Effect of Wind on the Relation of Leaf N, P Stoichiometry with Leaf Morphology in Quercus Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Growing Conditions
2.2. Experimental Design
2.3. Leaf Morphology and Leaf N and P Concentrations Measurements
2.4. Statistical Analysis
3. Results
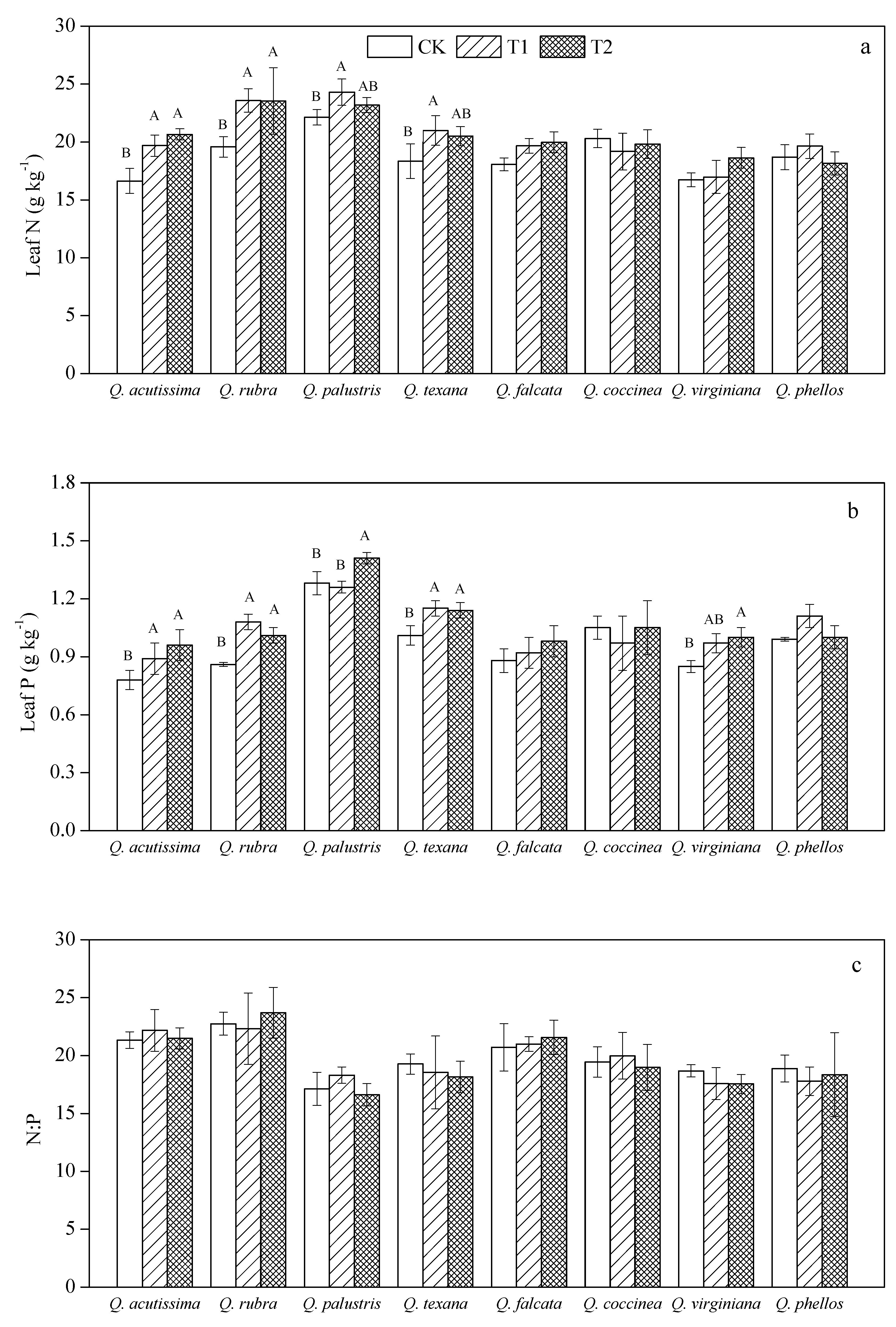
3.1. Effects of Wind on Leaf N, P Stoichiometry for Quercus Species
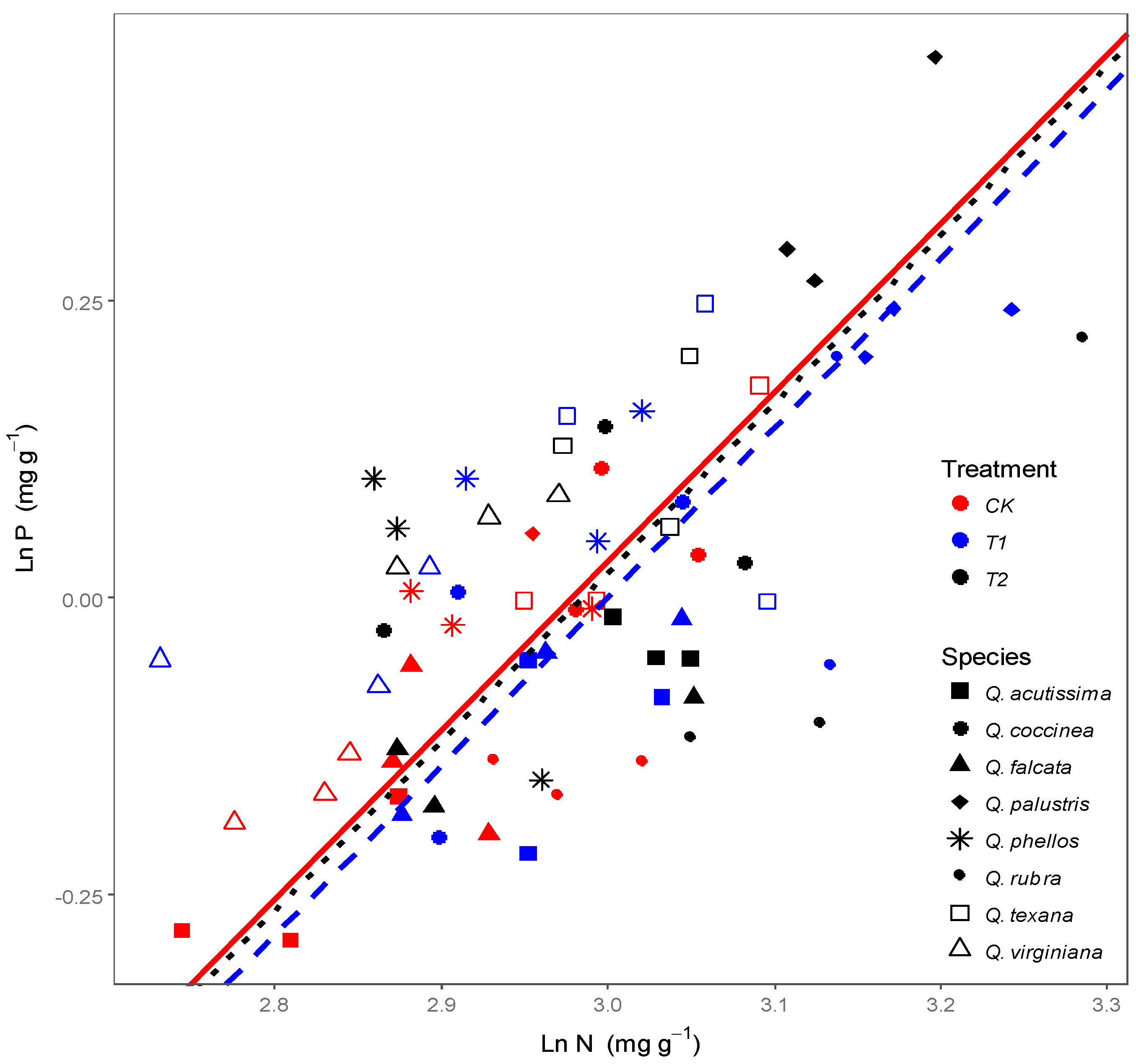
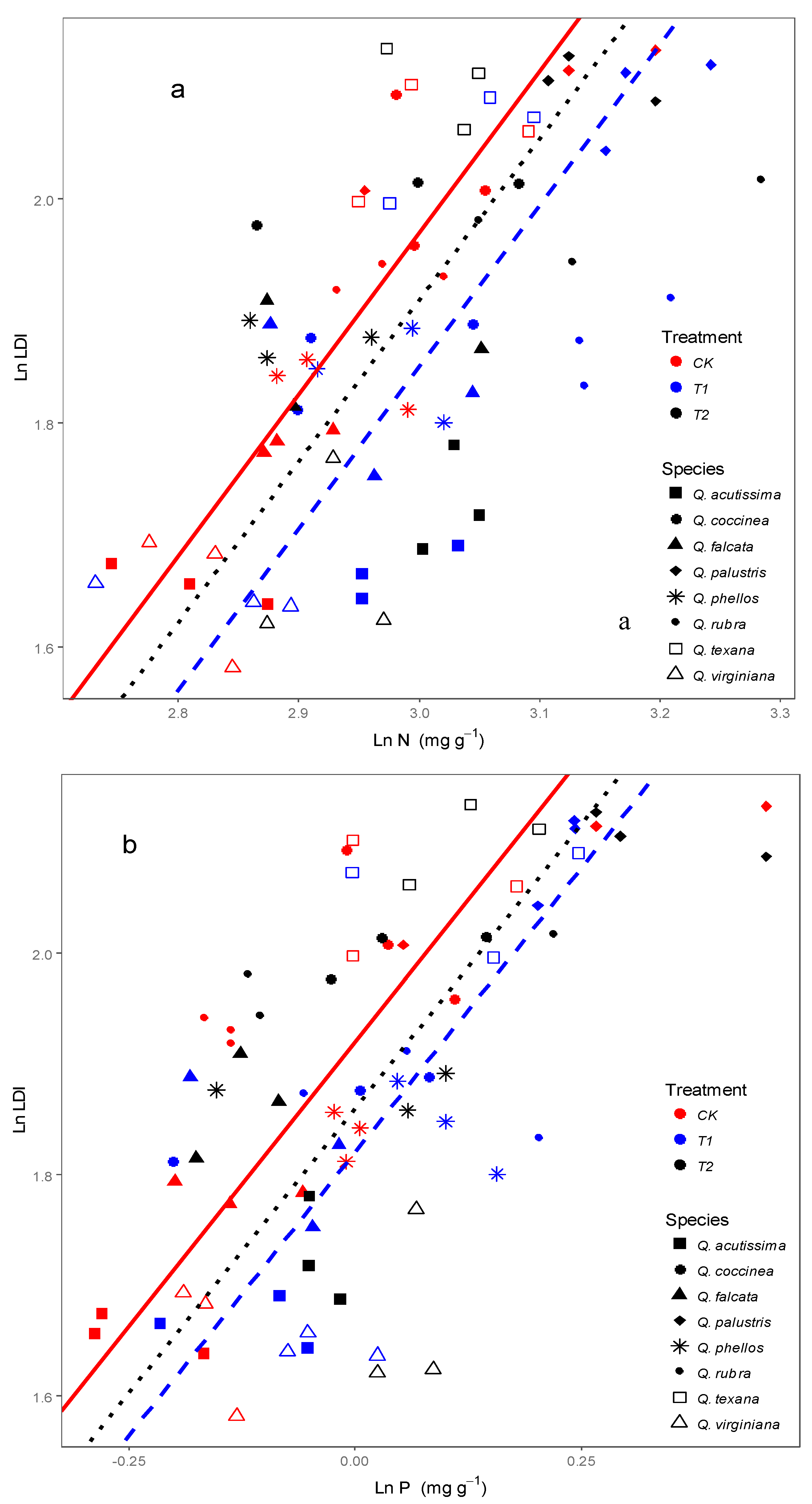
3.2. Effects of Wind on Relationships between Leaf N, P Stoichiometry and Leaf Morphology
4 Discussion
4.1. Response of Leaf N, P Stoichiometry to Wind
4.2. Response of Relationships between Leaf Stoichiometry and Morphology to Wind
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| N (g kg−1) | P (g kg−1) | N:P | ||
|---|---|---|---|---|
| N | 72 | 72 | 72 | |
| Normal Parameters a,b | Mean | 19.984 | 1.029 | 19.741 |
| Std. Deviation | 2.4026 | 0.1729 | 2.390 | |
| Asymp. Sig. (2-tailed) | 0.039 | 0.002 | 0.013 | |
| DF | PNUE (μmol m−2 s−1) | PPUE (μmol m−2 s−1) | |||||
|---|---|---|---|---|---|---|---|
| MS | F | P | MS | F | P | ||
| Tree species | 7 | 179410.515 | 39.239 | 0.000 | 57420784.71 | 25.746 | 0.000 |
| Treatment | 2 | 25103.133 | 5.490 | 0.007 | 12724003.31 | 5.705 | 0.006 |
References
- Niinemets, U. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol. 2015, 205, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Vasseur, F.; Violle, C.; Shipley, B.; Enquist, B.J.; Vile, D. Testing models for the leaf economics spectrum with leaf and whole-plant traits in Arabidopsis thaliana. AoB Plants 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Yu, G.; He, N.; Hou, J. Leaf morphological and anatomical traits from tropical to temperate coniferous forests: Mechanisms and influencing factors. Sci. Rep. 2016, 6, 19703. [Google Scholar] [CrossRef] [PubMed]
- Heberling, J.M.; Fridley, J.D. Biogeographic constrains on the world-wide leaf economic spectrum. Glob. Ecol. Biogeogr. 2012, 21, 1137–1146. [Google Scholar] [CrossRef]
- Osnas, J.L.D.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: Mass area and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, X.; Guo, W.; Yu, M.; Wu, T. Leaf N and P stoichiometry in relation to leaf shape and plant size for Quercus acutissima provenances across China. Sci. Rep. 2017, 7, 46133. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, M.; Wang, G.; Dong, Y.; Cheng, X. Leaf nitrogen and phosphorus stoichiometry across forty-two woody species in Southeast China. Biochem. Syst. Ecol. 2012, 44, 255–263. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Olmo, M.; Poorter, H.; Ubera, J.L.; Villar, R. Leaf mass per area (LMA) and its relationship with leaf structure and anatomy in 34 Mediterranean woody species along a water availability gradient. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.A.; Da, S.E.J.; Machado, G.C.; Bucci, S.J.; Scholz, F.G.; Goldstein, G.; Meinzer, F.C. Seasonal leaf dynamics across a tree density gradient in a Brazilian savanna. Oecologia 2005, 145, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Anten, N.P.R.; Alcala-Herrera, R.; Schieving, F.; Onoda, Y. Wind and mechanical stimuli differentially affect leaf traits in Plantago major. New Phytol. 2010, 188, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Royer, D.L.; Mcelwain, J.C.; Adams, J.M.; Wilf, P. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol. 2008, 179, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, P.; Zhang, L.; Wang, G.; Yu, M. Morphological response of eight Quercus species to simulated wind load. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Hoad, S.P.; Marzoli, A.; Grace, J.; Jeffree, C.E. Response of leaf surfaces and gas exchange to wind stress and acid mist in birch (Betula pubescens). Trees 1998, 13, 1–12. [Google Scholar] [CrossRef]
- Murren, C.J.; Pigliucci, M. Morphological responses to simulated wind in the genus Brassica (Brassicaceae): Allopolyploids and their parental species. Am. J. Bot. 2005, 92, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Kimmins, J.P. Forest Ecology; Addison-Wesley Press: New York, NY, USA, 2003. [Google Scholar]
- Wu, Q. Eight Oak Trees Growth Characteristics in Response to Simulated Wind Stimulus. Mater’s Theses, Hainan University, Haikou, China, 2014. [Google Scholar]
- Washusen, R.; Baker, T.; Menz, D.; Morrow, A. Effect of thinning and fertilizer on the cellulose crystallite width of Eucalyptus globulus. Wood Sci. Technol. 2005, 39, 569–578. [Google Scholar] [CrossRef]
- Shi, Z.; Tang, J.; Cheng, R.; Luo, D.; Liu, S. A review of nitrogen allocation in leaves and factors in its effects. Acta Ecol. Sin. 2015, 35, 5909–5919. [Google Scholar]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct. Ecol. 2004, 18, 419–425. [Google Scholar] [CrossRef]
- Cordero, R.A. Ecophysiology of Cecropia schreberiana saplings in two wind regimes in an elfin cloud forest: Growth, gas exchange, architecture and stem biomechanics. Tree Physiol. 1999, 19, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Woodward, F.I. Ecophysiological studies on the shrub Vaccinium myrtillus L. taken from a wide altitudinal range. Oecologia 1998, 70, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, J.C.; Sykes, M. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Zhao, N.; He, N.; Wang, Q.; Zhang, X.; Wang, R.; Xu, Z.; Yu, G. The altitudinal patterns of leaf C:N:P stoichiometry are regulated by plant growth form, climate and soil on Changbai mountain, China. PLoS ONE 2014, 9, e95196. [Google Scholar]
- Du, B.; Ji, H.; Peng, C.; Liu, X.; Liu, C. Altitudinal patterns of leaf stoichiometry and nutrient resorption in Quercus variablilis in the Baotianman Mountains, China. Plant Soil 2017, 413, 193–202. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Z.; Ji, C.; Liu, H.; Ma, W.; Mohhamot, A.; Shi, Z.; Sun, W.; Wang, T.; Wang, X.; et al. Scaling of nitrogen and phosphorus across plant organs in shrubland biomes across Northern China. Sci. Rep. 2014, 4, 5448. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Z.; Wu, T.; Yu, M.; Cheng, X.; Duan, X. Temporal and spacial distribution of wind in coastal area, Shanghai. Resour. Environ. Yangtze Basin 2013, 22, 40–45, (In Chinese with English abstract). [Google Scholar]
- Nicotra, A.B.; Cosgrove, M.; Cowling, A.; Schlichting, C.D.; Jones, C.S. Leaf shape linked to photosynthetic rates and temperature optima in South African Pelargonium species. Oecologia 2008, 154, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chen, Y.; Cao, Y. C:N:P stoichiometry and carbon storage in a naturally-regenerated secondary Qercus variabilis forest age sequence in the Qinling Mountains, China. Forests 2017, 8, 281. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, P.; Wu, Q.; Yan, H.; Yu, M.; Wang, G.G.; Wu, T. Effects of simulated wind load on leaf photosynthesis and carbohydrate allocation in eight Quercus species. J. Biobased Mater. Biol. 2017. [Google Scholar] [CrossRef]
- Buchanan, B.; Gruissem, W.; Jones, R. Biochemistry and Molecular Biology of Plants; American Society of Plant Physiologists: Rockeweel, MD, USA, 2000. [Google Scholar]
- Onoda, Y.; Schieving, F.; Anten, N.P.R. Effects of light and nutrient availability on leaf mechanical properties of Plantgo major: A conceptual approach. Ann. Bot. 2008, 101, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Coutand, C.; Dupraz, C.; Jaouen, G.; Ploquin, S.; Boris, A. Mechanical stimuli regulate the allocation of biomass in trees: Demonstration with young Prunus avium trees. Ann. Bot. 2008, 101, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J. Differences between Acer saccharum leaves from open and wind-protected sites. Ann. Bot. 1996, 78, 61–66. [Google Scholar] [CrossRef]
- Kume, T.K.; Laplace, S.; Komatsu, H.; Chu, C. Transpiration in response to wind speed: Can apparent leaf-type differences between conifer and broadleaf trees be a practical indicator? Trees 2015, 29, 605–612. [Google Scholar] [CrossRef]
- Schymanski, S.J.; Or, D. Wind increases leaf water use efficiency. Plant Cell Environ. 2016, 39, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, C.; Vooge, W. Plant Nutrition of Greenhouse Crops; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sardans, J.; Penuelas, J. Trees increase their P:N ratio with size. Glob. Ecol. Biogeogr. 2015, 24, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Su, J. Successional leaf traits of monsoon evergreen broad-leaved forest, Southerwest China. iForest 2017, 10, 391–396. [Google Scholar] [CrossRef]
- Li, H.; Crabbe, M.J.C.; Xu, F.; Wang, W.; Niu, R.; Gao, X.; Zhang, P.; Chen, H. Seasonal variations in carbon, nitrogen and phosphorus concentrations and C:N:P stoichiometry in the leaves of differently aged Larix principis-rupprechtii Mayr. plantations. Forests 2017, 8, 373. [Google Scholar] [CrossRef]
- Edwards, E.J.; Chatelet, D.S.; Sack, L.; Donoghue, M.J. Leaf life span and the leaf economic spectrum in the context of whole plant architecture. J. Ecol. 2014, 102, 328–336. [Google Scholar] [CrossRef]
- Hidaka, A.; Kitayama, K. Physiological linkage in co-variation of foliar nitrogen and phosphorus in tropical tree species along a gradient of soil phosphorus availability. J. Trop. Ecol. 2015, 31, 221–229. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, W.; Wang, G.; Yu, M.; Wu, T. Effects of environment and genetics on leaf N and P stoichiometry for Quercus acutissima across China. Eur. J. For. Res. 2016, 135, 795–802. [Google Scholar] [CrossRef]
- Kull, O.; Niinemets, U. Variations in leaf morphology and nitrogen concentration in Betula pendula Roth., Corylus avellana L. and Lonicera xylosteum L. Tree Physiol. 1993, 12, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Shipley, B.; Lechowicz, M.J. The functional co-ordination of leaf morphology, nitrogen concentration, and gas exchange in 40 wetland species. Ecosci. 2000, 7, 183–194. [Google Scholar] [CrossRef]
- Royer, D.L.; Wilf, P.; Janesko, D.A.; Kowalski, E.A.; Dilcher, D.L. Correlating climate and plant ecology to leaf size and shape: Potential proxies for the fossil record. Am. J. Bot. 2005, 92, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef]
- Santiago, L.S.; Kim, S.C. Correlated evolution of leaf shape and physiology in the woody Sonchus alliance (Asteraceae: Sonchinae) in Macaronesia. Int. J. Plant Sci. 2009, 170, 83–92. [Google Scholar] [CrossRef]
- Blonder, B.; Violle, C.; Enquist, B.J. Inclusion of vein traits improves predictive power for the leaf economic spectrum: A response to Sack et al. (2013). J. Exp. Bot. 2014, 65, 5109–5114. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.B.; Gerlach, J.P. Intraspecific growth and functional leaf trait responses to natural soil resource gradients for conifer species with contrasting leaf habit. Tree Physiol. 2013, 33, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Gornish, E.S.; Prather, C.M. Foliar functional traits that predict plant biomass response to warming. J. Veg. Sci. 2014, 25, 919–927. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wang, H.; Wu, Q.; Yu, M.; Wu, T. Effect of Wind on the Relation of Leaf N, P Stoichiometry with Leaf Morphology in Quercus Species. Forests 2018, 9, 110. https://doi.org/10.3390/f9030110
Zhang P, Wang H, Wu Q, Yu M, Wu T. Effect of Wind on the Relation of Leaf N, P Stoichiometry with Leaf Morphology in Quercus Species. Forests. 2018; 9(3):110. https://doi.org/10.3390/f9030110
Chicago/Turabian StyleZhang, Peng, Hua Wang, Qianting Wu, Mukui Yu, and Tonggui Wu. 2018. "Effect of Wind on the Relation of Leaf N, P Stoichiometry with Leaf Morphology in Quercus Species" Forests 9, no. 3: 110. https://doi.org/10.3390/f9030110
APA StyleZhang, P., Wang, H., Wu, Q., Yu, M., & Wu, T. (2018). Effect of Wind on the Relation of Leaf N, P Stoichiometry with Leaf Morphology in Quercus Species. Forests, 9(3), 110. https://doi.org/10.3390/f9030110





