 2015, 5, 17153. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Allison, R.B. Decay detection in red oak trees using a combination of visual inspection, acoustic testing, and resistance microdrilling. Arboric. Urban For. 2008, 34, 1–4. [Google Scholar]

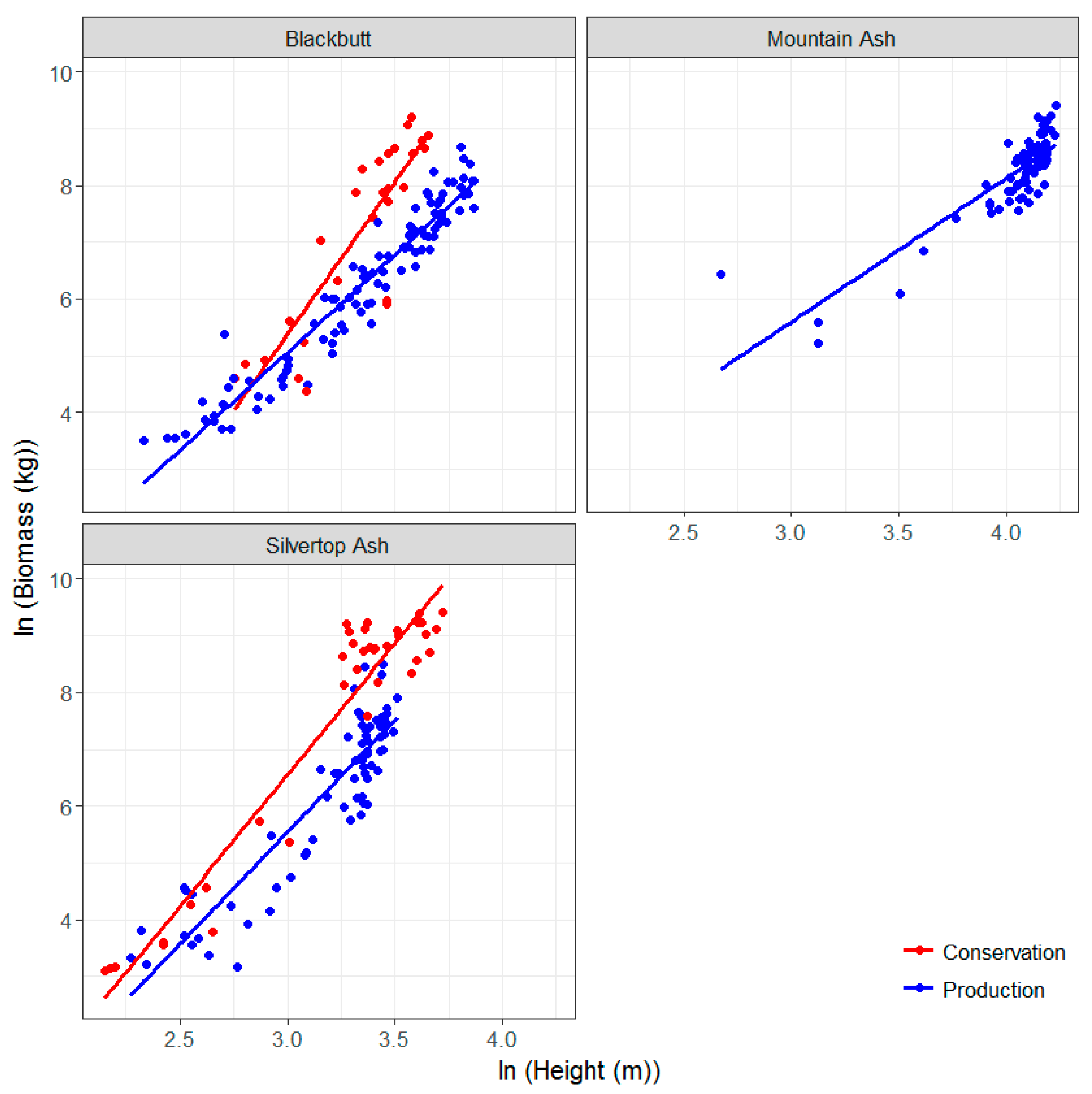
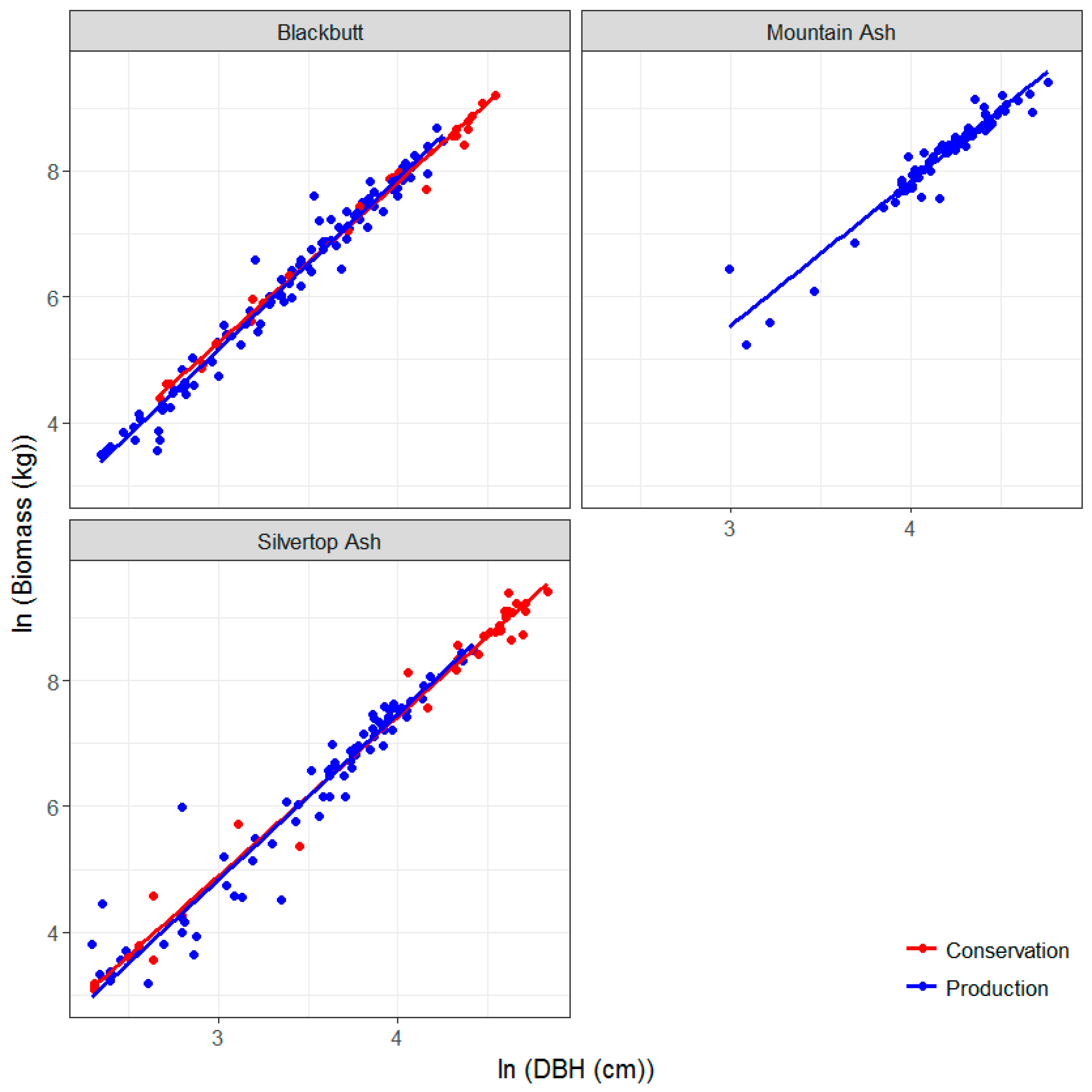
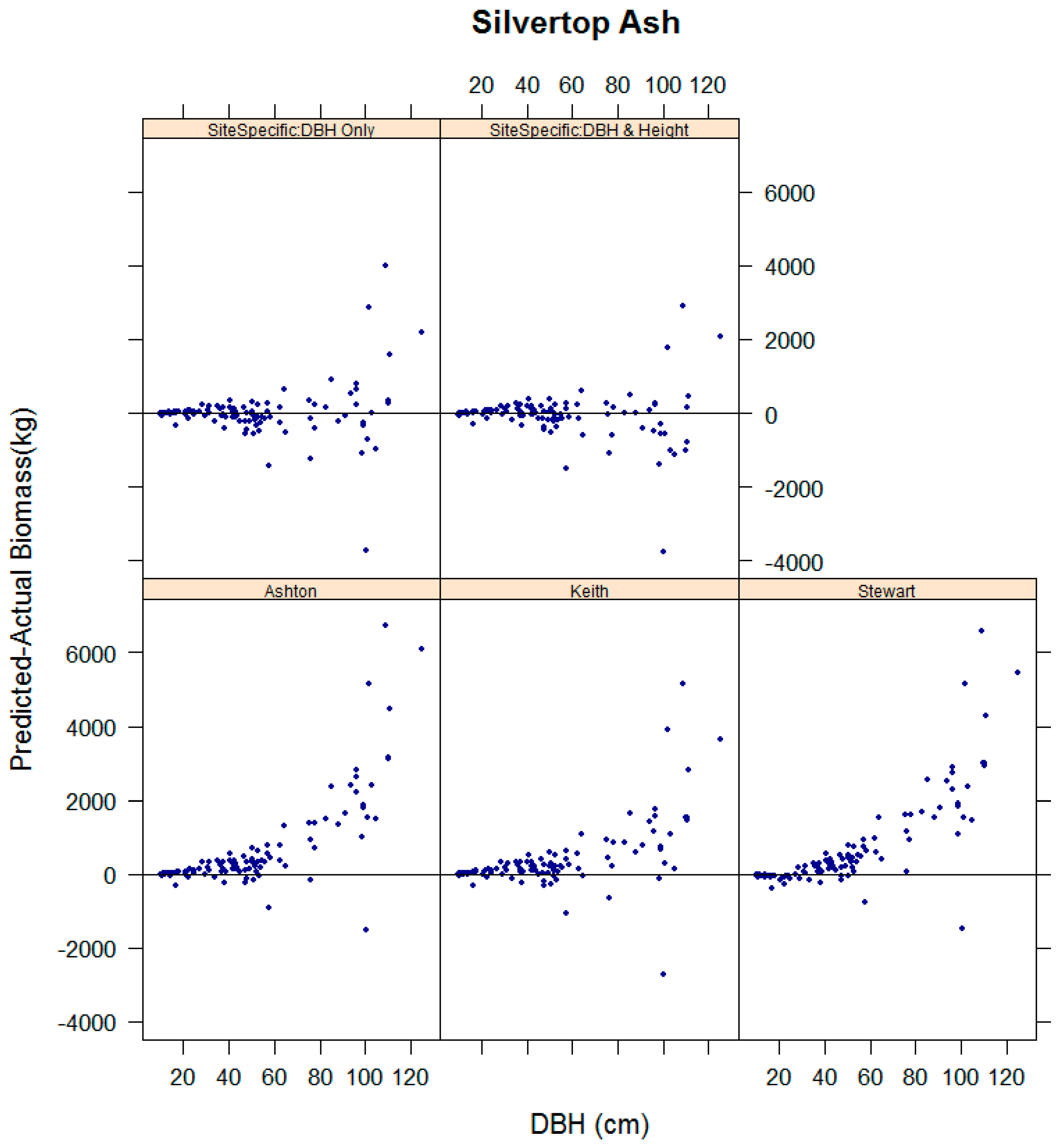

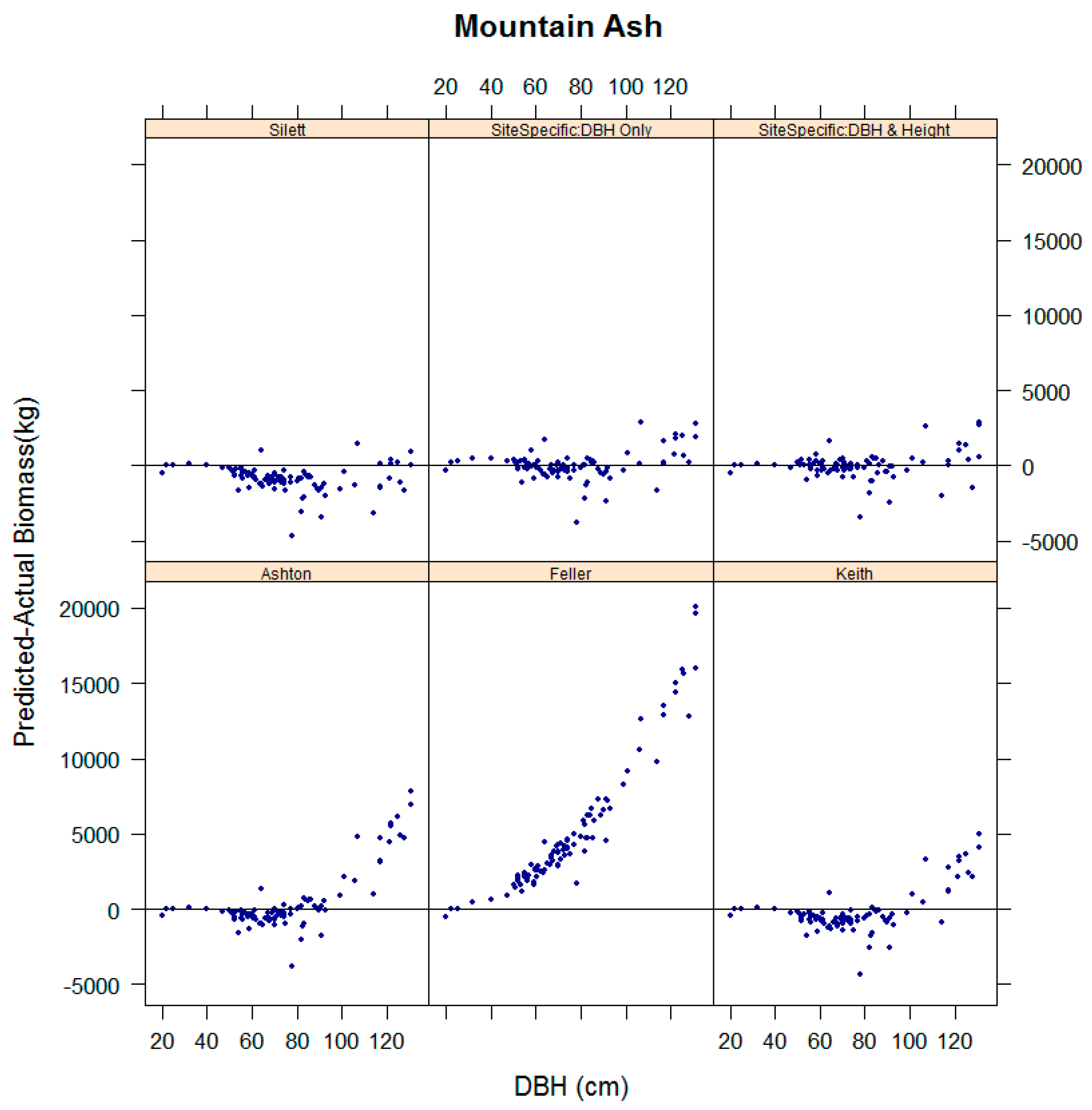
| Tenure | Dominant Species | Location Coordinates | Stand Age (Years, Circa) | Plot Dimensions and Area (m) | Mean Annual Rainfall (mm) | No. Trees |
|---|---|---|---|---|---|---|
| Production | Blackbutt | 56S 445400E 6544700N | 60 | 108 × 46.5 | 1283 | 153 |
| Conservation | Blackbutt | 56S 482200E 6501500N | 90; >200 * | 80 × 60 | 1548 | 128 |
| Production | Mountain ash | 55S 371474E 5845398N | 75 | 100 × 53 | 1372 | 122 |
| Conservation | Mountain ash | 55S 368757E 5847893N | 90; 110 * | 100 × 50.5 | 1372 | 91 |
| Production | Silvertop ash | 55S 737919E 5886641N | 60 | 100.3 × 46.8 | 1000 | 103 |
| Conservation | Silvertop ash | 55S 739065E 5885619N | 60; >200 * | 101 × 47.5 | 1000 | 80 |
| Species | Equation | Source |
|---|---|---|
| Blackbutt | ln M (kg) = −2.3267 + 2.485 ×ln DBH (cm) | Keith et al. [22] |
| ln M (kg) = −2.642 + 2.551 × ln DBH (cm) × 1.109 | Montagu et al. [23] 1 | |
| M (kg) = (0.000527127 × DBH (cm)^2.19699) × 710 | Mackowski [24] 2 | |
| log M (kg) = −1.3326 + 2.6934 × log DBH (cm) | Applegate [25] | |
| ln M (kg) = 0.0580 × DBH2.673 | This study | |
| ln M (kg) = 0.0311 × DBH2.405Ht0.465 | This study | |
| Mountain ash | log M (kg) = −2.43 + 2.58 log Girth (cm) | Ashton [26] |
| M (kg) = −45.6 + 248.9 DBH(m)^2 HGT (m) (Stem); M (kg) = −42.2 + 25.7 DBH (m)^2 HGT (m) (Branches); M (kg) = −16.9 + 6.4 × ln DBH (cm) (Leaves) | Feller [27] | |
| M (kg) = 0.8721 × DBH^2 − 9.4009 × DBH (cm) | Sillett et al. (derived); [28] | |
| ln M (kg) = 0.7555 × DBH2.038 | This study | |
| ln M (kg) = 0.0392 × DBH1.814Ht0.955 | This study | |
| Silvertop ash | log M (kg) = −2.43 + 2.58 log Girth (cm) | Ashton [26] |
| log M (kg) = −1.0373 + 2.3867 log DBH (cm) (Stem-wood); log M (kg) = −2.1434 + 2.7344 log DBH (cm) (Stem-bark); M (kg) = 4.7424 + 0.01026 DBH^2 (cm) (Leaves); M (kg) = −246.9228 + 0.2254 DBH^2 (cm) (Branches-wood); M (kg) = −69.5361 + 0.059 DBH^2 (cm) (Branch-bark); M (kg) = 3.4289 + 0.0133 DBH^2 (cm) (twigs) | Stewart et al. [29] | |
| ln M (kg) = −2.3267 + 2.485 × ln DBH (cm) | Keith et al. [22] | |
| log M (kg) = −2.43 + 2.58 log Girth (cm) | Ashton [26] | |
| ln M (kg) = 0.0564 × DBH2.579 | This study | |
| ln M (kg) = 0.0375 × DBH2.390Ht0.352 | This study |
| Site | DBH (Mean, SE, cm) 1 | Tree Height (Mean, SE, m) | Stand Density (Stems ha−2) 2 | Dead Trees (Stems ha−1) | Basal Area (m2 ha−1) |
|---|---|---|---|---|---|
| Blackbutt conservation | 53.1 (5.8) | 28.6 (1.3) | 242 | 25 | 39.3 |
| Blackbutt production | 33.7 (1.6) | 30.8 (1.1) | 270 | 38 | 25.0 |
| Mountain ash conservation | 102.3 (5.1) | 61.2 (2.4) | 160 | 22 | 74.0 |
| Mountain ash production | 68.4 (2.0) | 58.5 (1.2) | 198 | 40 | 62.3 |
| Silvertop ash conservation | 71.5 (6.5) | 26.5 (1.6) | 154 | 13 | 49.0 |
| Silvertop ash production | 39.2 (1.8) | 24.5 (0.8) | 191 | 28 | 25.4 |
| Site | Stump (t ha−1) | Stem (t ha−1) | Bark 1 (t ha−1) | Crown 2 (t ha−1) | Other 3 (t ha−1) | Total (t ha−1) | Total in the Dominant Species (t ha−1) |
|---|---|---|---|---|---|---|---|
| Blackbutt production | 12 | 123 | 17 | 35 | 71 | 258 | 124 |
| Blackbutt conservation | 11 | 91 | 34 | 148 | 134 | 418 | 347 |
| Mountain ash production | 34 | 581 | 31 | 61 | 38 | 745 | 737 |
| Mountain ash conservation 4 | 56 | 662 | 31 | 54 | 17 | 819 | 810 |
| Silvertop ash production | 9 | 105 | 30 | 46 | 15 | 205 | 180 |
| Silvertop ash conservation | 32 | 189 | 55 | 165 | 35 | 476 | 429 |
| Model | Species | Parameter | Parameter Value | Std. Error | t Value | p > |t| |
|---|---|---|---|---|---|---|
| ln M (kg) = 0.0580 × DBH2.673 | Blackbutt | Intercept | −2.847 | 0.108 | −26.428 | <0.001 |
| ln DBH | 2.673 | 0.031 | 87.258 | <0.001 | ||
| ln M (kg) = 0.0311 × DBH2.405 × Ht0.465 | Blackbutt | Intercept | −3.471 | 0.152 | −22.873 | <0.001 |
| ln DBH | 2.405 | 0.058 | 41.780 | <0.001 | ||
| ln Height | 0.465 | 0.086 | 5.401 | <0.002 | ||
| ln M (kg) = 0.7555 × DBH2.038 | Mountain ash | Intercept | −0.280 | 0.174 | −1.611 | 0.11 |
| ln DBH | 2.038 | 0.041 | 50.252 | <0.001 | ||
| ln M (kg) = 0.0392 × DBH1.814 × Ht0.955 | Mountain ash | Intercept | −3.239 | 0.203 | −15.986 | <0.001 |
| ln DBH | 1.814 | 0.054 | 33.345 | <0.001 | ||
| ln Height | 0.955 | 0.081 | 11.729 | <0.001 | ||
| ln M (kg) = 0.0564 × DBH2.579 | Silvertop ash | Intercept | −2.876 | 0.117 | −24.643 | <0.001 |
| ln DBH | 2.579 | 0.031 | 82.530 | <0.001 | ||
| ln M (kg) = 0.0375 × DBH2.390 × Ht0.352 | Silvertop ash | Intercept | −3.282 | 0.211 | −15.539 | <0.001 |
| ln DBH | 2.390 | 0.065 | 37.021 | <0.001 | ||
| ln Height | 0.352 | 0.121 | 2.904 | 0.004 |
| Species | Model | Slope | NSE | MAE (%) | Bias (%) |
|---|---|---|---|---|---|
| Blackbutt | Applegate [25] | 0.91 ** | 0.95 | 16.75 | −11.28 |
| Keith et al. [22] | 0.79 *** | 0.90 | 22.63 | −19.93 | |
| Mackowski [24] | 0.89 *** | 0.94 | 17.30 | −3.45 | |
| Montagu et al. [23] | 0.85 *** | 0.93 | 19.25 | −15.41 | |
| Site specific: DBH only | 1.04 * | 0.95 | 14.6 | 1.8 | |
| Site specific: DBH & Height | 0.96 * | 0.97 | 12.66 | −1.50 | |
| Mountain Ash | Ashton [26] | 1.17 ** | 0.60 | 21.88 | 8.73 |
| Feller [27] | 1.98 *** | −2.91 | 91.82 | 91.61 | |
| Keith et al. [22] | 1.04 ns | 0.81 | 18.84 | −3.06 | |
| Sillett et al. [28] | 0.87 ** | 0.88 | 17.00 | −14.91 | |
| Site specific: DBH only | 1.02 ns | 0.92 | 10.78 | 0.54 | |
| Site specific: DBH & Height | 1.02 ns | 0.94 | 9.01 | −0.22 | |
| Silvertop Ash | Ashton [26] | 1.28 *** | 0.79 | 30.82 | 27.81 |
| Keith et al. [22] | 1.13 ** | 0.91 | 19.95 | 15.11 | |
| Stewart et al. [29] | 1.28 *** | 0.79 | 32.18 | 28.15 | |
| Site specific: DBH only | 1.00 ns | 0.95 | 13.8 | 0.6 | |
| Site specific: DBH & Height | 0.96 * | 0.96 | 12.59 | −2.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Ximenes, F.; Kathuria, A.; McLean, M.; Coburn, R.; Sargeant, D.; Ryan, M.; Williams, J.; Boer, K.; Mo, M. Carbon in Mature Native Forests in Australia: The Role of Direct Weighing in the Derivation of Allometric Equations. Forests 2018, 9, 60. https://doi.org/10.3390/f9020060
A. Ximenes F, Kathuria A, McLean M, Coburn R, Sargeant D, Ryan M, Williams J, Boer K, Mo M. Carbon in Mature Native Forests in Australia: The Role of Direct Weighing in the Derivation of Allometric Equations. Forests. 2018; 9(2):60. https://doi.org/10.3390/f9020060
Chicago/Turabian StyleA. Ximenes, Fabiano, Amrit Kathuria, Michael McLean, Rebecca Coburn, David Sargeant, Michael Ryan, Justin Williams, Ken Boer, and Matthew Mo. 2018. "Carbon in Mature Native Forests in Australia: The Role of Direct Weighing in the Derivation of Allometric Equations" Forests 9, no. 2: 60. https://doi.org/10.3390/f9020060
APA StyleA. Ximenes, F., Kathuria, A., McLean, M., Coburn, R., Sargeant, D., Ryan, M., Williams, J., Boer, K., & Mo, M. (2018). Carbon in Mature Native Forests in Australia: The Role of Direct Weighing in the Derivation of Allometric Equations. Forests, 9(2), 60. https://doi.org/10.3390/f9020060





