Biochar Can Be a Suitable Replacement for Sphagnum Peat in Nursery Production of Pinus ponderosa Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media Components and Analysis of Individual Medium
2.1.1. Physical Properties
2.1.2. Chemical Properties
ACI_E(cmol∙kg−1)
2.2. Seedling Culture
2.2.1. Year One
2.2.2. Year Two
2.3. Statistical Analyses and Visualizations
3. Results
3.1. Media Characteristics
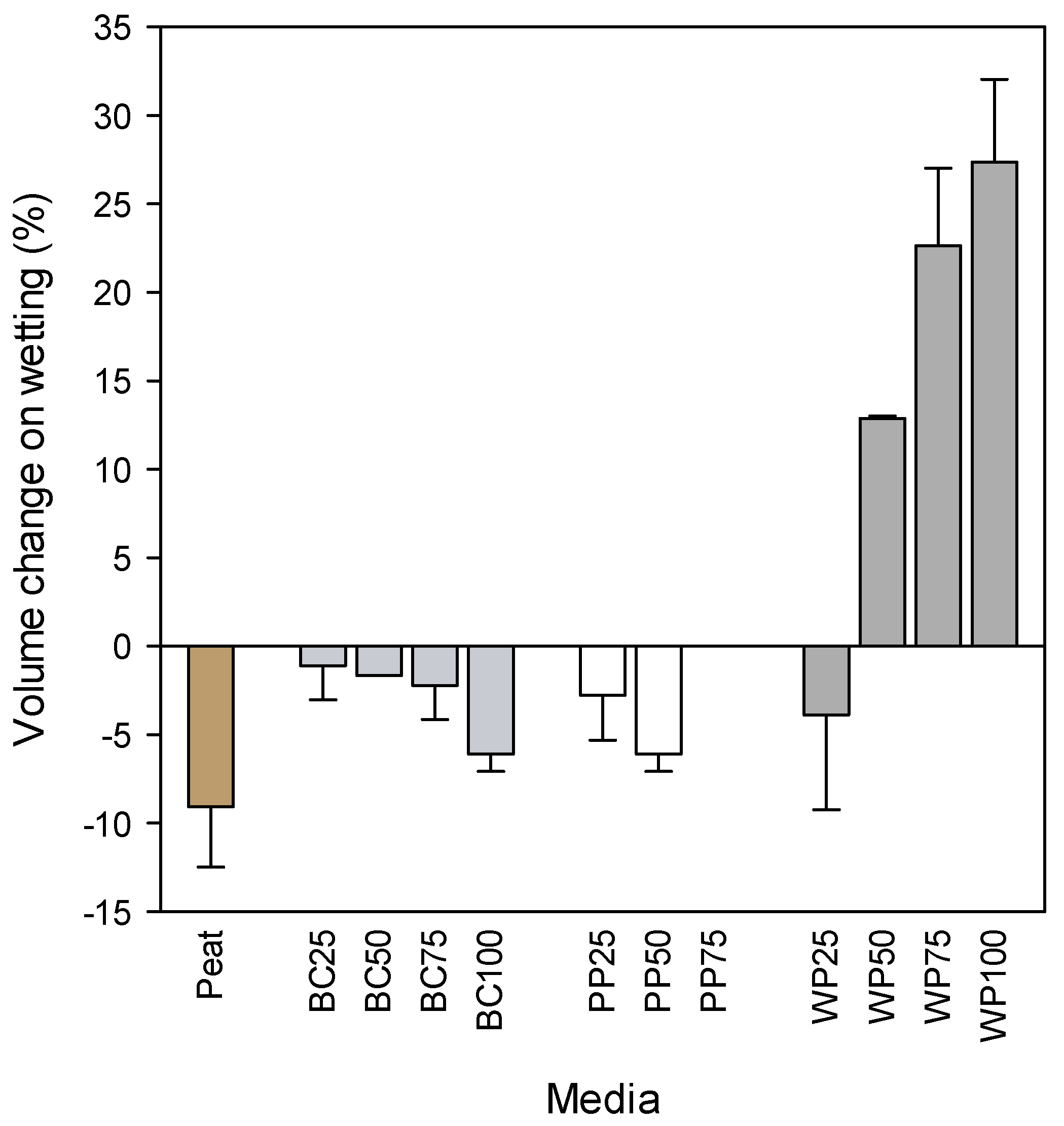
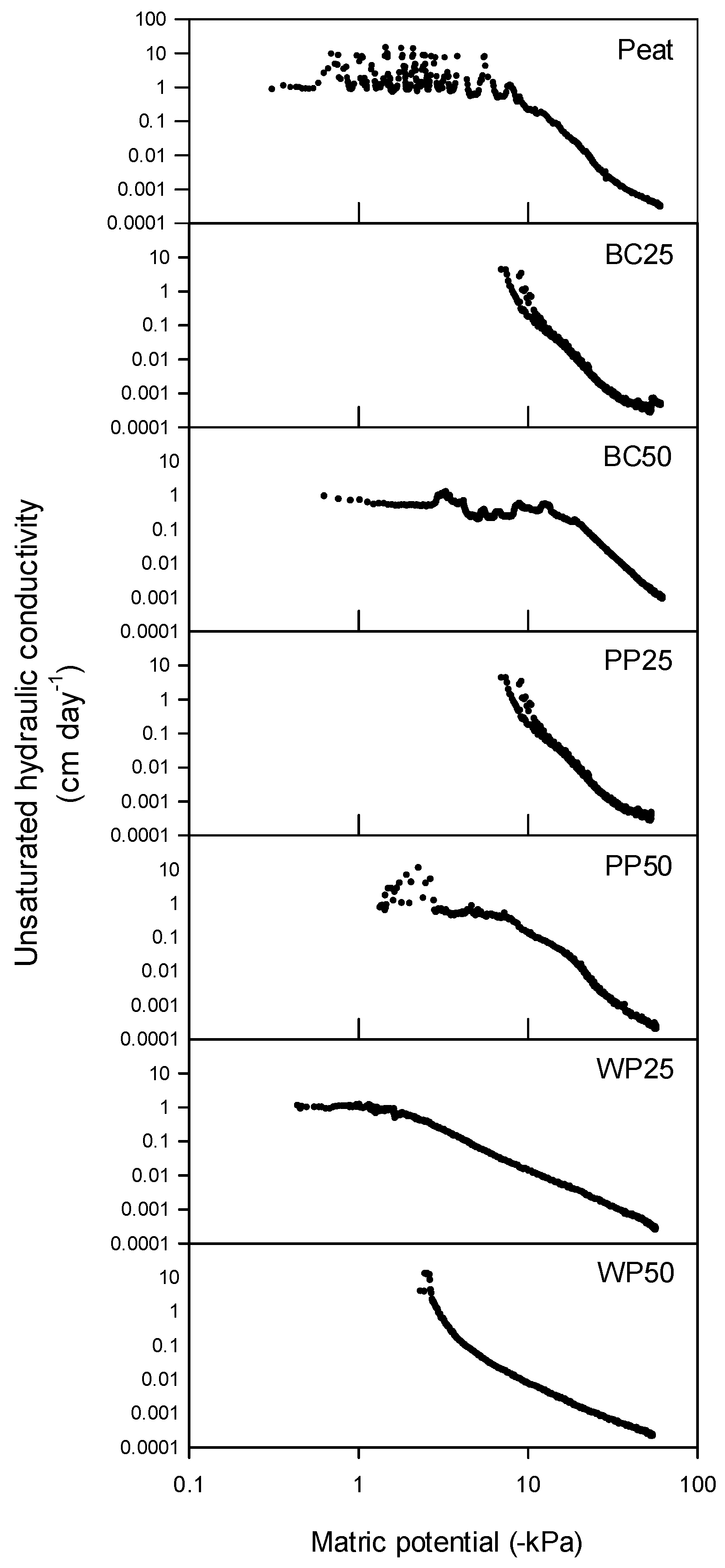
3.1.1. Physical Properties
3.1.2. Chemical Properties
3.2. Seedling Growth
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Palik, B.J.; Williams, M.I.; Dumroese, R.K.; Madsen, P. Forest restoration paradigms. J. Sustain. For. 2014, 33, S161–S194. [Google Scholar] [CrossRef]
- Haase, D.L.; Davis, A.S. Developing and supporting quality nursery facilities and staff are necessary to meet global forest and landscape restoration needs. Reforesta 2017, 4, 69–93. [Google Scholar] [CrossRef]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 144, 11645–11650. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, J. Effect of subirrigation on the growth of Norway spruce container seedlings in a greenhouse: A pilot study. J. Appl. Irrig. Sci. 2007, 42, 19–28. [Google Scholar]
- Schmal, J.L.; Dumroese, R.K.; Davis, A.S.; Pinto, J.R.; Jacobs, D.F. Subirrigation for production of native plants in nurseries—Concepts, current knowledge, and implementation. Nativ. Plants J. 2011, 12, 81–93. [Google Scholar] [CrossRef]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation: A review. Fert. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Page-Dumroese, D.S.; Salifu, K.F.; Jacobs, D.F. Exponential fertilization of Pinus monticola seedlings: Nutrient uptake efficiency, leaching fractions, and early outplanting performance. Can. J. For. Res. 2005, 35, 2961–2967. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Page-Dumroese, D.S.; Wenny, D.L. Managing Pesticide and Fertilizer Leaching and Runoff in a Container Nursery. In Proceedings of the Intermountain Forest Nursery Association, Park City, UT, USA, 12–16 August 1991; Landis, T.D., Ed.; USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1992; pp. 27–33. Available online: https://www.researchgate.net/publication/272819594 (accessed on 15 March 2018).
- Dumroese, R.K.; Wenny, D.L.; Page-Dumroese, D.S. Nursery Waste Water: The Problem and Possible Remedies. In Proceedings of the National Proceedings, Forest and Conservation Nursery Associations, Kearney, NE, USA, 7–11 August 1995; Landis, T.D., Cregg, B., Eds.; USDA Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; pp. 89–97. Available online: https://www.researchgate.net/publication/272819463 (accessed on 15 March 2018).
- Juntunen, M.-L.; Hammar, T.; Rikala, R. Leaching of nitrogen and phosphorus during production of forest seedlings in containers. J. Environ. Qual. 2002, 31, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Apostol, K.G.; Dumroese, R.K.; Pinto, J.R.; Davis, A.S. Response of conifer species from three latitudinal populations to light spectra generated by light-emitting diodes and high-pressure sodium lamps. Can. J. For. Res. 2015, 45, 1711–1719. [Google Scholar] [CrossRef]
- Riikonen, J.; Kettunen, N.; Gritsevich, M.; Hakala, T.; Särkkä, L.; Tahvonen, R. Growth and development of Norway spruce and Scots pine seedlings under different light spectra. Environ. Exp. Bot. 2016, 121, 112–120. [Google Scholar] [CrossRef]
- Montagnoli, A.; Dumroese, R.K.; Terzaghi, M.; Pinto, J.R.; Fulgaro, N.; Scippa, G.S.; Chiatante, D. Tree seedling response to LED spectra: Implications for forest restoration. Plant Biosyst. 2018, 152, 515–523. [Google Scholar] [CrossRef]
- Rose, R.; Haase, D.L. The use of coir as a containerized growing medium for Douglas-fir seedlings. Nativ. Plants J. 2000, 1, 107–111. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Page-Dumroese, D.S.; Brown, R.E. Allometry, nitrogen status, and carbon stable isotope composition of Pinus ponderosa seedlings in two growing media with contrasting nursery irrigation regimes. Can. J. For. Res. 2011, 41, 1091–1101. [Google Scholar] [CrossRef]
- Heiskanen, J. Effects of compost additive in sphagnum peat growing medium on Norway spruce container seedlings. New For. 2013, 44, 101–118. [Google Scholar] [CrossRef]
- Villa Castillo, J. Inoculating composted pine bark with beneficial organisms to make a disease suppressive compost for container production in Mexican forest nurseries. Nativ. Plants J. 2004, 5, 181–185. [Google Scholar] [CrossRef]
- Caron, J.; Rochefort, L. Use of peat in growing media: State of the art on industrial and scientific efforts envisioning sustainability. Acta Hortic. 2013, 982, 15–22. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Eller, F.J.; Evangelista, R.L.; Moser, B.R.; Lee, E.; Wagner, R.E.; Peterson, S.C. Evaluation of biochar-anaerobic potato digestate mixtures as renewable components of horticultural potting media. Ind. Crop Prod. 2015, 65, 467–471. [Google Scholar] [CrossRef]
- Landis, T.D.; Morgan, N. Growing Media Alternatives for Forest and Native Plant Nurseries. In Proceedings of the National Proceedings, Forest and Conservation Nursery Associations, Missoula, MT, USA, 23–25 June 2008; Dumroese, R.K., Riley, L.E., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2009; pp. 26–31. Available online: https://www.fs.usda.gov/treesearch /pubs/20894 (accessed on 15 March 2018).
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Dumroese, R.K.; Heiskanen, J.; Englund, K.; Tervahauta, A. Pelleted biochar: Chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenergy 2011, 35, 2018–2027. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Zhang, L. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind. Crop Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Matt, C.P.; Keyes, C.R.; Dumroese, R.K. Biochar effects on the nursery propagation of 4 northern Rocky Mountain native plant species. Nativ. Plants J. 2018, 19, 14–25. [Google Scholar] [CrossRef]
- Headlee, W.L.; Brewer, C.E.; Hall, R.B. Biochar as a substitute for vermiculite in potting mix for hybrid poplar. Bioenergy Res. 2014, 7, 120–131. [Google Scholar] [CrossRef]
- Nemati, M.R.; Simard, F.; Fortin, J.-P.; Beaudoin, J. Potential use of biochar in growing media. Vadose Zone J. 2015, 14. [Google Scholar] [CrossRef]
- Ennis, C.J.; Evans, A.G.; Islam, M.; Ralebitso-Senior, T.K.; Senior, E. Biochar: Carbon sequestration, land remediation, and impacts on soil microbiology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2311–2364. [Google Scholar] [CrossRef] [Green Version]
- McCarl, B.A.; Peacocke, C.; Chrisman, R.; Kung, C.-C.; Sands, R.D. Chapter 19: Economics of Biochar Production, Utilization and Greenhouse Gas Offsets. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan: Stirling, VA, USA, 2009; pp. 341–358. ISBN 978-1-84407-658-1. [Google Scholar]
- Page-Dumroese, D.S.; Anderson, N.M.; Windell, K.; Englund, K.; Jump, K. Development and Use of a Commercial-Scale Biochar Spreader; General Technical Report RMRS-GTR-354; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2016; p. 10. Available online: https://www.fs.usda.gov/treesearch/pubs/52309 (accessed on 20 March 2018).
- McElligott, K.M. Biochar Amendments to Forest Soils: Effects on Soil Properties and Tree Growth. Master of Science Thesis, University of Idaho, Moscow, ID, USA, 2011. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; Agronomy Monograph 9; Klute, A., Ed.; American Society of Agronomy and Academic Press: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Heiskanen, J. Comparison of three methods for determining the particle density of soil with liquid pycnometers. Commun. Soil Sci. Plant Anal. 1992, 23, 841–846. [Google Scholar] [CrossRef]
- Ball, D.F. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. Eur. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; Agronomy Monograph 9; American Society of Agronomy and Academic Press: Madison, WI, USA, 1986. [Google Scholar]
- Heiskanen, J. Variation in water retention characteristics of peat growth media used in tree nurseries. Silva Fenn. 1993, 27, 77–97. [Google Scholar] [CrossRef]
- White, J.W.; Mastalerz, J.W. Soil moisture as related to “container capacity”. Am. Soc. Hortic. Sci. 1996, 89, 758–765. [Google Scholar]
- Wilson, G.C.S. The physico-chemical and physical properties of horticultural substrates. Acta Hortic. 1983, 150, 19–32. [Google Scholar] [CrossRef]
- Schindler, U. Ein Schnellverfahren zur Messung der Wasserleitfähigkeit im teilgesättigten Boden an Stechzylinderproben. Arch. Acker Pflanzenbau Bodenkd. 1980, 24, 1–7. (In German) [Google Scholar]
- Schindler, U.; Müller, L. Simplifying the evaporation method for quantifying soil hydraulic properties. J. Plant Nutr. Soil Sci. 2006, 169, 623–629. [Google Scholar] [CrossRef]
- Mahler, R.L.; Naylor, D.V.; Fredrickson, M.K. Hot water extraction of boron from soils using sealed plastic pouches. Commun. Soil Sci. Plant Anal. 1984, 15, 479–492. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Montville, M.E.; Pinto, J.R. Using container weights to determine irrigation needs: A simple method. Nativ. Plants J. 2015, 16, 67–71. Available online: https://www.fs.usda.gov/treesearch/pubs/48087 (accessed on 15 March 2018). [CrossRef]
- Timmer, V.R.; Aidelbaum, A.S. Manual for Exponential Nutrient Loading of Seedlings to Improve Outplanting Performance on Competitive Forest Sites; NODA/NFP Technical Report TR-25; Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Center: Marie, ON, Canada, 1996; Available online: http://www.cfs.nrcan.gc.ca/pubwarehouse/pdfs/9567.pdf (accessed on 15 March 2018).
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Haase, D.L.; Rose, R. Vector analysis and its use for interpreting plant nutrient shifts in response to silvicultural treatments. For. Sci. 1995, 41, 54–66. [Google Scholar]
- Landis, T.D.; Tinus, R.W.; McDonald, S.E.; Barnett, J.P. Seedling Nutrition and Irrigation. The Container Tree Nursery Manual: Agriculture Handbook 674; USDA Forest Service: Washington, DC, USA, 1989; Volume 4, 119p, Available online: https://rngr.net/publications/ctnm/volume-4 (accessed on 18 March 2018).
- Landis, T.D.; Haase, D.L.; Dumroese, R.K. Plant Nutrient Testing and Analysis in Forest and Conservation Nurseries. In Proceedings of the National Proceedings, Forest and Conservation Nursery Associations―2004, Charleston, NC, USA, 12–15 July 2004; Dumroese, R.K., Riley, L.E., Landis, T.D., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2005; pp. 76–83. Available online: https://www.fs.usda.gov/treesearch/pubs/20894 (accessed on 15 March 2018).
- Bunt, A.C. Media and Mixes for Container-Grown Plants. A Manual on the Preparation and Use of Growing Media for Pot Plants, 2nd ed.; Unwin Hyman: London, UK, 1988; ISBN 978-94-011-7904-1. [Google Scholar]
- Rikala, R.; Jozefek, H.J. Effect of dolomite lime and wood ash on peat subtrate and develoment of tree seedlings. Silva Fenn. 1990, 24, 323–334. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Skjemstad, J.O.; Czimczik, C.I.; Glaser, B.; Prentic, K.M.; Gelina, Y.; Kuhlbusch, T.A. Comparative analysis of black carbon in soils. Glob. Biogeochem. Cycles 2001, 15, 163–167. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar Effects on Soil Nutrient Transformations. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK; New York, NY, USA, 2015; pp. 421–454. ISBN 978-0-415-70415-1. [Google Scholar]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C.A. Quantitative review of the effects of biochar applications to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Altland, J.E.; Locke, J.C. Gasified rice hull biochar is a source of phosphorus and potassium for container-grown plants. J. Environ. Hortic. 2013, 31, 138–144. [Google Scholar] [CrossRef]
- Wrobel-Tobiszewska, A.; Boersma, M.; Sargison, J.P.; Adams, P.; Singh, B.; Franks, S.; Birch, C.J.; Close, D.C. Nutrient changes in potting mix and Eucalyptus nitens leaf tissue under macadamia biochar amendments. J. For. Res. 2018, 29, 383–393. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.-Y.; Tian, Y.; Gong, X.-G. Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci. Hortic. 2014, 176, 70–78. [Google Scholar] [CrossRef]
- Beets, P.N.; Oliver, G.R.; Kimberley, M.O.; Pearce, S.H.; Rodgers, B. Genetic and soil factors associated with variation in visual magnesium deficiency symptoms in Pinus radiata. For. Ecol. Manag. 2004, 189, 263–279. [Google Scholar] [CrossRef]
- Heiskanen, J. Favourable water and aeration conditions for growth media used in containerized tree seedling production: A review. Scand. J. For. Res. 1993, 8, 337–358. [Google Scholar] [CrossRef]
- Conversa, G.; Bonasia, A.; Lazzizera, C.; Elia, A. Influence of biochar, mycorrhizal inoculation, and fertilizer rate on growth and flowering of Pelargonium (Pelargonium zonale L.) plants. Front. Plant Sci. 2015, 6, 429. [Google Scholar] [CrossRef] [PubMed]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Wenny, D.L.; Dumroese, R.K. A Growing Regime for Containerized Ponderosa Pine Seedlings; University of Idaho, Idaho Forest, Wildlife and Range Experiment Station: Moscow, ID, USA, 1987; p. 9. Available online: https://www.researchgate.net/publication/272828130 (accessed on 16 April 2018).
- Landis, T.D.; Tinus, R.W.; McDonald, S.E.; Barnett, J.P. Seedling Propagation. The Container Tree Nursery Manual: Agriculture Handbook 674; USDA Forest Service: Washington, DC, USA, 1998; Volume 6, 166p. Available online: https://rngr.net/publications/ctnm/volume-6 (accessed on 16 April 2018).
- Frenkel, O.; Jaiswal, A.K.; Elad, Y.; Lew, B.; Kammann, C.; Graber, E.R. The effect of biochar on plant diseases: What should we learn while designing biochar substrates? J. Environ. Eng. Landsc. 2017, 25, 105–113. [Google Scholar] [CrossRef]
- Kope, H.H.; Axelrood, P.E.; Sutherland, J.; Reddy, M.S. Prevalence and incidence of the root-inhabiting fungi, Fusarium, Cylindrocarpon and Pythium, on container-grown Douglas-fir and spruce seedlings in British Columbia. New For. 1996, 12, 55–67. [Google Scholar] [CrossRef]
- Unestam, T.; Beyer-Ericson, L.; Strand, M. Involvement of Cylindrocarpon destructans in root death of Pinus sylvestris seedlings: Pathogenic behaviour and predisposing factors. Scand. J. For. Res. 1989, 4, 521–535. [Google Scholar] [CrossRef]
- Sutherland, J.R.; Shrimpton, G.M.; Sturrock, R.N. Diseases and Insects in British Columbian Forest Seedling Nurseries; FRDA Report 065; Forestry Canada and British Columbia Ministry of Forests: Victoria, BC, Canada, 1989; p. 85. Available online: https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/forestry/forest-health/diseases_and_insects_in_bc_forest_seedling_nurseries-complete.pdf (accessed on 18 March 2018).
- Dumroese, R.K.; James, R.L. Root diseases in bareroot and container nurseries of the Pacific Northwest: Epidemiology, management, and effects on outplanting performance. New For. 2005, 30, 185–202. [Google Scholar] [CrossRef]
- Heiskanen, J. Water status of sphagnum peat and a peat–perlite mixture in containers subjected to irrigation regimes. HortScience 1995, 30, 281–284. [Google Scholar]
- Heiskanen, J. Irrigation regime affects water and aeration conditions in peat growth medium and the growth of containerized Scots pine seedings. New For. 1995, 9, 181–195. [Google Scholar] [CrossRef]
- Pinto, J.R.; Dumroese, R.K.; Davis, A.S.; Landis, T.D. Conducting seedling stocktype trials: A new approach to an old question. J. For. 2011, 109, 293–299. Available online: https://www.fs.usda.gov/treesearch/pubs/38391 (accessed on 22 March 2018).




| Growing Media Designation | (v v−1) | (w w−1) a | pH | Db (g∙cm−3) | ECEC (cmol∙kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Peat (%) | Biochar Amendment (%) | Biochar Amendment (%) | |||||||
| Peat | |||||||||
| Peat (control) | 100 | 0 | - | 3.9 | g | 0.099 | j | 49.6 | a |
| Peat + biochar (BC) | |||||||||
| BC25 | 75 | 25 | 10 | 5.0 | e | 0.173 | i | 31.0 | b |
| BC50 | 50 | 50 | 70 | 5.9 | c | 0.251 | g | 23.8 | c |
| BC75 | 25 | 75 | 90 | 6.7 | b | 0.294 | f | 15.4 | de |
| BC100 | 0 | 100 | 100 | - | 0.331 | d | 7.2 | gh | |
| Peat + pyrolized softwood pellets (PP) | |||||||||
| PP25 | 75 | 25 | 7 | 4.5 | f | 0.179 | i | 31.8 | b |
| PP50 | 50 | 50 | 69 | 5.4 | d | 0.264 | g | 17.8 | d |
| PP75 | 25 | 75 | 90 | 7.0 | a | 0.313 | e | 11.1 | f |
| PP100 | 0 | 100 | 100 | - | 0.318 | de | 5.2 | h | |
| Peat + wood-biochar pellets (WP) | |||||||||
| WP25 | 75 | 25 | 44 | 4.4 | f | 0.223 | h | 22.7 | c |
| WP50 | 50 | 50 | 81 | 4.7 | ef | 0.387 | c | 16.8 | de |
| WP75 | 25 | 75 | 94 | 5.2 | de | 0.469 | b | 13.2 | ef |
| WP100 | 0 | 100 | 100 | - | 0.527 | a | 10.4 | fg | |
| P values | <0.0001 | <0.0001 | <0.0001 | ||||||
| Mean Particle Size Distribution (%) | |||||
|---|---|---|---|---|---|
| (mm) | |||||
| <0.5 | 0.5−1 | 1−2 | 2−5 | >5 | |
| Peat | 30.8 | 22.7 | 27.6 | 13.3 | 5.6 |
| BC | 92.5 | 6.6 | 0.7 | 0.2 | 0.0 |
| PP | 4.7 | 2.5 | 2.4 | 44.9 | 45.5 |
| WP | 8.0 | 2.7 | 4.3 | 65.9 | 19.2 |
| Media | Total N | NH4 | NO3 | Organic N | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 15 | Day 29 | Day 1 | Day 15 | Day 29 | |||||||||||
| Peat | 17.9 | a | 13.0 | a | 1.60 | a | 0.70 | a | 0.27 | a | 5.04 | d | 3.85 | d | 3.19 | c |
| BC25 | 7.5 | bc | 1.1 | bc | 1.53 | ab | 0.01 | b | 0.01 | b | 5.60 | d | 5.91 | cd | 6.30 | b |
| BC50 | 6.9 | bc | 0.3 | c | 0.93 | abc | 0.01 | b | 0.01 | b | 4.56 | d | 7.00 | cd | 7.43 | b |
| BC75 | 3.5 | c | <0.1 | c | 0.43 | c | 0.01 | b | 0.01 | b | 2.52 | e | 3.99 | d | 3.37 | c |
| PP25 | 19.7 | a | 15.3 | a | 0.03 | c | 0.03 | b | 0.03 | ab | 5.73 | d | 3.81 | d | 3.63 | c |
| PP50 | 10.8 | b | 6.7 | b | 0.02 | c | 0.02 | b | 0.04 | ab | 4.66 | d | 3.74 | d | 3.75 | c |
| PP75 | 5.5 | bc | 1.8 | bc | 0.07 | c | 0.04 | b | 0.03 | ab | 4.24 | de | 3.79 | d | 2.99 | c |
| WP25 | 9.3 | bc | <0.1 | c | 0.49 | bc | 0.01 | b | 0.01 | b | 10.67 | c | 8.76 | c | 7.96 | b |
| WP50 | 17.7 | a | 0.2 | c | 1.53 | ab | 0.05 | b | 0.06 | ab | 22.20 | b | 13.51 | b | 15.31 | a |
| WP75 | 19.7 | a | 0.1 | c | 1.02 | abc | 0.26 | ab | 0.07 | ab | 27.45 | a | 17.70 | a | 14.28 | a |
| Al | B | Ca | Cd | Cr | Cu | Fe | K | Mg | Mn | Na | Ni | P | Pb | S | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ||||||||||||||||
| Peat | 1036 | 5.5 | 6615 | 0.11 | 1.4 | 2.3 | 1619 | 446 | 1131 | 150 | 82 | 1.8 | 523 | 2.2 | 2111 | 22 |
| BC | 164 | 17.0 | 4694 | <0.11 | 98.4 | 8.0 | 1108 | 4340 | 509 | 139 | 82 | 10.7 | 179 | 3.3 | 117 | 16 |
| PP | 178 | 8.2 | 999 | <0.11 | 0.9 | 3.1 | 230 | 1868 | 165 | 100 | 856 | 0.5 | 238 | <2.1 | 45 | 9.6 |
| WP | 93 | 10.6 | 2642 | <0.11 | 98.7 | 5.8 | 1168 | 2540 | 363 | 98 | 148 | 5.2 | 277 | 2.6 | 140 | 18 |
| Soluble | ||||||||||||||||
| Peat | 26 | 2.8 | 5347 | - | - | - | 9.5 | 361 | 1045 | 1344 | 136 | - | 91 | - | 338 | - |
| BC25 | 18 | 5.8 | 3666 | - | - | - | 8.9 | 2570 | 435 | 76 | 79 | - | 44 | - | 156 | - |
| BC50 | 16 | 6.6 | 3062 | - | - | - | 12.8 | 3044 | 274 | 62 | 64 | - | 36 | - | 104 | - |
| BC75 | 13 | 7.0 | 2440 | - | - | - | 22.2 | 3040 | 177 | 53 | 52 | - | 32 | - | 77 | - |
| BC100 | 3 | 7.1 | 1522 | - | - | - | 93.9 | 2890 | 120 | 41 | 43 | - | 25 | - | 51 | - |
| PP25 | 11 | 3.9 | 3614 | - | - | - | 3.7 | 1081 | 780 | 101 | 426 | - | 106 | - | 254 | - |
| PP50 | 6 | 4.5 | 1928 | - | - | - | 1.2 | 1512 | 333 | 65 | 586 | - | 103 | - | 152 | - |
| PP75 | 6 | 5.1 | 1074 | - | - | - | 0.6 | 1580 | 168 | 48 | 634 | - | 114 | - | 102 | - |
| PP100 | 11 | 5.4 | 809 | - | - | - | 2.3 | 1524 | 114 | 43 | 621 | - | 117 | - | 91 | - |
| WP25 | 20 | 5.7 | 2492 | - | - | - | 11.8 | 1408 | 360 | 67 | 140 | - | 87 | - | 109 | - |
| WP50 | 17 | 6.2 | 1964 | - | - | - | 14.6 | 1680 | 267 | 57 | 140 | - | 99 | - | 72 | - |
| WP75 | 13 | 6.2 | 1456 | - | - | - | 31.9 | 1690 | 191 | 46 | 131 | - | 106 | - | 49 | - |
| WP100 | 5 | 6.2 | 1186 | - | - | - | 48.3 | 1590 | 137 | 39 | 128 | - | 108 | - | 57 | - |
| Press water extract | ||||||||||||||||
| Peat | 0.38 | 0.1 | 20 | <0.001 | 0.01 | 0.000 | 0.5 | 11 | 7 | 1 | 7 | 0.00 | 5 | <0.015 | 34 | 0.04 |
| BC25 | 0.53 | 0.5 | 7 | <0.001 | 0.05 | 0.013 | 1.7 | 93 | 1 | <1 | 5 | <0.01 | 8 | <0.015 | 20 | 0.02 |
| BC50 | 0.67 | 0.5 | 12 | <0.001 | 0.12 | 0.037 | 2.9 | 155 | 1 | <1 | 6 | 0.02 | 10 | <0.015 | 7 | 0.02 |
| BC75 | 0.12 | 0.3 | 42 | <0.001 | 0.07 | 0.005 | 1.7 | 250 | 7 | 1 | 8 | 0.01 | 4 | <0.015 | 3 | <0.01 |
| BC100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| PP25 | 0.39 | 0.3 | 6 | <0.001 | <0.01 | 0.006 | 0.5 | 60 | 2 | <1 | 39 | <0.01 | 21 | <0.005 | 44 | 0.02 |
| PP50 | 0.40 | 0.4 | 4 | <0.001 | <0.01 | 0.007 | 0.5 | 78 | 1 | <1 | 51 | <0.01 | 30 | <0.005 | 38 | 0.02 |
| PP75 | 0.30 | 0.3 | 3 | <0.001 | <0.01 | 0.007 | 0.3 | 75 | 1 | <1 | 52 | <0.01 | 19 | <0.005 | 24 | 0.02 |
| PP100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| WP25 | 0.58 | 0.4 | 23 | <0.001 | 0.04 | 0.009 | 2.7 | 135 | 5 | 1 | 16 | 0.01 | 16 | <0.015 | 27 | 0.07 |
| WP50 | 1.75 | 0.6 | 53 | <0.001 | 0.12 | 0.005 | 6.3 | 368 | 14 | 1 | 38 | 0.03 | 36 | <0.015 | 28 | 0.18 |
| WP75 | 1.12 | 0.6 | 77 | <0.001 | 0.11 | 0.008 | 6.9 | 430 | 20 | 2 | 4 | 0.04 | 30 | <0.015 | 20 | 0.26 |
| WP100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Independent Variables | Height | Stem Diameter | Shoot Biomass | Root Biomass |
|---|---|---|---|---|
| N fertilization (F) | 0.2672 | 0.1341 | 0.0784 | 0.6250 |
| Medium (M) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| F × M | 0.6368 | 0.0143 | <0.0001 | 0.0335 |
| Percentage of Peat Replaced (v v−1) | ||||
|---|---|---|---|---|
| 0 | 25 | 50 | 75 | |
| Peat | 100 | |||
| BC | 88 | 75 | 88 | |
| PP | 101 | 102 | 100 | |
| WP | 58 | ― | ― | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumroese, R.K.; Pinto, J.R.; Heiskanen, J.; Tervahauta, A.; McBurney, K.G.; Page-Dumroese, D.S.; Englund, K. Biochar Can Be a Suitable Replacement for Sphagnum Peat in Nursery Production of Pinus ponderosa Seedlings. Forests 2018, 9, 232. https://doi.org/10.3390/f9050232
Dumroese RK, Pinto JR, Heiskanen J, Tervahauta A, McBurney KG, Page-Dumroese DS, Englund K. Biochar Can Be a Suitable Replacement for Sphagnum Peat in Nursery Production of Pinus ponderosa Seedlings. Forests. 2018; 9(5):232. https://doi.org/10.3390/f9050232
Chicago/Turabian StyleDumroese, R. Kasten, Jeremiah R. Pinto, Juha Heiskanen, Arja Tervahauta, Katherine G. McBurney, Deborah S. Page-Dumroese, and Karl Englund. 2018. "Biochar Can Be a Suitable Replacement for Sphagnum Peat in Nursery Production of Pinus ponderosa Seedlings" Forests 9, no. 5: 232. https://doi.org/10.3390/f9050232





