Vaccinia virus in Feces and Urine of Wild Rodents from São Paulo State, Brazil
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Wild Rodent Capture and Sample Collection
3.2. Polymerase Chain Reaction (PCR) and Sequencing
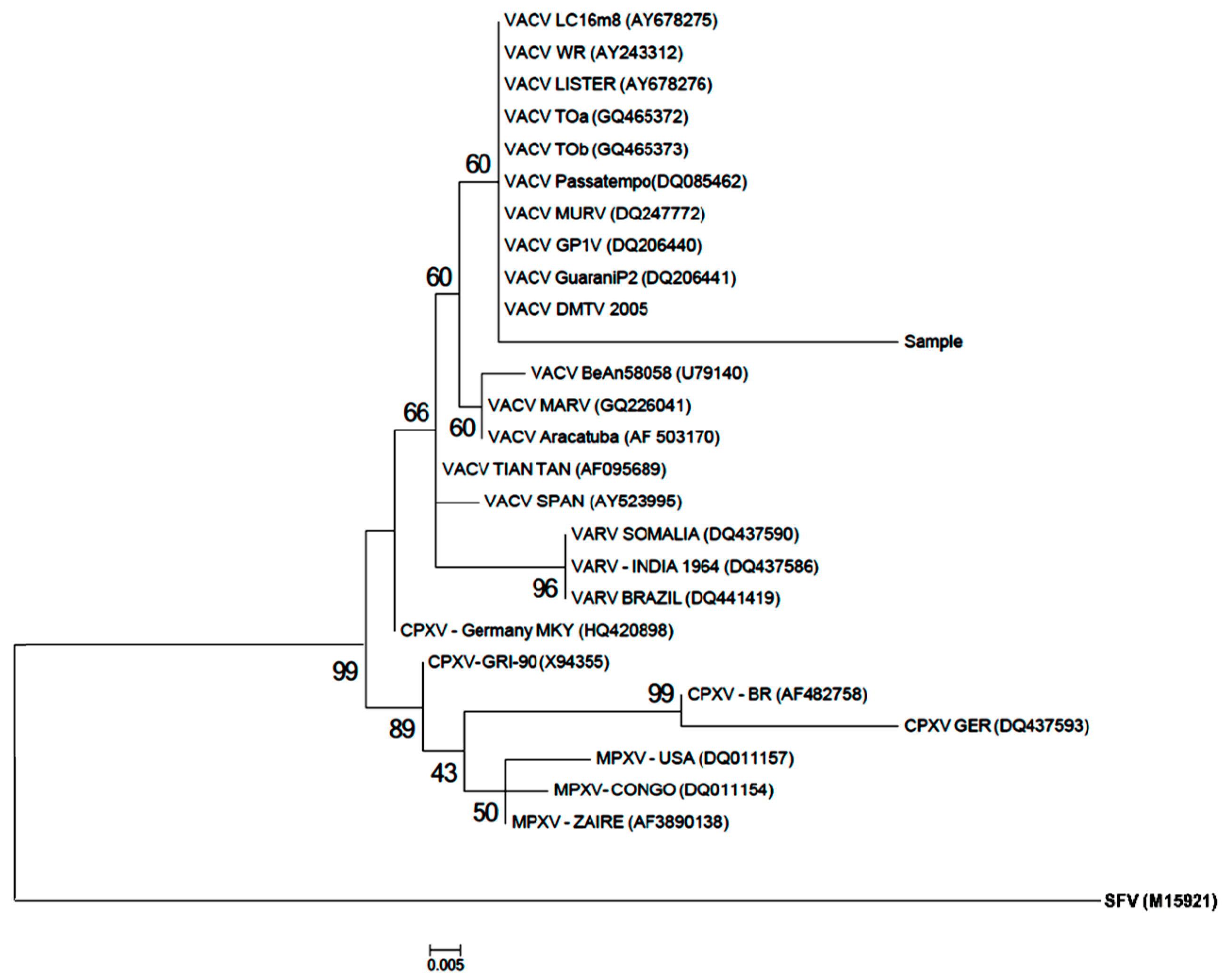
3.3. Phylogenetic Analysis
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Damaso, C.R.; Esposito, J.J.; Condit, R.C.; Moussatché, N. An emergent poxvirus from Humans and Cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virollogy 2000, 277, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.P.; Fonseca, F.G.; Santos, J.R.; Madureira, M.C.; Guedes, M.I.M.C.; Ferreira, J.M.S.; Bonjardim, C.A.; et al. Passatempo Virus, a Vaccinia Virus Strain, Brazil. J. Emerg. Infect. Dis. 2005, 11, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.L.; Vinhote, W.M.; Barbosa, J.D.; Oliveira, C.H.S.; Oliveira, C.M.G.; Campos, K.F.; Silva, N.S.; Trindade, G.S.; Abrahão, J.S.; Kroon, E.G. Reemergence of Vaccinia vírus during zoonotic outbreak, Pará State, Brazil. Emerg. Infect. Dis. 2013, 19, 2017–2020. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.B.; Assis, F.L.; Ferreira, P.C.P.; Bonjardim, C.A.; Trindade, G.S.; Kroon, E.G.; Abrahão, J.S. Short Report: Group 1 Vaccinia vírus zoonotic outbreak in Maranhão State, Brazil. Am. J. Trop. Med. Hyg. 2013, 89, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Campos, R.K.; Trindade, G.S.; Fonseca, F.G.; Ferreira, P.C.P.; Kroon, E.G. Outbreak of severe zoonotic Vaccinia vírus infection, Southeastern Brazil. Emerg. Infect. Dis. 2015, 21, 695–698. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Guedes, M.I.M.; Trindade, G.S.; Fonseca, F.G.; Campos, R.K.; Mota, B.F.; Lobato, Z.I.P.; Silva-Fernandes, A.T.; Rodrigues, G.O.L.; Lima, L.S.; et al. One more piece in the VACV ecological puzzle: Could peridomestic rodents be the link between wildlife and bovine Vaccinia outbreaks in Brazil? PLoS ONE 2009, 4, e7428. [Google Scholar] [CrossRef]
- Ferreira, J.M.S.; Abrahão, J.S.; Drumond, B.P.; Oliveira, F.M.; Alves, P.A.; Pascoal-Xavier, M.A.; Lobato, Z.I.P.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Vaccinia vírus: Shedding and horizontal transmission in a murine model. J. Gen. Virol. 2008, 89, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Trindade, G.S.; Ferreira, J.M.S.; Campos, R.K.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Long-lasting stability of Vaccinia virus strains in murine feces: Implications for virus circulation and environmental maintenance. Arch. Virol. 2009, 154, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Megid, J.; Appolinário, C.M.; Langoni, H.; Pituco, E.M.; Okuda, L.H. Short Report: Vaccinia virus in humans and cattle in southwest region of São Paulo State, Brazil. Am. J. Trop. Med. Hyg. 2008, 79, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Megid, J.; Borges, I.A.; Abrahão, J.S.; Trindade, G.S.; Appolinário, C.M.; Ribeiro, M.G.; Allendorf, S.D.; Antunes, J.M.A.P.; Silva-Fernandes, A.T.; Kroon, E.G. Vaccinia Virus, Zoonotic Infection, São Paulo State, Brazil. J. Emerg. Infect. Dis. 2012, 18, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Lima, L.S.; Assis, F.L.; Alves, P.A.; Silva-Fernandes, A.T.; Cota, M.M.G.; Ferreira, V.M.; Campos, R.K.; Mazur, C.; Lobato, Z.I.P.; et al. Nested-multiplex PCR detection of Orthopoxvirus and Parapoxvirus directly from exanthematic clinical samples. Virol. J. 2009, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Drumond, B.P.; Trindade, G.S.; Silva-Fernandes, A.T.; Ferreira, M.S.; Alves, P.A.; Campos, R.K.; Siqueira, L.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Rapid Detection of Orthopoxvirus by Semi-Nested PCR Directly From Clinical Specimens: A Useful Alternative for Routine Laboratories. J. Med. Virol. 2010, 82, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; Allendorf, S.D.; Antunes, J.M.A.P. Serological study of vaccínia virus reservoirs in areas with and without official reports of outbreaks in cattle and humans in São Paulo, Brazil. Arch. Virol. 2013, 158, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- D’Anunciação, L.; Guedes, M.I.M.; Oliveira, T.L.; Rehfeld, I.; Bonjardim, C.A.; Ferreira, P.P.; Trindade, G.S.; Lobato, Z.P.; Kroon, E.G.; Abrahão, J.S. Filling one more gap: Experimental evidence of horizontal transmission of Vaccinia virus between bovines and rodents. Vector Borne Zoonotic Dis. 2012, 12, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Delariva, R.L.; Agostinho, A.A. Introdução de espécies: Uma síntese comentada. Acta Sci. 1999, 21, 255–262. [Google Scholar] [CrossRef]
- Flores, E.F. Virologia Veterinária; Editora UFSM: Santa Maria, Brazil, 2012. [Google Scholar]
- Ninove, L.; Domart, Y.; Vervel, C.; Voinot, C.; Salez, N.; Raoult, D.; Meyer, H.; Capek, I.; Zandotti, C.; Charrel, R.N. Cowpox virus transmission from pet rats to humans, France. J. Emerg. Infect. Dis. 2009, 15, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Campe, H.; Zimmermann, P.; Glos, K.; Bayer, M.; Bergemann, H.; Dreweck, C.; Bayer, M.; Bergemann, H.; Dreweck, C.; Graf, P.; et al. Cowpox virus transmission from pet rats to humans, Germany. J. Emerg. Infect. Dis. 2009, 15, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, I.S.; Guedes, M.I.M.C.; Matos, A.C.D.; Oliveira, T.M.L.; Rivetti Junior, A.V.; Moura, A.C.J.; Paes, P.R.O.; Lago, L.A.; Kroon, E.G.; Lobato, Z.I.P. Clinical, hematological and biochemical parameters of dairy cows experimentally infected with Vaccinia virus. Res. Vet. Sci. 2013, 95, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Damaso, C.R.A.; Reis, S.A.; Jesus, D.M.; Lima, P.S.F.; Moussatché, N. A PCR-based assay for detection of emerging vaccínia-like viruses isolated in Brazil. Diagn. Microbiol. Infect. Dis. 2007, 57, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.G.; Barros, C.B.; Appolinário, C.M.; Antunes, J.M.A.P.; Mioni, M.S.R.; Bacchiega, T.S.; Allendorf, S.D.; Vicente, A.F.; Fonseca, C.R.; Megid, J. Dogs and opossums positive for vaccinia virus during outbreak affecting cattle and humans, São Paulo State, Brazil. J. Emerg. Infect. Dis. 2016, 22, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Silva-Fernandes, A.T.; Lima, L.S.; Campos, R.K.; Guedes, M.I.M.C.; Cota, M.M.G.; Assis, F.L.; Borges, I.A.; Souza-Júnior, M.F.; Lobato, Z.I.P.; et al. Vaccinia Virus Infection in Monkeys, Brazilian Amazon. J. Emerg. Infect. Dis. 2010, 16, 976–979. [Google Scholar] [CrossRef] [PubMed]


| Species | Captured | Analyzed Samples | ||||
|---|---|---|---|---|---|---|
| Feces | Urine | |||||
| n | (%) * | n | (%) * | n | (%) * | |
| J. pictipes | 1 | (0.7) | 0 | (0.0) | 0 | (0.0) |
| N. lasiurus | 1 | (0.7) | 1 | (0.9) | 1 | (1.8) |
| C. tener | 4 | (2.9) | 4 | (3.5) | 1 | (1.8) |
| N. squamipes | 4 | (2.9) | 3 | (2.6) | 2 | (3.6) |
| A. montensis | 8 | (5.8) | 6 | (5.2) | 2 | (3.6) |
| S. agouya | 14 | (10.1) | 14 | (12.2) | 9 | (16.4) |
| O. flavescens | 31 | (22.5) | 24 | (20.9) | 11 | (20.0) |
| O. nigripes | 75 | (54.3) | 63 | (54.8) | 29 | (52.7) |
| TOTAL | 138 | (100.0) | 115 | (83.3) | 55 | (39.8) |
| Species | Anhembi | Bofete | Torre de Pedra | TOTAL | ||||
|---|---|---|---|---|---|---|---|---|
| n | Positive (%) | n | Positive (%) | n | Positive (%) | n | Positive (%) | |
| N. lasiurus | 0 | 0 (0.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 1 | 0 (0.0) |
| N. squamipes | 2 | 0 (0.0) | 1 | 0 (0.0) | 0 | 0 (0.0) | 3 | 0 (0.0) |
| C. tener | 2 | 0 (0.0) | 2 | 0 (0.0) | 0 | 0 (0.0) | 4 | 0 (0.0) |
| A. montensis | 0 | 0 (0.0) | 6 | 0 (0.0) | 0 | 0 (0.0) | 6 | 0 (0.0) |
| S. agouya | 13 | 2 (15.4) | 1 | 0 (0.0) | 0 | 0 (0.0) | 14 | 2 (14.3) |
| O. flavescens | 14 | 0 (0.0) | 9 | 2 (22.2) | 1 | 0 (0.0) | 24 | 2 (8.3) |
| O. nigripes | 42 | 1 * (2.3) | 18 | 1 (5.5) | 3 | 0(0.0) | 63 | 2 (3.7) |
| TOTAL | 73 | 3 (4.1) | 38 | 3 (7.9) | 4 | 0 (0.0) | 115 | 6 (5.2) |
| Species | Anhembi | Bofete | Torre de Pedra | TOTAL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Positive | n | Positive | n | Positive | n | Positive | |||||
| DNA | SN 1 | DNA | SN 1 | DNA | SN 1 | DNA | SN 1 | |||||
| N. lasiurus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| N. squamipes | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| C. tener | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| A. montensis | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 |
| S. agouya | 13 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | 2 | 2 |
| O. flavescens | 14 | 0 | 1 | 9 | 2 * | 2 * | 1 | 0 | 0 | 24 | 2 | 3 |
| O. nigripes | 42 | 1 | 2 | 18 | 1 | 2 | 3 | 0 | 0 | 63 | 2 | 4 |
| TOTAL | 73 | 3 | 5 | 38 | 3 | 4 | 4 | 0 | 0 | 115 | 6 | 9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, M.G.; Bacchiega, T.S.; Appolinário, C.M.; Vicente, A.F.; Mioni, M.S.R.; Ribeiro, B.L.D.; Fonseca, C.R.S.; Pelícia, V.C.; Ferreira, F.; Abrahão, J.S.; et al. Vaccinia virus in Feces and Urine of Wild Rodents from São Paulo State, Brazil. Viruses 2018, 10, 51. https://doi.org/10.3390/v10020051
Peres MG, Bacchiega TS, Appolinário CM, Vicente AF, Mioni MSR, Ribeiro BLD, Fonseca CRS, Pelícia VC, Ferreira F, Abrahão JS, et al. Vaccinia virus in Feces and Urine of Wild Rodents from São Paulo State, Brazil. Viruses. 2018; 10(2):51. https://doi.org/10.3390/v10020051
Chicago/Turabian StylePeres, Marina G., Thais S. Bacchiega, Camila M. Appolinário, Acácia F. Vicente, Mateus S. R. Mioni, Bruna L. D. Ribeiro, Clóvis R. S. Fonseca, Vanessa C. Pelícia, Fernando Ferreira, Jonatas S. Abrahão, and et al. 2018. "Vaccinia virus in Feces and Urine of Wild Rodents from São Paulo State, Brazil" Viruses 10, no. 2: 51. https://doi.org/10.3390/v10020051





