A Review on the Dynamics of Prescribed Fire, Tree Mortality, and Injury in Managing Oak Natural Communities to Minimize Economic Loss in North America
Abstract
:1. Introduction
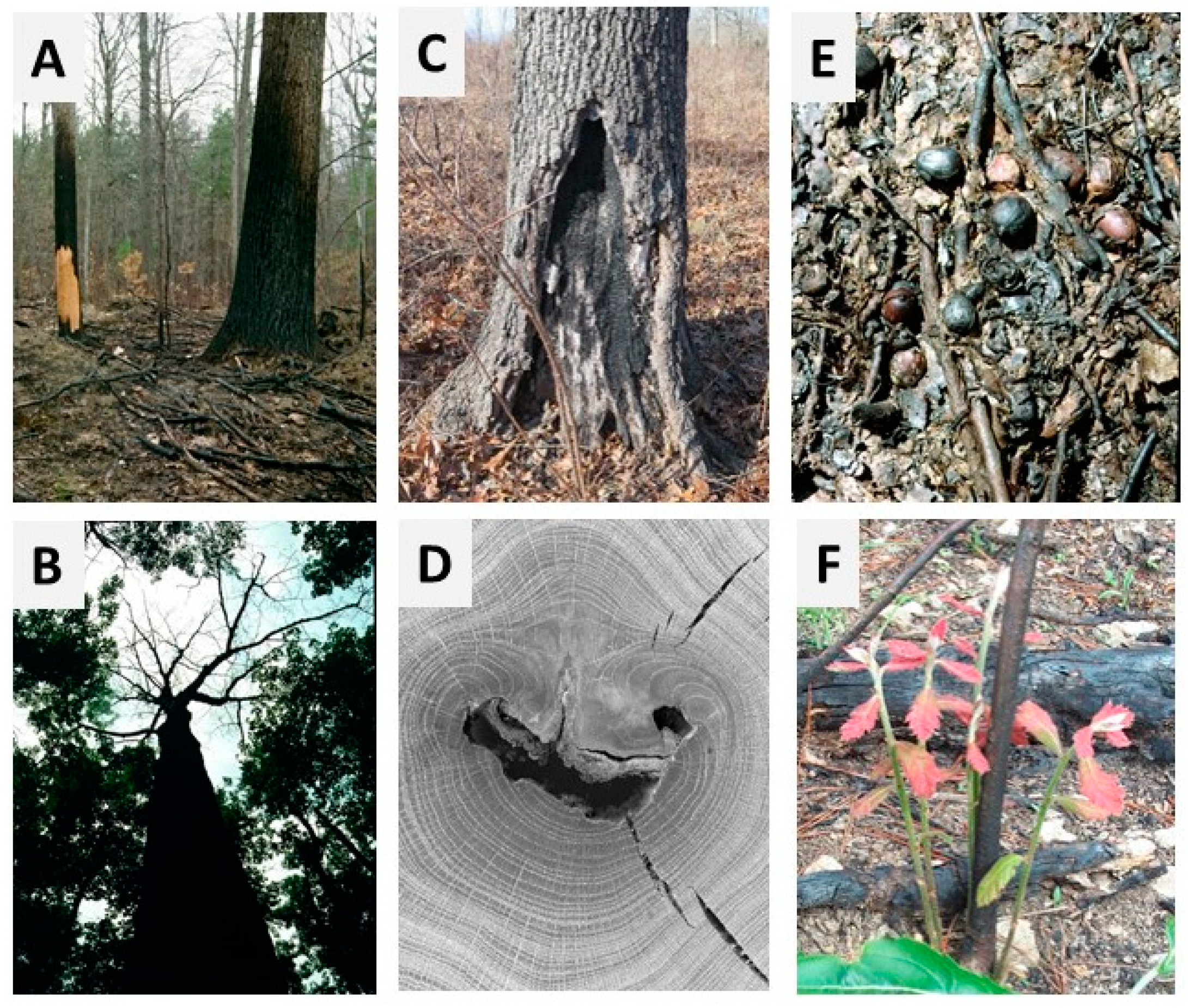
2. Types of Fire Injury and Damage
2.1. Tree Mortality
2.2. Stem Top-Kill
2.3. Bole Wounding and Decay
3. Determinants of Fire Injury and Damage
3.1. Tree Species
3.2. Tree Size
3.3. Bark Characteristics
3.4. Defense against Decay
3.4.1. Compartmentalization
3.4.2. Heartwood Decay Resistance
3.5. Scar Size and Time Since Wounding
4. Fire in Oak Management
4.1. Scenario 1: Mature Forest with No Oak Advance Reproduction
4.2. Scenario 2: Mature Forests with Abundant Small Oak Advance Reproduction
4.3. Scenario 3: Stand Initiation Stage after Final Shelterwood Removal or Clearcutting
4.4. Scenario 4: Stem Exclusion Stage, Crown Closure
4.5. Scenario 5: Stands Managed by Uneven-Aged Methods
4.6. Scenario 6: Savanna and Woodland Restoration
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crepet, W.L.; Nixon, K.C. Earliest megafossil evidence of Fagaceae: Phylogenetic and biogeographic implications. Am. J. Bot. 1989, 76, 842–855. [Google Scholar] [CrossRef]
- Crepet, W.L.; Nixon, K.C. Extinct transitional Fagaceae from the Oligocene and their phylogenetic implications. Am. J. Bot. 1989, 76, 1493–1505. [Google Scholar] [CrossRef]
- Patterson, W.A., III. The paleoecology of fire and oaks in eastern forests. In Fire in Eastern Oak Forests: Delivering Science to Land Managers; Dickinson, M.B., Ed.; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2006; pp. 2–19. [Google Scholar]
- Nixon, K.C. Global and neotropical distribution and diversity of oak (genus Quercus) and oak forests. In Ecological Studies 185, Ecology and Conservation of Neotropical Montane Oak Forests; Kappelle, M., Ed.; Springer: Berlin, Germany, 2006; pp. 3–13. ISBN 3-540-28908-9. [Google Scholar]
- Delcourt, P.A.; Delcourt, H.R. The influence of prehistoric human-set fires on oak-chestnut forests in the southern Appalachians. Castanea 1998, 63, 337–345. [Google Scholar]
- Delcourt, P.A.; Delcourt, H.R.; Ison, C.R.; Sharp, W.E.; Gremillion, K.J. Prehistoric human use of fire, the Eastern Agricultural Complex, and Appalachian oak-chestnut forests: Paleoecology of Cliff Palace Pond, Kentucky. Am. Antiq. 1998, 63, 263–278. [Google Scholar] [CrossRef]
- Foster, D.R.; Clayden, S.; Orwig, D.A.; Hall, B.; Barry, S. Oak, chestnut and fire: Climatic and cultural controls of long-term forest dynamics in New England, USA. J. Biogeogr. 2002, 29, 1359–1379. [Google Scholar] [CrossRef]
- Purcell, K.L.; Stephens, S.L. Changing fire regimes and the avifauna of California oak woodlands. Stud. Avian Biol. 2005, 30, 30–45. [Google Scholar]
- Guyette, R.P.; Spetich, M.A. Fire history of oak-pine forests in the lower Boston Mountains, Arkansas, USA. For. Ecol. Manag. 2003, 180, 463–474. [Google Scholar] [CrossRef]
- DeSantis, R.D.; Hallgren, S.W.; Stahle, D.W. Historic fire regime of an upland oak forest in south-central North America. Fire Ecol. 2010, 6, 45–61. [Google Scholar] [CrossRef]
- Brose, P.H.; Guyette, R.P.; Marschall, J.M.; Stambaugh, M.C. Fire history reflects human history in the Pine Creek Gorge of north-central Pennsylvania. Nat. Areas J. 2015, 35, 214–223. [Google Scholar] [CrossRef]
- Guyette, R.P.; Muzika, R.M.; Dey, D.C. Dynamics of an anthropogenic fire regime. Ecosystems 2002, 5, 472–486. [Google Scholar] [CrossRef]
- Stambaugh, M.C.; Marschall, J.M.; Abadir, E.R.; Jones, B.C.; Brose, P.H.; Dey, D.C.; Guyette, R.P. Wave of fire: An anthropogenic signal in historical fire regimes across central Pennsylvania, USA. Ecosphere 2018, 9, e02222. [Google Scholar] [CrossRef]
- Steen, H.K. The U.S. Forest Service: A History; University Washington Press: Seattle, WA, USA, 1976; 356p, ISBN 0295955236. [Google Scholar]
- Pyne, S.J. Fire in America: A Cultural History of Wildland and Rural Fire; Princeton University Press: Princeton, NJ, USA, 1982; 654p, ISBN1 0691083002. ISBN2 9780691083001. [Google Scholar]
- Pyne, S.J.; Andrews, P.L.; Laven, R.D. Introduction to Wildland Fire, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1996; 769p, ISBN1 0471549134. ISBN2 9780471549130. [Google Scholar]
- Nuzzo, V.A. Extent and status of Midwest oak savanna: Presettlement and 1985. Nat. Areas J. 1986, 6, 6–36. [Google Scholar]
- Noss, R.F.; LaRoe, E.T.; Scott, J.M. Endangered Ecosystems of the United States: A Preliminary Assessment of Loss and Degradation; USDI, National Biological Service: Washington, DC, USA, 1995; Volume 28. [Google Scholar]
- Johnson, P.S.; Shifley, S.R.; Rogers, R. The Ecology and Silviculture of Oaks, 2nd ed.; CABI Publishing: New York, NY, USA, 2009; 580p, ISBN 10 1845934741. [Google Scholar]
- Nelson, P.W. The Terrestrial Natural Communities of Missouri; Missouri Natural Areas Committee: Jefferson City, MO, USA, 2010; 550p. [Google Scholar]
- Dey, D.C. Sustaining oak forests in eastern North America: Regeneration and recruitment, the pillars of sustainability. For. Sci. 2014, 60, 926–942. [Google Scholar] [CrossRef]
- Marschall, J.M.; Guyette, R.P.; Stambaugh, M.C.; Stevenson, A.P. Fire damage effects on red oak timber product value. For. Ecol. Manag. 2014, 320, 182–189. [Google Scholar] [CrossRef]
- Dey, D.C.; Schweitzer, C.J. Timing fire to minimize damage in managing oak ecosystems. In Proceedings of the 17th Biennial Southern Silvicultural Research Conference, Shreveport, LA, USA, 5–7 March 2013; Holley, A.G., Connor, K.F., Haywood, J.D., Eds.; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2015; pp. 143–153. [Google Scholar]
- Stambaugh, M.C.; Smith, K.T.; Dey, D.C. Fire scar growth and closure rates in white oak (Quercus alba) and the implications for prescribed burning. For. Ecol. Manag. 2017, 391, 396–403. [Google Scholar] [CrossRef]
- Kaufert, F.H. Fire and decay injury in the southern bottomland hardwoods. J. For. 1933, 31, 64–67. [Google Scholar] [CrossRef]
- Hepting, G.H. Decay in Merchantable Oak, Yellow Poplar, and Basswood in the Appalachian Region; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1937; 30p.
- Gustafson, R.O. Cull as determined from basal wounds in Kentucky highlands timber. J. For. 1944, 42, 181–184. [Google Scholar] [CrossRef]
- Burns, P.Y. Fire Scars and Decay in Missouri Oaks; University of Missouri, College of Agriculture, Agriculture Experiment Station: Columbia, MO, USA, 1955; 8p. [Google Scholar]
- Knapp, B.O.; Marschall, J.M.; Stambaugh, M.C. Effects of long-term prescribed burning on timber value in hardwood forests of the Missouri Ozarks. In Proceedings of the 20th Central Hardwood Forest Conference, Columbia, MO, USA, 28 March–1 April 2016; Kabrick, J.M., Dey, D.C., Knapp, B.O., Larsen, D.R., Shifley, S.R., Stelzer, H.E., Eds.; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2017; pp. 304–313. [Google Scholar]
- Hepting, G.H. Prediction of cull following fire in Appalachian oaks. J. Agric. Res. 1941, 62, 109–120. [Google Scholar]
- Loomis, R.M. Predicting the Losses in Sawtimber Volume and Quality from Fires in Oak-hickory Forests; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1974; 6p.
- Stambaugh, M.; Guyette, R. Prescribed Fire Effects on the Wood Quality of Three Common Oaks in the Ozark Region, Q. coccinea, Q. velutina, Q. alba; Missouri Department of Conservation: Jefferson City, MO, USA, 2008; 23p. [Google Scholar]
- Stambaugh, M.C.; Guyette, R.P. Long-term growth and climate response of shortleaf pine at the Missouri Ozark forest ecosystem project. In Proceedings of the 14th Central Hardwood Forest Conference, Wooster, OH, USA, 16–19 March 2004; Yaussy, D.A., Hix, D.M., Long, R.P., Goebel, P.C., Eds.; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2004; pp. 448–458. [Google Scholar]
- Guyette, R.P.; Stambaugh, M.C.; Dey, D.C.; Muzika, R.M. Predicting fire frequency with chemistry and climate. Ecosystems 2012, 15, 322–335. [Google Scholar] [CrossRef]
- EVALIDator. Forest Inventory EVALIDator Web-Application Version 1.6.0.03a. U.S. Department of Agriculture, Forest Service, Northern Research Station. 2015. Available online: https://apps.fs.usda.gov/Evalidator/evalidator.jsp (accessed on 14 February 2018).
- Insurance Information Institute. Wildfires by State: 2010 to 2016. Available online: https://www.iii.org/table-archive/23284 (accessed on 12 February 2018).
- Miles, P.D. Forest Inventory EVALIDator Web-Application Version 1.5.1.04; U.S. Department of Agriculture Forest Service, Northern Research Station: St. Paul, MN, USA. Available online: http://apps.fs.fed.us/Evalidator/tmattribute.jsp (accessed on 14 March 2013).
- USDA Forest Service. Mark Twain National Forest Plan 2005; U.S. Department of Agriculture, Forest Service, Mark Twain National Forest: Rolla, MO, USA, 2005.
- USDA Forest Service. Revised Land and Resource Management Plan Ozark-St; Francis National Forests, U.S. Department of Agriculture, Forest Service, Southern Region: Asheville, NC, USA, 2005; 296p.
- Upper Mississippi River and Great Lakes Joint Venture. Landbird Habitat Conservation Strategy. Available online: http://www.uppermissgreatlakesjv.org/docs/UMRGLR_JV_LandbirdHCS.pdf (accessed on 13 February 2018).
- Schulte, L.A.; Mladenoff, D.J.; Crow, T.R.; Merrick, L.C.; Cleland, D.T. Homogenization of northern U.S. Great Lakes forests due to land use. Landsc. Ecol. 2007, 22, 1089–1103. [Google Scholar] [CrossRef] [Green Version]
- Hunter, M.L.; Schmiegelow, F.K.A. Wildlife, Forests and Forestry, 2nd ed.; Prentice Hall: New York, NY, USA, 2011; 288p, ISBN 10 0135014328. [Google Scholar]
- Brandt, L.; He, H.; Iverson, L.; Thompson, F.R.; Butler, P.; Handler, S.; Janowiak, M.; Shannon, P.D.; Swanston, C.; Albrecht, M.; et al. Central Hardwoods Ecosystem Vulnerability Assessment and Synthesis: A Report from the Central Hardwoods Climate Change Response Framework Project; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2014; 254p.
- Butler, P.L.; Iverson, L.; Thompson, F.R.; Brandt, L.; Handler, S.; Janowiak, M.; Shannon, P.D.; Swanston, C.; Karriker, K.; Bartig, J.; et al. Central Appalachians Forest Ecosystem Vulnerability Assessment and Synthesis: A Report from the Central Appalachians Climate Change Response Framework Project; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2015; 310p.
- Brandt, L.A.; Butler, P.R.; Handler, S.D.; Janowiak, M.K.; Shannon, P.D.; Swanston, C.W. Integrating science and management to assess forest ecosystem vulnerability to climate change. J. For. 2017, 115, 212–221. [Google Scholar] [CrossRef]
- Davis, M.A.; Peterson, D.W.; Reich, P.B.; Crozier, M.; Query, T.; Mitchell, E.; Huntington, J.; Bazakas, P. Restoring savanna using fire: Impact on the breeding bird community. Restor. Ecol. 2000, 8, 30–40. [Google Scholar] [CrossRef]
- Brawn, J.D.; Robinson, S.K.; Thompson, F.R. The role of disturbance in the ecology and conservation of birds. Ann. Rev. Ecol. Syst. 2001, 32, 251–276. [Google Scholar] [CrossRef]
- Brawn, J.D. Effects of restoring oak savannas on bird communities and populations. Conserv. Biol. 2006, 20, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.C.; Buehler, D.A.; Canterbury, R.A.; Confer, J.L.; Hamel, P.B. Conservation of disturbance-dependent birds in eastern North America. Wildl. Soc. Bull. 2001, 29, 440–455. [Google Scholar]
- Greenberg, C.H.; Collins, B.S.; Thompson, F.R. Sustaining Young Forest Communities: Ecology and Management of Early Successional Habitats in the Central Hardwood Region; Springer: New York, NY, USA, 2011; 312p. [Google Scholar]
- King, D.I.; Schlossberg, S. Synthesis of the conservation value of the early-successional stage in forests of eastern North America. For. Ecol. Manag. 2014, 324, 186–195. [Google Scholar] [CrossRef]
- Collins, S.L.; Knapp, A.K.; Briggs, J.M.; Blair, J.M.; Steinauer, E.M. Modulation of diversity by grazing and mowing in native tallgrass prairie. Science 1998, 280, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.K.; Givnish, T.J. Gradients in the composition, structure, and diversity of remnant oak savannas in southern Wisconsin. Ecol. Monogr. 1999, 69, 353–374. [Google Scholar] [CrossRef]
- Peterson, D.W.; Reich, P.B. Fire frequency and tree canopy structure influence plant species diversity in a forest-grassland ecotone. Plant Ecol. 2008, 194, 5–16. [Google Scholar] [CrossRef]
- McShea, W.J.; Healy, W.M.; Devers, P.; Fearer, T.; Koch, F.H.; Stauffer, D.; Waldon, J. Forestry matters: Decline of oaks will impact wildlife in hardwood forests. J. Wildl. Manag. 2007, 71, 1717–1728. [Google Scholar] [CrossRef]
- Fox, V.L.; Buehler, C.P.; Byers, C.M.; Drake, S.E. Forest composition, leaf litter, and songbird communities in oak- vs. maple-dominated forests in the eastern United States. For. Ecol. Manag. 2010, 259, 2426–2432. [Google Scholar] [CrossRef]
- Reidy, J.L.; Thompson, F.R.; Kendrick, S.W. Breeding bird response to habitat and landscape factors across a gradient of savanna, woodland, and forest in the Missouri Ozarks. For. Ecol. Manag. 2014, 313, 34–46. [Google Scholar] [CrossRef]
- Starbuck, C.A.; Amelon, S.K.; Thompson, F.R. Relationships between bat occupancy and habitat and landscape structure along a savanna, woodland, forest gradient in the Missouri Ozarks. Wildl. Soc. Bull. 2015, 39, 20–30. [Google Scholar] [CrossRef]
- Hansen, R.A. Effects of habitat complexity and composition on a diverse litter microarthropod assemblage. Ecology 2000, 81, 1120–1132. [Google Scholar] [CrossRef]
- Rubbo, M.J.; Kiesecker, J.M. Leaf litter composition and community structure: Translating regional species changes into local dynamics. Ecology 2004, 85, 2519–2525. [Google Scholar] [CrossRef]
- Tallamy, D.W.; Shropshire, K.J. Ranking Lepidoptera use of native versus introduced plants. Conserv. Biol. 2009, 23, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Stoler, A.B.; Relyea, R.A. Living in the litter: The influence of tree leaf litter on wetland communities. Oikos 2011, 120, 862–872. [Google Scholar] [CrossRef]
- Arthur, M.A.; Alexander, H.D.; Dey, D.C.; Schweitzer, C.J.; Loftis, D.L. Refining the oak-fire hypothesis for management of oak-dominated forests of the eastern United States. J. For. 2012, 110, 257–266. [Google Scholar] [CrossRef]
- Cole, K.L.; Klick, K.F.; Pavlovic, N.B. Fire temperature monitoring during experimental burns at Indiana Dunes National Lakeshore. Nat. Areas J. 1992, 12, 177–183. [Google Scholar]
- Franklin, S.B.; Robertson, P.A.; Fralish, J.S. Small-scale fire temperature patterns in upland Quercus communities. J. Appl. Ecol. 1997, 34, 613–630. [Google Scholar] [CrossRef]
- Iverson, L.R.; Hutchinson, T.F. Soil temperature and moisture fluctuations during and after prescribed fire in mixed-oak forests, USA. Nat. Areas J. 2002, 22, 296–304. [Google Scholar]
- Dey, D.C.; Hartman, G. Returning fire to Ozark Highland forest ecosystems: Effects on advance regeneration. For. Ecol. Manag. 2005, 217, 37–53. [Google Scholar] [CrossRef]
- Hutchinson, T.F.; Sutherland, E.K.; Yaussy, D.A. Effects of repeated prescribed fires on the structure, composition, and regeneration of mixed-oak forests in Ohio. For. Ecol. Manag. 2005, 218, 210–228. [Google Scholar] [CrossRef]
- Elliott, K.J.; Vose, J.M. Effects of understory prescribed burning on shortleaf pine (Pinus echinata Mill.)/mixed-hardwood forests. J. Torrey Bot. Soc. 2005, 132, 236–251. [Google Scholar] [CrossRef]
- Guyette, R.P.; Stambaugh, M.C. Post-oak fire scars as a function of diameter, growth, and tree age. For. Ecol. Manag. 2004, 198, 183–192. [Google Scholar] [CrossRef]
- Smith, K.T.; Sutherland, E.K. Fire-scar formation and compartmentalization in oak. Can. J. For. Res. 1999, 29, 166–171. [Google Scholar] [CrossRef]
- Regelbrugge, J.C.; Smith, D.W. Postfire tree mortality in relation to wildfire severity in mixed oak forests in the Blue Ridge of Virginia. North. J. Appl. For. 1994, 11, 90–97. [Google Scholar] [CrossRef]
- Smith, K.T.; Sutherland, E.K. Resistance of eastern hardwood stems to fire injury and damage. In Fire in Eastern Oak Forests: Delivering Science to Land Managers; Dickinson, M.B., Ed.; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2006; pp. 210–217. [Google Scholar]
- Kinkead, C.S.; Stambaugh, M.C.; Kabrick, J.M. Mortality, scarring, and growth in an oak woodland following prescribed fire and commercial thinning in the Ozark Highlands. For. Ecol. Manag. 2017, 403, 12–26. [Google Scholar] [CrossRef]
- Schweitzer, C.J.; Dey, D.C.; Wang, Y. Hardwood-pine mixedwoods stand dynamics following thinning and prescribed burning. Fire Ecol. 2016, 12, 85–104. [Google Scholar] [CrossRef]
- Brose, P.; Van Lear, D. Effects of seasonal prescribed fires on residual overstory trees in oak-dominated shelterwood stands. South. J. Appl. For. 1999, 23, 88–93. [Google Scholar] [CrossRef]
- Waldrop, T.A.; White, D.L.; Jones, S.M. Fire regimes for pine-grassland communities in the southeastern United States. For. Ecol. Manag. 1992, 47, 195–210. [Google Scholar] [CrossRef]
- Arthur, M.A.; Blankenship, B.A.; Schörgendorfer, A.; Loftis, D.L.; Alexander, H.D. Changes in stand structure and tree vigor with repeated prescribed fire in an Appalachian hardwood forest. For. Ecol. Manag. 2015, 340, 46–61. [Google Scholar] [CrossRef]
- Knapp, B.O.; Stephan, K.; Hubbart, J.A. Structure and composition of an oak-hickory forest after over 60 years of repeated prescribed burning in Missouri, U.S.A. For. Ecol. Manag. 2015, 344, 95–109. [Google Scholar] [CrossRef]
- Hare, R.C. The contribution of bark to fire resistance of southern trees. J. For. 1965, 63, 248–251. [Google Scholar] [CrossRef]
- Brose, P.H.; Dey, D.C.; Phillips, R.J.; Waldrop, T.A. A meta-analysis of the fire-oak hypothesis: Does prescribed burning promote oak reproduction in Eastern North America? For. Sci. 2013, 59, 322–334. [Google Scholar] [CrossRef]
- Schweitzer, C.J.; Dey, D.C.; Wang, Y. Thinning and prescribed fire alters hardwood seedling sprouting in the William B Bankhead National Forest, Alabama. In Proceedings of the 19th Central Hardwood Forest Conference, Carbondale, IL, USA, 10–12 March 2014; Groninger, J.W., Holzmueller, E.J., Nielsen, C.K., Dey, D.C., Eds.; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2014; pp. 137–138. [Google Scholar]
- Stambaugh, M.C.; Marschall, J.M.; Guyette, R.P. Linking fire history to successional change of xeric oak woodlands. For. Ecol. Manag. 2014, 320, 83–95. [Google Scholar] [CrossRef]
- Iverson, L.R.; Yaussy, D.A.; Rebbeck, J.; Hutchinson, T.F.; Long, R.P.; Prasad, A.M. A comparison of thermocouples and temperature paints to monitor spatial and temporal characteristics of landscape-scale prescribed fires. Int. J. Wildland Fire 2004, 13, 311–322. [Google Scholar] [CrossRef]
- Stevenson, A.P.; Muzika, R.M.; Guyette, R.P. Fire scars and tree vigor following prescribed fires in Missouri Ozark upland forests. In Proceedings of the 16th Central Hardwood Forest Conference, West Lafayette, IN, USA, 8–9 April 2008; Jacobs, D.F., Michler, C.H., Eds.; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2008; pp. 525–534. [Google Scholar]
- Loomis, R.M.; Paananen, D.M. Appraising fire effects. In Central Hardwood Notes; Clark, F.B., Hutchinson, J.G., Eds.; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1989. [Google Scholar]
- Paulsell, L.K. Effects of Burning on Ozark Hardwood Timberland; University of Missouri, College of Agriculture, Agricultural Experiment Station: Columbia, MO, USA, 1957; 24p. [Google Scholar]
- McEwan, R.W.; Hutchinson, T.F.; Ford, R.D.; McCarthy, B.C. An experimental evaluation of fire history reconstruction using dendrochronology in white oak (Quercus alba). Can. J. For. Res. 2007, 37, 806–816. [Google Scholar] [CrossRef]
- Stambaugh, M.C.; Guyette, R.P.; Grabner, K.W.; Kolaks, J. Understanding Ozark forest litter variability through a synthesis of accumulation rates and fire events. In Fuels Management-How to Measure Success: Conference Proceedings; Andrews, P.L., Butler, B.W., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. 321–332. [Google Scholar]
- Fan, Z.; Larsen, D.R.; Shifley, S.R.; Thompson, F.R.; Spetich, M.A. Distribution of cavity trees in Midwestern old-growth and second-growth forests. Can. J. For. Res. 2003, 33, 1481–1494. [Google Scholar] [CrossRef]
- Johnson, P.S. Survival and Growth of Northern Red Oak Seedlings Following a Prescribed Burn; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1974; 3p.
- Brose, P.H.; Van Lear, D.H. Survival of Hardwood Regeneration during Prescribed Fires: The Importance of Root Development and Root Collar Location; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2004.
- Iverson, L.R.; Hutchinson, T.F.; Prasad, A.M.; Peters, M.P. Thinning, fire and oak regeneration across a heterogeneous landscape in the eastern US: 7-year results. For. Ecol. Manag. 2008, 255, 3035–3050. [Google Scholar] [CrossRef]
- Dey, D.C. A Comprehensive Ozark Regenerator. Ph.D. Thesis, University of Missouri, Columbia, MO, USA, 1991; 283p. Available online: https://elibrary.ru/item.asp?id=5808201 (accessed on 29 July 2018).
- Dey, D.C.; Johnson, P.S.; Garrett, H.E. Modeling the regeneration of oak stands in the Missouri Ozark Highlands. Can. J. For. Res. 1996, 6, 573–583. [Google Scholar] [CrossRef]
- Green, S.R.; Arthur, M.A.; Blankenship, B.A. Oak and red maple seedling survival and growth following periodic prescribed fire on xeric ridgetops on the Cumberland Plateau. For. Ecol. Manag. 2010, 259, 2256–2266. [Google Scholar] [CrossRef]
- Kruger, E.L.; Reich, P.B. Responses of hardwood regeneration to fire in mesic forest openings. I. Post-fire community dynamics. Can. J. For. Res. 1997, 27, 1822–1831. [Google Scholar] [CrossRef]
- Brose, P.H.; Van Lear, D.H. Responses of hardwood advance regeneration to seasonal prescribed fires in oak-dominated shelterwood stands. Can. J. For. Res. 1998, 28, 331–339. [Google Scholar] [CrossRef]
- Blankenship, B.A.; Arthur, M.A. Stand structure over nine years in burned and fire-excluded oak stands on the Cumberland Plateau, Kentucky. For. Ecol. Manag. 2006, 225, 134–145. [Google Scholar] [CrossRef]
- Chiang, J.; Arthur, M.A.; Blankenship, B.A. The effect of prescribed fire on gap fraction in an oak forest on the Cumberland Plateau. J. Torrey Bot. Soc. 2005, 132, 432–441. [Google Scholar] [CrossRef]
- Scowcroft, P.G. The Effects of Fire on the Hardwood Forests of the Missouri Ozarks. Master’s Thesis, University of Missouri, Columbia, MO, USA, 1966; 126p. Available online: https://www.researchgate.net/publication/36026587_The_effects_of_fire_on_the_hardwood_forests_of_the_Missouri_Ozarks_microform (accessed on 29 July 2018).
- Forest Products Laboratory. Comparative Decay Resistance of Heartwood of Native Species; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1967.
- Vines, R.G. Heat transfer through bark, and the resistance of trees to fire. Aust. J. Bot. 1968, 16, 499–514. [Google Scholar] [CrossRef]
- Pausas, J.G. Bark thickness and fire regime. Funct. Ecol. 2015, 29, 315–327. [Google Scholar] [CrossRef]
- Sutherland, E.K.; Smith, K.T. Resistance is not futile: The response of hardwoods to fire-caused wounding. In Proceedings: Workshop on Fire, People, and the Central Hardwoods Landscape; Yaussy, D.A., Ed.; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2000; pp. 111–115. [Google Scholar]
- Harmon, M.E. Survival of trees after low-intensity surface fires in the Great Smokey Mountains National Park. Ecology 1984, 65, 796–802. [Google Scholar] [CrossRef]
- Hengst, G.E.; Dawson, J.O. Bark properties and fire resistance of selected tree species from the central hardwood region of North America. Can. J. For. Res. 1994, 24, 688–696. [Google Scholar] [CrossRef]
- Wiedenbeck, J.K.; Schuler, T.M. Effects of prescribed fire on the wood quality and marketability of four hardwood species in the central Appalachian region. In Proceedings of the 19th Central Hardwood Forest Conference, Carbondale, IL, USA, 10–12 March 2014; Groninger, J.W., Holzmueller, E.J., Nielsen, C.K., Dey, D.C., Eds.; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2014; pp. 202–212. [Google Scholar]
- Smith, K.T. Compartmentalization, resource allocation, and wood quality. Curr. For. Rep. 2015, 1, 8–15. [Google Scholar] [CrossRef]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and durability—A review. Wood Fiber Sci. 2002, 34, 587–611. [Google Scholar]
- Hesterberg, G.A. Deterioration of Sugar Maple Following Logging Damage; U.S. Department of Agriculture, Forest Service, Lake States Forest Experiment Station: St. Paul, MN, USA, 1957; 58p.
- Dey, D.C.; Jacobs, D.; McNabb, K.; Miller, G.; Baldwin, V.; Foster, G. Artificial regeneration of major oak (Quercus) species in the eastern United States—A review of the literature. For. Sci. 2008, 54, 77–106. [Google Scholar] [CrossRef]
- Dey, D.C.; Gardiner, E.S.; Schweitzer, C.J.; Kabrick, J.M.; Jacobs, D.F. Underplanting to sustain future stocking of oak (Quercus) in temperate deciduous forests. New For. 2012, 43, 955–978. [Google Scholar] [CrossRef]
- Schuler, T.M.; Thomas Van-Gundy, M.; Adams, M.B.; Ford, W.M. Seed Bank Response to Prescribed Fire in the Central Appalachians; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2010.
- Auchmoody, L.R.; Smith, H.C. Survival of Acorns after Fall Burning; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1993.
- Greenberg, C.H.; Keyser, T.L.; Zarnoch, S.J.; Connor, K.; Simon, D.M.; Warburton, G.S. Acorn viability following prescribed fire in upland hardwood forests. For. Ecol. Manag. 2012, 275, 79–86. [Google Scholar] [CrossRef]
- Lockhart, B.R.; Hodges, J.D.; Gardiner, E.S. Response of advance cherrybark oak reproduction to midstory removal and shoot clipping. South. J. Appl. For. 2000, 24, 45–50. [Google Scholar] [CrossRef]
- Miller, G.W.; Kochenderfer, J.N.; Gottschalk, K.W. Effect of Pre-Harvest Shade Control and Fencing on Northern Red Oak Seedling Development in the Central Appalachians; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2004; pp. 182–189.
- Schweitzer, C.J.; Dey, D.C. The conundrum of creating understory light conditions conducive to promoting oak regeneration: midstory herbicide treatment versus prescribed fire. In Proceedings of the 17th Biennial Southern Silvicultural Research Conference, Shreveport, LA, USA, 5–7 March 2013; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2015; pp. 45–56. [Google Scholar]
- Brose, P.H. Root development of acorn-origin oak seedlings in shelterwood stands on the Appalachian Plateau of northern Pennsylvania: 4-Year results. For. Ecol. Manag. 2008, 255, 3374–3381. [Google Scholar] [CrossRef]
- Brose, P.H. A comparison of the effects of different shelterwood harvest methods on the survival and growth of acorn origin oak seedlings. Can. J. For. Res. 2011, 41, 2359–2374. [Google Scholar] [CrossRef]
- Dey, D.C.; Parker, W.C. Morphological indicators of stock quality and field performance of red oak (Quercus rubra L.) seedlings underplanted in a central Ontario shelterwood. New For. 1997, 14, 145–156. [Google Scholar] [CrossRef]
- Knapp, B.O.; Wang, G.G.; Van Lear, D.L.; Walker, J.L. Predicting root biomass of burned and unburned white oak advance reproduction from diameter and height. South. J. Appl. For. 2006, 30, 40–45. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics, update ed.; John Wiley & Sons: New York, NY, USA, 1996; ISBN 10 0471138339. [Google Scholar]
- Dey, D.C.; Jensen, R.G.; Wallendorf, M.J. Single-tree harvesting reduces survival and growth of oak stump sprouts in the Missouri Ozark Highlands. In Proceedings of the 16th Central Hardwood Forest Conference, West Lafayette, IN, USA, 8–9 April 2008; Jacobs, D.F., Michler, C.H., Eds.; Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2008; pp. 26–37. [Google Scholar]
- Shifley, S.R.; Smith, W.B. Diameter Growth, Survival, and Volume Estimates for Missouri Trees; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1982.
- Loewenstein, E.F. Conversion of uniform broadleaved stands to an uneven-aged structure. For. Ecol. Manag. 2005, 215, 103–112. [Google Scholar] [CrossRef]
- Weigel, D.R.; Parker, G.R. Tree regeneration response to the group selection method in southern Indiana. North. J. Appl. For. 1997, 14, 90–94. [Google Scholar] [CrossRef]
- Jenkins, M.A.; Parker, G.R. Composition and diversity of woody vegetation in silvicultural openings of southern Indiana forests. For. Ecol. Manag. 1998, 109, 57–74. [Google Scholar] [CrossRef]
- Dey, D.C.; Kabrick, J.M. Restoration of Midwestern oak woodlands and savannas. In Restoration of Boreal and Temperate Forests, 2nd ed.; Stanturf, J.A., Ed.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 20; pp. 401–428. ISBN 10 1482211963. [Google Scholar]
- Dey, D.C.; Kabrick, J.M.; Schweitzer, C.J. Silviculture to Restore Oak Savannas and Woodlands. J. For. 2017, 115, 202–211. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, D.C.; Schweitzer, C.J. A Review on the Dynamics of Prescribed Fire, Tree Mortality, and Injury in Managing Oak Natural Communities to Minimize Economic Loss in North America. Forests 2018, 9, 461. https://doi.org/10.3390/f9080461
Dey DC, Schweitzer CJ. A Review on the Dynamics of Prescribed Fire, Tree Mortality, and Injury in Managing Oak Natural Communities to Minimize Economic Loss in North America. Forests. 2018; 9(8):461. https://doi.org/10.3390/f9080461
Chicago/Turabian StyleDey, Daniel C., and Callie Jo Schweitzer. 2018. "A Review on the Dynamics of Prescribed Fire, Tree Mortality, and Injury in Managing Oak Natural Communities to Minimize Economic Loss in North America" Forests 9, no. 8: 461. https://doi.org/10.3390/f9080461



