Resistance of a Local Ecotype of Castanea sativa to Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Southern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Morphological Characterization for the Identification of Plants and Fruits
2.3. Evaluation of Resistance
2.3.1. Buds
2.3.2. Shoots and Galls
2.3.3. Evaluation of Percentage of Parasitization
2.3.4. Statistical Analysis
3. Results
3.1. Morphological Characterization for the Identification of Plants and Fruits
3.2. Evaluation of Resistance
3.2.1. Buds
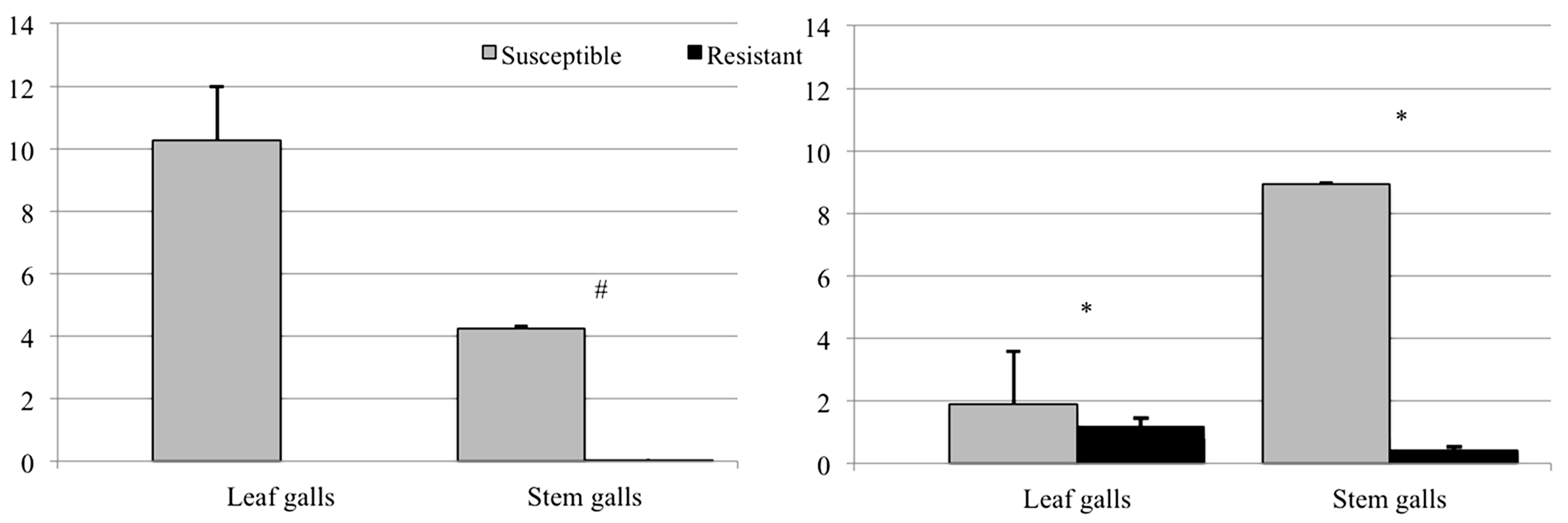
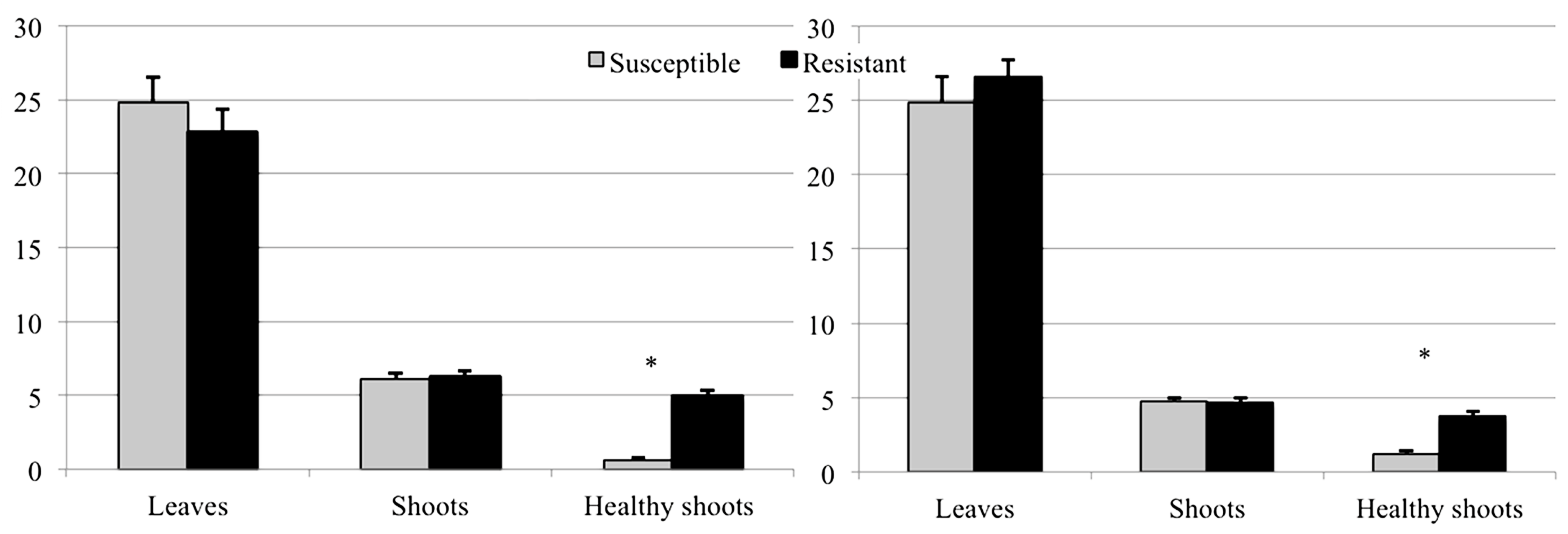
3.2.2. Shoots and Galls
3.2.3. Evaluation of Percentage of Parasitization
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, R.; Candresse, T.; Dormannsné Simon, E.; Gilioli, G.; Grégoire, J.-C.; Jeger, M.J.; Karadjova, O.E.; Lövei, G.; Makowski, D.; Manceau, C.; et al. EFSA Panel on Plant Health (PLH). Risk assessment of the oriental chestnut gall wasp, Dryocosmus kuriphilus for the EU territory on request from the European Commission. EFSA J. 2010, 8. [Google Scholar] [CrossRef]
- Bernardo, U.; Gebiola, M.; Xiao, Z.; Zhu, C.-D.; Pujade-Villar, J.; Viggiani, G. Description of Synergus castaneus n. sp. (Hymenoptera: Cynipidae: Synergini) associated with an unknown gall on Castanea spp. (Fagaceae) in China. Ann. Entomol. Soc. Am. 2013, 106, 437–446. [Google Scholar] [CrossRef]
- Zhu, D.-H.; Liu, Z.; Lu, P.-F.; Yang, X.-H.; Su, C.-Y.; Liu, P. New gall wasp species attacking chestnut trees: Dryocosmus zhuili n. sp. (Hymenoptera: Cynipidae) on Castanea henryi from Southeastern China. J. Insect Sci. 2015, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Melika, G.; Stone, G. The diversity and phylogeography of cynipid gallwasps (Hymenoptera: Cynipidae) of the oriental and eastern Palearctic regions, and their associated communities. Orient. Insects 2007, 41, 169–212. [Google Scholar] [CrossRef]
- Cho, D.Y.; Lee, S.O. Ecological studies on the chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu and observations on the chestnut trees by its insect. Korean J. Appl. Entomol. 1963, 2, 47–54. [Google Scholar]
- Graziosi, I.; Santi, F. Chestnut gall wasp (Dryocosmus kuriphilus): Spreading in Italy and new records in Bologna province. Bull. Insectol. 2008, 61, 343–348. [Google Scholar]
- Kos, K.; Kriston, E.; Melika, G. Invasive chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae), its native parasitoid community and association with oak gall wasps in Slovenia. Eur. J. Entomol. 2015, 112, 698–704. [Google Scholar] [CrossRef]
- Michaelakis, A.; Papachristos, D.; Chytas, D.A.; Antonopoulou, P.D.; Milonas, P.G.; Avtzis, D.N. First record of Dryocosmus kuriphilus in Greece. EPPO Bull. 2016, 46, 290–294. [Google Scholar] [CrossRef]
- Radócz, L.; Szilagyi, A.; Nagy, M.; Kovács, G.; Melika, G. Asian sweet chestnut gallwasp, Dryocosmus kuriphilus (Hymenoptera, Cynipidae): First record for Romania. North-West. J. Zool. 2016, 12, 201–204. [Google Scholar]
- Brussino, G.; Bosio, G.; Baudino, M.; Giordano, R.; Ramello, F.; Melika, G. Pericoloso insetto esotico per il castagno europeo. Inf. Agrar. 2002, 37, 59–62. [Google Scholar]
- EPPO (European Plant Protection Organization). First Report of Dryocosmus kuriphilus in the Czech Republic; EPPO Reporting Service; EPPO: Paris, France, 2012; Volume 7, p. 5. [Google Scholar]
- EPPO (European Plant Protection Organization). First Report of Dryocosmus kuriphilus in Portugal; EPPO Reporting Service; EPPO: Paris, France, 2014; Volume 6, p. 103. [Google Scholar]
- MAGRAMA (Ministerio de Agricultura, Alimentación y Medio Ambiente of Spain). BOE-A-2013-8565 Catálogo Español de Especies Exóticas Invasoras. Real Decreto 630/2013. 2013. Available online: http://www.boe.es/buscar/pdf/2013/BOE-A-2013-8565consolidado.pdf (accessed on 23 August 2017).
- Pástor, M.; Juhásová, G.; Juhás, D.; Bakay, L.; Ján, K.; Benčať, T. Occurrence of oriental chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera, Cynipidae) in Slovakia. Plant Prot. Sci. 2017, 53, 243–246. [Google Scholar] [CrossRef]
- Bernardo, U.; Iodice, L.; Sasso, R.; Tutore, V.A.; Cascone, P.; Guerrieri, E. Biology and monitoring of Dryocosmus kuriphilus on Castanea sativa in Southern Italy. Agric. For. Entomol. 2013, 15, 65–76. [Google Scholar] [CrossRef]
- Aebi, A.; Schonrogge, K.; Melika, G.; Alma, A.; Bosio, G.; Quacchia, A.; Picciau, L.; Abe, Y.; Moriya, S.; Yaras, K.; et al. Parasitoid recruitment to the globally invasive chestnut gall wasp Dryocosmus kuriphilus. In Galling Arthropods and Their Associates, Ecology and Evolution; Ozaki, K., Yukwa, J., Ohgushi, T., Price, P.W., Eds.; Springer: Tokyo, Japan, 2006; pp. 103–121. [Google Scholar]
- Cooper, W.R.; Rieske, L.K. Community associates of an exotic gallmaker, Dryocosmus kuriphilus (Hymenoptera: Cynipidae), in eastern North America. Ann. Entomol. Soc. Am. 2007, 100, 236–244. [Google Scholar] [CrossRef]
- Panzavolta, T.; Bernardo, U.; Bracalini, M.; Cascone, P.; Croci, F.; Gebiola, M.; Iodice, L.; Tiberi, R.; Guerrieri, E. Native parasitoids associated with Dryocosmus kuriphilus in Tuscany, Italy. Bull. Insectol. 2013, 66, 195–201. [Google Scholar]
- Quacchia, A.; Ferracini, C.; Nicholls, J.A.; Piazza, E.; Saladini, M.A.; Tota, F.; Melika, G.; Alma, A. Chalcid parasitoid community associated with the invading pest Dryocosmus kuriphilus in north-western Italy. Insect Conserv. Divers. 2013, 6, 114–123. [Google Scholar] [CrossRef]
- Colombari, F.; Battisti, A. Native and introduced parasitoids in the biocontrol of Dryocosmus kuriphilus in Veneto (Italy). EPPO Bull. 2016, 46, 275–285. [Google Scholar] [CrossRef]
- Moriya, S.; Inoue, K.; Otake, A.; Shiga, M.; Mabuchi, M. Decline of the chestnut gall wasp population, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) after the establishment of Torymus sinensis Kamijo (Hymenoptera: Torymidae). Appl. Entomol. Zool. 1989, 24, 231–233. [Google Scholar] [CrossRef]
- Murakami, Y.; Toda, S.; Gyoutoku, Y. Colonization of imported Torymus (Syntomaspis) sinensis Kamijo (Hymenoptera: Torymidae) parasitic on the chestnut gall wasp (Hymenoptera: Cynipidae). Success in the eighteenth year after release in Kumamoto. Proc. Assoc. Plant Prot. Kyushu 2001, 47, 132–134. [Google Scholar] [CrossRef]
- Quacchia, A.; Moriya, S.; Bosio, G.; Scapin, I.; Alma, A. Rearing, release and settlement prospect in Italy of Torymus sinensis, the biological control agent of the chestnut gall wasp Dryocosmus kuriphilus. BioControl 2008, 53, 829–839. [Google Scholar] [CrossRef]
- Quacchia, A.; Moriya, S.; Bosio, G. Effectiveness of Torymus sinensis in the biological control of Dryocosmus kuriphilus in Italy. Acta Hortic. 2014, 1043, 199–204. [Google Scholar] [CrossRef]
- Bosio, G.; Mauro, A.; Moriya, S. Verso il controllo biologico del cinipide del castagno. Inf. Agrar. 2013, 14, 60–63. [Google Scholar]
- Bernardo, U.; Nugnes, F.; Gualtieri, L.; Scarpato, S.; Gargiulo, G.; Griffo, R. Cinipide del castagno, cresce il controllo biologico in Campania. Inf. Agrar. 2017, 27, 51–53. [Google Scholar]
- Yasumatsu, K. A new Dryocosmus injurious to chestnut trees in Japan (Hym., Cynipidae). Mushi 1951, 22, 89–93. [Google Scholar]
- Murakami, Y. The parasitoids of Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) in Japan and the introduction of a promising natural enemy from China (Hymenoptera: Chalcidoidea). J. Fac. Agric. Kyushu Univ. 1981, 25, 167–174. [Google Scholar]
- Sartor, C.; Torello Marinoni, D.; Beccaro, G.L.; Bounous, G.; Botta, R.; Quacchia, A. Valutazione della sensibilità varietale al cinipide galligeno in cultivar di castagno e meccanismi molecolari di risposta all’insetto. In Proceedings of the Castanea 2009—Atti del 5 Convegno Nazionale Castagno, Cuneo, Italy, 13–16 October 2009; pp. 132–139. [Google Scholar]
- Sartor, C.; Dini, F.; Marinoni, D.T.; Mellano, M.G.; Beccaro, G.L.; Alma, A.; Quacchia, A.; Botta, R. Impact of the Asian wasp Dryocosmus kuriphilus (Yasumatsu) on cultivated chestnut: Yield loss and cultivar susceptibility. Sci. Hortic. 2015, 197, 454–460. [Google Scholar] [CrossRef]
- Dini, F.; Sartor, C.; Botta, R. Detection of a hypersensitive reaction in the chestnut hybrid “Bouche de Bétizac” infested by Dryocosmus kuriphilus Yasumatsu. Plant Physiol. Biochem. 2012, 60, 67–73. [Google Scholar] [CrossRef] [PubMed]
- MIPAAF (Ministero delle Politiche Agricole, Alimentari e Forestali). Piano del Settore Castanicolo 2010/2013; MIPAAF—Ministero delle Politiche Agricole, Alimentari e Forestali: Rome, Italy, 2013; p. 324. ISBN 978-88-8145-253-8. [Google Scholar]
- UPOV (International Union for the Protection of New Varieties of Plants). Chestnut (Castanea mollissima Blume; Castanea crenata Siebold & Zucc.; Castanea sativa Mill.); Guidelines for the Conduct of Tests for Distinctness Homogeneity and Stability; UPOV: Geneva, Switzerland, 2017. [Google Scholar]
- Kogan, M.; Ortman, E.F. Antixenosis—A new term proposed to define painter’s “nonpreference” modality of resistance. Bull. Entomol. Soc. Am. 1978, 24, 175–176. [Google Scholar] [CrossRef]
- Manugistics. Statgraphics Plus Version 3.0; Manugistics: Rockville, MD, USA, 1997. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; W.H. Freeman and Co.: New York, NY, USA, 1981; p. 859. [Google Scholar]
- Panda, N.; Khush, G.A. Host Plant Resistance to Insects; CAB International: Wallingford, UK, 1995; p. 431. [Google Scholar]
- Koch, K.G.; Chapman, K.; Louis, J.; Heng-Moss, T.; Sarath, G. Plant tolerance: A unique approach to control Hemipteran pests. Front. Plant Sci. 2016, 7, 1363. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y. A history of studies on the chestnut gall wasp in Japan. In A Global Serious Pest of Chestnut Trees, Dryocosmus kuriphilus: Yesterday, Today and Tomorrow, Japan; National Agricultural Research Center: Ibaraki, Japan, 2010; pp. 38–40. [Google Scholar]
- Stone, G.N.; Schonrogge, K.; Atkinson, R.J.; Bellido, D.; Pujade-Villar, J. The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 2002, 47, 633–668. [Google Scholar] [CrossRef] [PubMed]
- Viggiani, G.; Tesone, T. Phenology and monitoring of Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) in Campania. Frustula Entomol. 2009, 32, 93–100. [Google Scholar]
- Cooper, W.R.; Rieske, L.K. Gall structure affects ecological associations of Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Environ. Entomol. 2010, 39, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Geng, G.M.; Zhu, C.C.; Zhou, J.Y. Resistance of Castanea mollissima Shuhe-WYL strain to Dryocosmus kuriphilus and its molecular mechanism. Genet. Mol. Res. 2015, 14, 11456–11461. [Google Scholar] [CrossRef] [PubMed]
- Matsui, S.; Torikata, H. Studies on the resistance of chestnut trees (Castanea spp.) to chestnut gall wasps (Dryocosmus kuriphilus Yasumatsu). IX. Polyphenolic contents in susceptible and resistant varieties. J. Jpn. Soc. Hortic. Sci. 1976, 45, 7–14. [Google Scholar] [CrossRef]
- Trigiano, R.N.; Windham, M.T.; Windham, A.S. Plant Pathology Concepts and Laboratory Exercises; CRC Press: Boca Raton, FL, USA, 2004; p. 413. [Google Scholar]





| Plant | Leaf | |||
|---|---|---|---|---|
| Vigor (UPOV 1) | Habit (UPOV 2) | Size (UPOV 12) | Base Shape (UPOV 21) | Margin (UPOV 22) |
| Strong | Semi-upright | Medium | Obtuse | Acute |
| Pericarp | ||||
| Color of Skin (UPOV 37) | Shape (UPOV 31) | Area of pubescence on upper part (UPOV 32) | Area of hilum (UPOV 33) | Shape of border line of helium (UPOV 34) |
| Reddish brown | Broad ovate | Medium | Medium | Straight |
| Seed | ||||
| Embryo (UPOV 28) | Degree of penetration of seed coat into embryo (UPOV 30) | Seed coat: adherence to kernel (UPOV 39) | Kernel: color of flesh | |
| Mono-embryonic | Weak | Weak | White | |
| Date of Sampling | Value | df | X2 | p | ||
|---|---|---|---|---|---|---|
| Scars | 2.12.16 | Susceptible | 85 | 1 | 0.15 | 0.703 |
| Resistant | 82 | |||||
| Larvae | 2.12.16 | Susceptible | 94 | 1 | 14.52 | 0.001 |
| Resistant | 73 | |||||
| Alterations | 2.12.16 | Susceptible | 100 | 1 | 1.35 | 0.245 |
| Resistant | 97 | |||||
| Scars | 8.10.16 | Susceptible | 99 | 1 | - | - |
| Resistant | 99 | |||||
| Eggs | 8.10.16 | Susceptible | 97 | 1 | 4.61 | 0.032 |
| Resistant | 88 | |||||
| Alterations | 8.10.16 | Susceptible | 88 | 1 | 6.22 | 0.013 |
| Resistant | 98 | |||||
| Scars | 2.22.17 | Susceptible | 74 | 1 | 1.9 | 0.168 |
| Resistant | 83 | |||||
| Larvae | 2.22.17 | Susceptible | 97 | 1 | 4.61 | 0.032 |
| Resistant | 88 | |||||
| Alterations | 2.22.17 | Susceptible | 90 | 1 | 4.34 | 0.037 |
| Resistant | 98 |
| Date of Sampling | Healthy Larvae | Parasitized Larvae | df | X2 | p | |
|---|---|---|---|---|---|---|
| 5.25.17 | Susceptible | 127 | 16 | 1 | 35.14 | <0.001 |
| Resistant | 77 | 59 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugnes, F.; Gualtieri, L.; Bonsignore, C.P.; Parillo, R.; Annarumma, R.; Griffo, R.; Bernardo, U. Resistance of a Local Ecotype of Castanea sativa to Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Southern Italy. Forests 2018, 9, 94. https://doi.org/10.3390/f9020094
Nugnes F, Gualtieri L, Bonsignore CP, Parillo R, Annarumma R, Griffo R, Bernardo U. Resistance of a Local Ecotype of Castanea sativa to Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Southern Italy. Forests. 2018; 9(2):94. https://doi.org/10.3390/f9020094
Chicago/Turabian StyleNugnes, Francesco, Liberata Gualtieri, Carmelo Peter Bonsignore, Rita Parillo, Regina Annarumma, Raffaele Griffo, and Umberto Bernardo. 2018. "Resistance of a Local Ecotype of Castanea sativa to Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Southern Italy" Forests 9, no. 2: 94. https://doi.org/10.3390/f9020094








