Invertebrate Iridoviruses: A Glance over the Last Decade
Abstract
1. Introduction
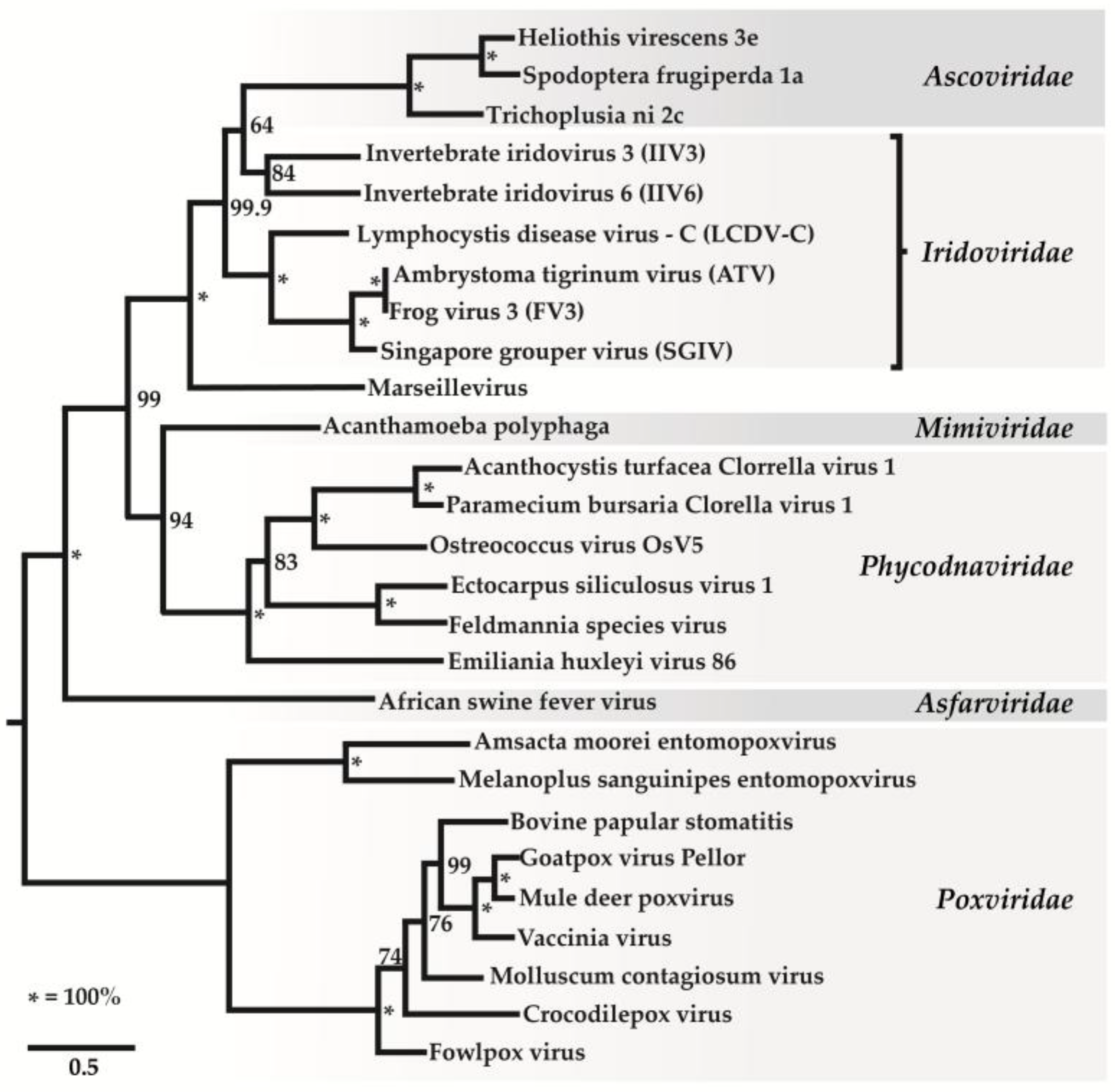
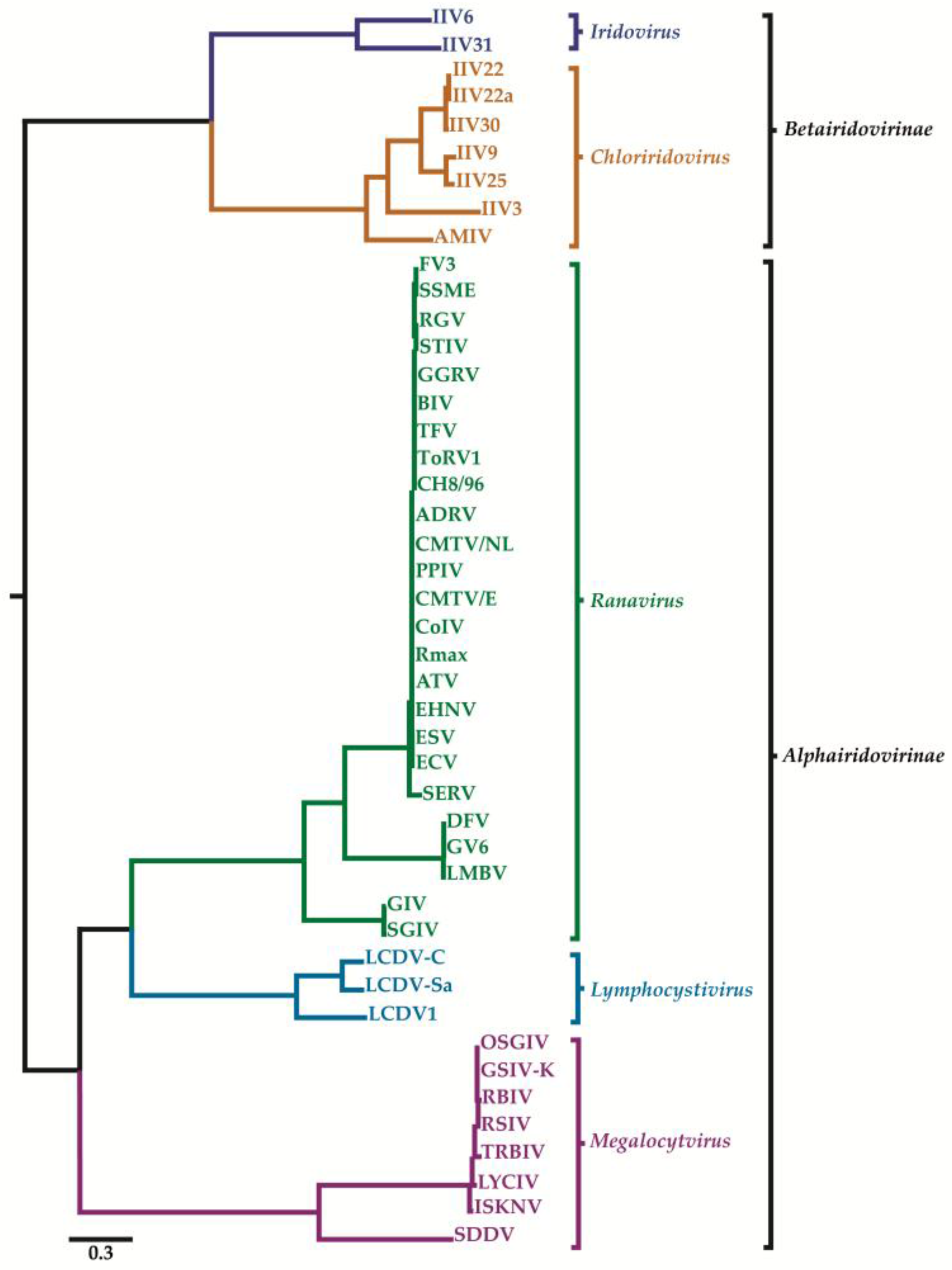
2. Classification of Iridovirids
3. Morphology and Composition
4. IIV-6 Persistence and Sensitivity to External Factors
5. Host Range and Pathology
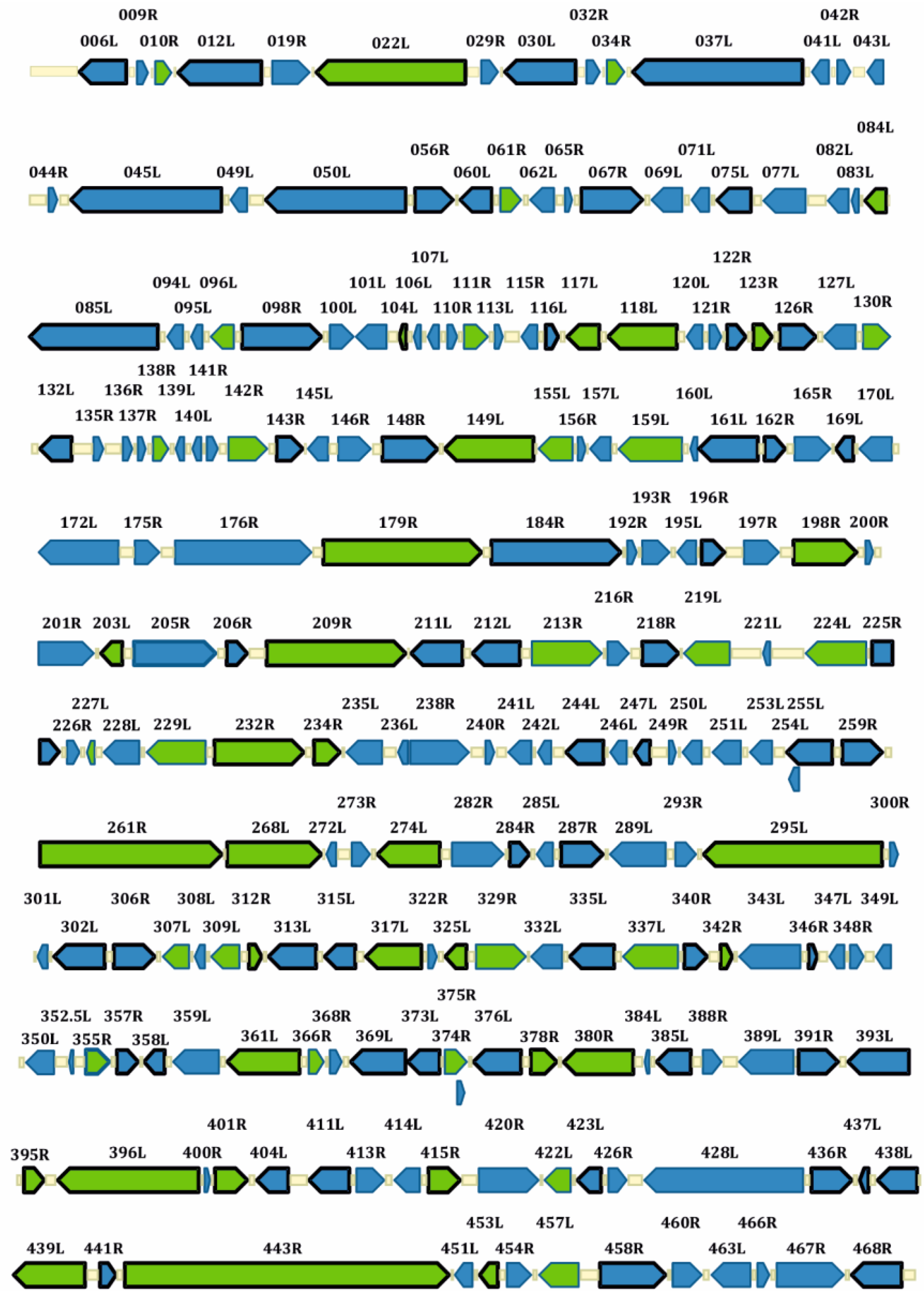
6. Genomic Organization and Codon Usage
7. Virion Proteins
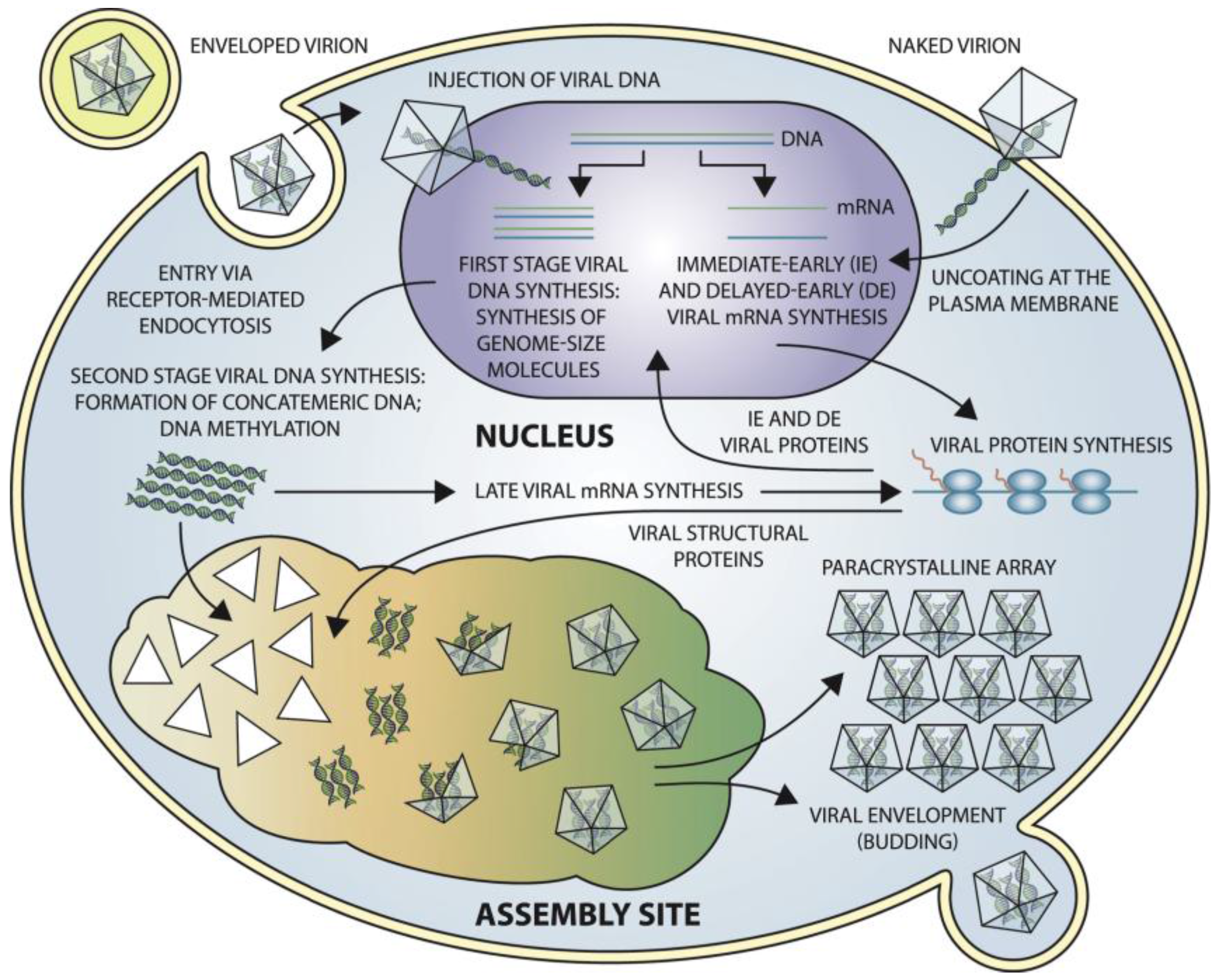
8. Viral Entry, Replication, and Release Strategy
9. Transcriptional Regulation
10. Promoter Elements and Transcription Initiation Sites
11. Induction/Inhibition of Apoptosis in Infections
12. Concluding Remarks
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; et al. ICTV Virus Taxonomy Profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Waltzek, T.B.; Subramaniam, K. Ranaviruses and other members of the family Iridoviridae: Their place in the virosphere. Virology 2017, 511, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.; Essbauer, S.; He, J.; Hyatt, A.; Miyazaki, T.; Seligy, V.; Williams, T. Family Iridoviridae. In Virus Taxonomy Eighth Report of the International Committee on Taxonomy of Viruses; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 145–162. [Google Scholar]
- King, A.M.; Lefkowitz, E.; Adams, M.J.; Carstens, E.B. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Xeros, N. A second virus disease of the leatherjacket, Tipula paludosa. Nature 1954, 174, 562–563. [Google Scholar] [CrossRef]
- Fukaya, M.; Nasu, S. An iridescent virus (CIV) from the rice stem borer, Chilo suppressalis Walker (Lepidoptera: Pyralidae). Appl. Entomol. Zool. 1966, 1, 69–72. [Google Scholar] [CrossRef]
- Jakob, N.J.; Muller, K.; Bahr, U.; Darai, G. Analysis of the first complete DNA sequence of an invertebrate iridovirus: Coding strategy of the genome of Chilo iridescent virus. Virology 2001, 286, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Yutin, N.; Pagnier, I.; Barrassi, L.; Fournous, G.; Espinosa, L.; Robert, C.; Azza, S.; Sun, S.; Rossmann, M.G.; et al. Giant Marseillevirus highlights the role of amoebae as a melting pot in emergence of chimeric microorganisms. Proc. Natl. Acad. Sci. USA 2009, 106, 21848–21853. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Piégu, B.; Guizard, S.; Yeping, T.; Cruaud, C.; Asgari, S.; Bideshi, D.K.; Federici, B.A.; Bigot, Y. Genome sequence of a crustacean iridovirus, IIV31, isolated from the pill bug, Armadillidium vulgare. J. Gen. Virol. 2014, 95, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Delhon, G.; Tulman, E.R.; Afonso, C.L.; Lu, Z.; Becnel, J.J.; Moser, B.A.; Kutish, G.F.; Rock, D.L. Genome of Invertebrate iridescent virus type 3 (mosquito iridescent virus). J. Virol. 2006, 80, 8439–8449. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Young, V.L.; Kleffmann, T.; Ward, V.K. Genomic and proteomic analysis of Invertebrate iridovirus type 9. J. Virol. 2011, 85, 7900–7911. [Google Scholar] [CrossRef] [PubMed]
- Piégu, B.; Guizard, S.; Spears, T.; Cruaud, C.; Couloux, A.; Bideshi, D.K.; Federici, B.A.; Bigot, Y. Complete genome sequence of invertebrate iridescent virus 22 isolated from a blackfly larva. J. Gen. Virol. 2013, 94, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Piégu, B.; Guizard, S.; Yeping, T.; Cruaud, C.; Couloux, A.; Bideshi, D.K.; Frederici, B.A.; Bigot, Y. Complete genome sequence of invertebrate iridovirus IIV22A, a variant of IIV22, isolated originally from a blackfly larva. Stand Genom. Sci. 2014, 9, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Piégu, B.; Guizard, S.; Spears, T.; Cruaud, C.; Couloux, A.; Bideshi, D.K.; Federici, B.A.; Bigot, Y. Complete genome sequence of invertebrate iridovirus IIV25 isolated from a blackfly larva. Arch. Virol. 2014, 159, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Piégu, B.; Guizard, S.; Spears, T.; Cruaud, C.; Couloux, A.; Bideshi, D.K.; Federici, B.A.; Bigot, Y. Complete genome sequence of invertebrate iridovirus IIV30 isolated from the corn earworm, Helicoverpa zea. J. Invertebr. Pathol. 2014, 116, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gui, J.; Gao, X.; Pei, C.; Hong, Y.; Zhang, Q. Genome architecture changes and major gene variations of Andrias davidianus ranavirus (ADRV). BMC Vet. Res. 2013, 44, 101. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Mao, J.; Chinchar, V.G.; Wyatt, C.; Case, S.T.; Kumar, S.; Valente, G.; Subramanian, S.; Davidson, E.W.; Collins, J.P.; et al. Genomic sequence of a ranavirus (family Iridoviridae) associated with salamander mortalities in North America. Virology 2003, 316, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Hick, P.M.; Subramaniam, K.; Thompson, P.; Whittington, R.J.; Waltzek, T.B. Complete genome sequence of a Bohle iridovirus isolate from ornate burrowing frogs (Limnodynastes ornatus) in Australia. Genome Announc. 2016, 4, e00632-16. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, A.C.; López-Bueno, A.; Blahak, S.; Caeiro, M.F.; Rosa, G.M.; de Matos, A.P.A.; Martel, A.; Alejo, A.; Marschang, R.E. Phylogeny and differentiation of reptilian and amphibian ranaviruses detected in Europe. PLoS ONE 2015, 10, e0118633. [Google Scholar] [CrossRef] [PubMed]
- Mavian, C.; López-Bueno, A.; Balseiro, A.; Casais, R.; Alcamí, A.; Alejo, A. The genome sequence of the emerging common midwife toad virus identifies an evolutionary intermediate within ranaviruses. J. Virol. 2012, 86, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Van Beurden, S.J.; Hughes, J.; Saucedo, B.; Rijks, J.; Kik, M.; Haenen, O.L.; Engelsma, M.Y.; Gröne, A.; Verheije, M.H.; Wilkie, G. Complete genome sequence of a common midwife toad virus-like ranavirus associated with mass mortalities in wild amphibians in The Netherlands. Genome Announc. 2014, 2, e01293-14. [Google Scholar] [CrossRef] [PubMed]
- Ariel, E.; Steckler, N.K.; Subramaniam, K.; Olesen, N.J.; Waltzek, T.B. Genomic sequencing of ranaviruses isolated from turbot (Scophthalmus maximus) and Atlantic cod (Gadus morhua). Genome Announc. 2016, 4, e01393-16. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Bremont, M.; Touchman, J.W.; Jacobs, B.L. Evidence for multiple recent host species shifts among the ranaviruses (Family Iridoviridae). J. Virol. 2010, 84, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Mavian, C.; López-Bueno, A.; Somalo, M.P.F.; Alcamí, A.; Alejo, A. Complete genome sequence of the European sheatfish virus. J. Virol. 2012, 86, 6365–6366. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.G.; Barkman, T.J.; Chinchar, V.G.; Essani, K. Comparative genomic analyses of Frog virus 3, type species of the genus Ranavirus (family Iridoviridae). Virology 2004, 323, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Ting, J.W.; Wu, M.H.; Wu, M.F.; Guo, C.; Chang, C.Y. Complete genome sequence of the grouper iridovirus and comparison of genomic organization with those of other iridoviruses. J. Virol. 2005, 79, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Davidson, E.W.; Parameswaran, N.; Mao, J.; Chinchar, V.G.; Collins, J.P.; Jacobs, B.L.; Storfer, A. Evidence for emergence of an amphibian iridoviral disease because of human-enhanced spread. Mol. Ecol. 2005, 14, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, R.; Subramaniam, K.; Steckler, N.K.; Claytor, S.C.; Ariel, E.; Waltzek, T.B. Genome sequence of a Ranavirus isolated from pike-perch Sander lucioperca. Genome Announc. 2016, 4, e01295-16. [Google Scholar] [CrossRef] [PubMed]
- Claytor, S.C.; Subramaniam, K.; Landrau-Giovannetti, N.; Chinchar, V.G.; Gray, M.J.; Miller, D.L.; Mavian, C.; Salemi, M.; Wisely, S.; Waltzek, T.B. Ranavirus phylogenomics: Signatures of recombination and inversions among bullfrog ranaculture isolates. Virology 2017, 511, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Ariel, E.; Subramaniam, K.; Imnoi, K.; Sriwanayos, P.; Ahasan, M.S.; Olesen, N.J.; Amedeo, M.; Toffan, A.; Waltzek, T.B. Genomic sequencing of ranaviruses isolated from edible frogs (Pelophylax esculentus). Genome Announc. 2017, 5, e01015-17. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.Y.; Ou, T.; Zhu, R.L.; Zhang, Q.Y. Sequencing and analysis of the complete genome of Rana grylio virus (RGV). Arch. Virol. 2012, 157, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Epstein, B.; Storfer, A. Comparative genomics of an emerging amphibian virus. G3 Genes Genomes Genet. 2016, 6, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; Toffan, A.; Cappellozza, E.; Steckler, N.K.; Olesen, N.J.; Ariel, E.; Waltzek, T.B. Genomic sequence of a ranavirus isolated from short-finned eel (Anguilla australis). Genome Announc. 2016, 4, e00843-16. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Qin, Q.W.; Qiu, J.; Huang, C.H.; Wang, F.; Hew, C.L. Functional genomics analysis of Singapore grouper iridovirus: Complete sequence determination and proteomic analysis. J. Virol. 2004, 78, 12576–12590. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.A.; Garner, S.; Echaubard, P.; Lesbarrères, D.; Kyle, C.J.; Brunetti, C.R. Complete genome analysis of a Frog virus 3 (FV3) isolate and sequence comparison with isolates of differing levels of virulence. Virol. J. 2014, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, X.; Liu, H.; Gong, J.; Ouyang, Z.; Cui, H.; Cao, J.; Zhao, Y.; Wang, X.; Jiang, Y. Complete sequence determination of a novel reptile iridovirus isolated from soft-shelled turtle and evolutionary analysis of Iridoviridae. BMC Genom. 2009, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- He, J.G.; Lü, L.; Deng, M.; He, H.H.; Weng, S.P.; Wang, X.H.; Zhou, S.Y.; Long, Q.X.; Wang, X.Z.; Chan, S.M. Sequence analysis of the complete genome of an iridovirus isolated from the tiger frog. Virology 2002, 292, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Tidona, C.A.; Darai, G. The complete DNA sequence of lymphocystis disease virus. Virology 1997, 230, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Xiao, F.; Xie, J.; Li, Z.Q.; Gui, J.F. Complete genome sequence of Lymphocystis disease virus isolated from China. J. Virol. 2004, 78, 6982–6994. [Google Scholar] [CrossRef] [PubMed]
- López-Bueno, A.; Mavian, C.; Labella, A.M.; Castro, D.; Borrego, J.J.; Alcami, A.; Alejo, A. Concurrence of iridovirus, polyomavirus, and a unique member of a new group of fish papillomaviruses in lymphocystis disease-affected gilthead Sea Bream. J. Virol. 2016, 90, 8768–8779. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Wen, C.M.; Wu, J.L.; Su, Y.C.; Hong, J.R. Giant seaperch iridovirus (GSIV) induces mitochondria-mediated cell death that is suppressed by bongkrekic acid and cycloheximide in a fish cell line. Virus Res. 2016, 213, 37–45. [Google Scholar] [CrossRef] [PubMed]
- He, J.G.; Deng, M.; Weng, S.P.; Li, Z.; Zhou, S.Y.; Long, Q.X.; Wang, X.Z.; Chan, S.M. Complete genome analysis of the mandarin fish infectious spleen and kidney necrosis iridovirus. Virology 2001, 291, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Kurita, J.; Nakajima, K. Megalocytiviruses. Viruses 2012, 4, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Lü, L.; Zhou, S.Y.; Chen, C.; Weng, S.P.; Chan, S.M.; He, J.G. Complete genome sequence analysis of an iridovirus isolated from the orange-spotted grouper, Epinephelus coioides. Virology 2005, 339, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Do, J.W.; Moon, C.H.; Kim, H.J.; Ko, M.S.; Kim, S.B.; Son, J.H.; Kim, J.S.; An, E.J.; Kim, M.K.; Lee, S.K.; et al. Complete genomic DNA sequence of Rock bream iridovirus. Virology 2004, 325, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kunita, J. Red sea bream iridoviral disease. Uirusu 2005, 55, 115–125. [Google Scholar] [CrossRef] [PubMed]
- De Groof, A.; Guelen, L.; Deijs, M.; van der Wal, Y.; Miyata, M.; Ng, K.S.; van Grinsven, L.; Simmelink, B.; Biermann, Y.; Grisez, L.; et al. A novel virus causes scale drop disease in Lates calcarifer. PLoS Pathog. 2015, 11, e1005074. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Jia, K.T.; Yang, B.; Huang, J. Complete genome sequence of a Megalocytivirus (family Iridoviridae) associated with turbot mortality in China. Virol. J. 2010, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.; Zhao, Q.; Pei, G.; An, X.; Guo, X.; Zhou, H.; Zhang, Z.; Zhang, J.; Tong, Y. Isolation and characterization of a novel invertebrate iridovirus from adult Anopheles minimus (AMIV) in China. J. Invertebr. Pathol. 2015, 127, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, M.M.; Wang, R.Y.; Wan, X.Y.; Li, C.; Zhang, Q.L.; Dong, X.; Yang, B.; Xiang, J.H.; Huang, J. Complete genome sequence of shrimp hemocyte iridescent virus (SHIV) isolated from white leg shrimp, Litopenaeus vannamei. Arch. Virol. 2018, 163, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Eaton, H.E.; Metcalf, J.; Penny, E.; Tcherepanov, V.; Upton, C.; Brunetti, C.R. Comparative genomic analysis of the family Iridoviridae: Re-annotating and defining the core set of iridovirus genes. Virol. J. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Lin, C.H.; Chang, C.Y. Analysis of codon usage bias and base compositional constraints in iridovirus genomes. Virus Res. 2007, 126, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Magrane, M.; Consortium, U. UniProt Knowledgebase: A hub of integrated protein data. Database J. Biol. Databases Curation Database 2011, 2011, Bar009. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Cory, J.S. Proposals for a new classification of iridescent viruses. J. Gen. Virol. 1994, 75, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Webby, R.; Kalmakoff, J. Sequence comparison of the major capsid protein gene from 18 diverse iridoviruses. Arch. Virol. 1998, 143, 1949–1966. [Google Scholar] [CrossRef] [PubMed]
- Webby, R.J.; Kalmakoff, J. Comparison of the major capsid protein genes, terminal redundancies, and DNA-DNA homologies of two New Zealand iridoviruses. Virus Res. 1999, 59, 179–189. [Google Scholar] [CrossRef]
- Muttis, E.; Miele, S.A.; Belaich, M.N.; Micieli, M.V.; Becnel, J.J.; Ghiringhelli, P.D.; Garcia, J.J. First record of a mosquito iridescent virus in Culex pipiens L. (Diptera: Culicidae). Arch. Virol. 2012, 157, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; De Lamballerie, X.; Yutin, N.; Asgari, S.; Bigot, Y.; Bideshi, D.K.; Cheng, X.W.; Federici, B.A.; Van Etten, J.L.; Koonin, E.V. “Megavirales”, a proposed new order for eukaryotic nucleocytoplasmic large DNA viruses. Arch. Virol. 2013, 158, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Qin, Q.; Zhang, Q.Y.; Chinchar, V.G. Ranavirus replication: Molecular, Cellular, and Immunological events. In Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates; Gray, M.J., Chinchar, V.G., Eds.; Springer: New York, NY, USA, 2015; pp. 105–139. [Google Scholar]
- Piégu, B.; Asgari, S.; Bideshi, D.; Federici, B.A.; Bigot, Y. Evolutionary relationships of iridoviruses and divergence of ascoviruses from invertebrate iridoviruses in the superfamily Megavirales. Mol. Phylogenet. Evol. 2015, 84, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tidona, C.A.; Schnitzler, P.; Kehm, R.; Darai, G. Is the major capsid protein of iridoviruses a suitable target for the study of viral evolution? Virus Genes 1998, 16, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Eaton, H.E.; Ring, B.A.; Brunetti, C.R. The genomic diversity and phylogenetic relationship in the family Iridoviridae. Viruses 2010, 2, 1458–1475. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Li, C.; Zhang, Q.L.; Wang, R.Y.; Cheng, D.Y.; Dong, X.; Yang, B.; Wang, X.H.; et al. Characterization of a new member of Iridoviridae, Shrimp hemocyte iridescent virus (SHIV), found in white leg shrimp (Litopenaeus vannamei). Sci. Rep. 2017, 7, 11834. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Olson, N.H.; Van Etten, J.L.; Bergoin, M.; Rossmann, M.G.; Baker, T.S. Structure and assembly of large lipid-containing dsDNA viruses. Nat. Struct. Mol. Biol. 2000, 7, 101–103. [Google Scholar]
- Yan, X.; Yu, Z.; Zhang, P.; Battisti, A.J.; Holdaway, H.A.; Chipman, P.R.; Bajaj, C.; Bergoin, M.; Rossmann, M.G.; Baker, T.S. The capsid proteins of a large, icosahedral dsDNA virus. J. Mol. Biol. 2009, 385, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Williams, T. Invertebrate iridescent viruses. In The Insect Viruses; Miller, L., Ball, A., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 31–68. [Google Scholar]
- Balange-Orange, N.; Devauchelle, G. Lipid composition of an Iridescent virus type 6 (CIV). Arch. Virol. 1982, 73, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Low, M.G. Glycosyl-phosphatidylinositol: A versatile anchor for cell surface proteins. FASEB J. 1989, 3, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Thompson, I. Fatty acid profiles of invertebrate iridescent viruses. Arch. Virol. 1995, 140, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- ViralZone. SIB Swiss Institute of Bioinformatics. Available online: www.expasy.org/viralzone (accessed on 28 March 2018).
- Tonka, T.; Weiser, J. Iridovirus infection in mayfly larvae. J. Invertebr. Pathol. 2000, 76, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Marina, C.F.; Feliciano, J.M.; Valle, J.; Williams, T. Effect of temperature, pH, ion concentration, and chloroform treatment on the stability of Invertebrate iridescent virus 6. J. Invertebr. Pathol. 2000, 75, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Christian, P.; Valle, J.; Williams, T. Persistence of Invertebrate iridescent virus 6 in soil. BioControl 2004, 49, 433–440. [Google Scholar] [CrossRef]
- Day, M.; Mercer, E. Properties of an iridescent virus from the beetle, Sericesthis pruinosa. Aust. J. Biol. Sci. 1964, 17, 892–902. [Google Scholar] [CrossRef]
- Martinez, G.; Christian, P.; Marina, C.; Williams, T. Sensitivity of Invertebrate iridescent virus 6 to organic solvents, detergents, enzymes and temperature treatment. Virus Res. 2003, 91, 249–254. [Google Scholar] [CrossRef]
- Hernandez, A.; Marina, C.F.; Valle, J.; Williams, T. Persistence of Invertebrate iridescent virus 6 in tropical artificial aquatic environments. Arch. Virol. 2005, 150, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Constantino, M.; Christian, P.; Marina, C.F.; Williams, T. A comparison of techniques for detecting Invertebrate iridescent virus 6. J. Virol. Methods 2001, 98, 109–118. [Google Scholar] [CrossRef]
- Wu, J.; Chan, R.; Wenk, M.R.; Hew, C.L. Lipidomic study of intracellular Singapore grouper iridovirus. Virology 2010, 399, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Williams, T. Natural invertebrate hosts of iridoviruses (Iridoviridae). Neotrop. Entomol. 2008, 37, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Federici, B. Isolation of an iridovirus from two terrestrial isopods, the pill bug, Armadillidium vulgare and the sow bug, Porcellio dilatatus. J. Invertebr. Pathol. 1980, 36, 373–381. [Google Scholar] [CrossRef]
- Karasawa, S.; Takatsuka, J.; Kato, J. Report on iridovirus IIV31 (Iridoviridae, Iridovirus) infecting terrestrial isopods (Isopoda, Oniscidea) in Japan. Crustaceana 2012, 85, 1269–1278. [Google Scholar] [CrossRef]
- Lupetti, P.; Montesanto, G.; Ciolfi, S.; Marri, L.; Gentile, M.; Paccagnini, E.; Lombardo, B.M. Iridovirus infection in terrestrial isopods from Sicily (Italy). Tissue Cell 2013, 45, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.L.; Longdon, B.; Lewis, S.H.; Obbard, D.J. Twenty-five new viruses associated with the Drosophilidae (Diptera). Evol. Bioinform. Online 2016, 12, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.R.; Latimer, K.S.; Pennick, K.E.; Benson, K.; Moore, T. Novel iridovirus in a nautilus (Nautilus spp.). J. Vet. Diagn. Investig. 2006, 18, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Devauchelle, G. Ultrastructural characterization of an iridovirus from the marine worm Nereis diversicolor (O. F. Müller) Architecture of the virion and virus morphogenesis. Virology 1977, 81, 237–246. [Google Scholar] [CrossRef]
- Hunter, W.; Lapointe, S.; Sinisterra, X.; Achor, D.; Funk, C. Iridovirus in the root weevil Diaprepes abbreviatus. J. Insect Sci. 2003, 3, 1–6. [Google Scholar] [CrossRef][Green Version]
- Hernandez, O.; Maldonado, G.; Williams, T. An epizootic of patent iridescent virus disease in multiple species of blackflies in Chiapas, Mexico. Med. Vet. Entomol. 2000, 14, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Marina, C.F.; Arredondo-Jimenez, J.; Castillo, A.; Williams, T. Sublethal effects of iridovirus disease in a mosquito. Oecologia 1999, 119, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Marina, C.F.; Ibarra, J.E.; Arredondo-Jimenez, J.I.; Fernandez-Salas, I.; Valle, J.; Williams, T. Sublethal iridovirus disease of the mosquito Aedes aegypti is due to viral replication not cytotoxicity. Med. Vet. Entomol. 2003, 17, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Williams, T. Covert iridovirus infection of blackfly larvae. Proc. R. Soc. Lond. B 1993, 251, 225–230. [Google Scholar] [CrossRef]
- Williams, T. Patterns of covert infection by invertebrate pathogens: Iridescent viruses of blackflies. Mol. Ecol. 1995, 4, 447–458. [Google Scholar] [CrossRef]
- Williams, T.; Valle, J.; Viñuela, E. Is the naturally-derived insecticide Spinosad compatible with insect natural enemies? Biocontrol. Sci. Technol. 2003, 13, 459–475. [Google Scholar] [CrossRef]
- Bromenshenk, J.J.; Henderson, C.B.; Wick, C.H.; Stanford, M.F.; Zulich, A.W.; Jabbour, R.E.; Deshpande, S.V.; McCubbin, P.E.; Seccomb, R.A.; Welch, P.M.; et al. Iridovirus and microsporidian linked to honey bee colony decline. PLoS ONE 2010, 5, e13181. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, R.; Firth, C.; Street, C.; Cox-Foster, D.L.; Lipkin, W.I. Lack of evidence for an association between Iridovirus and colony collapse disorder. PLoS ONE 2011, 6, e21844. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.J. Interpretation of data underlying the link between colony collapse disorder (CCD) and an invertebrate iridescent virus. Mol. Cell Proteom. 2011, 10, M110.006387. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.J.; Hunter, W.B.; Achor, D.S. Replication of insect iridescent virus 6 in a whitefly cell line. J. Invertebr. Pathol. 2001, 77, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.B.; Patte, C.P.; Sinisterra, X.H.; Achor, D.S.; Funk, C.J.; Polston, J.E. Discovering new insect viruses: Whitefly iridovirus (Homoptera: Aleyrodidae: Bemisia tabaci). J. Invertebr. Pathol. 2001, 78, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.; Johnson, C.; Lodhi, S.; Bilimoria, S. Replication of Chilo iridescent virus in the cotton boll weevil, Anthonomus grandis, and development of an infectivity assay. Arch. Virol. 2001, 146, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Belloncik, S.; Petcharawan, O.; Couillard, M.; Charpentier, G.; Larue, B.; Guardado, H.; Chareonsak, S.; Imanishi, S. Development and characterization of a continuous cell line, AFKM-On-H, from hemocytes of the European corn borer Ostrinia nubilalis (Hübner) (Lepidoptera, Pyralidae). In Vitro Cell. Dev. Biol. Anim. 2007, 43, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Just, F.; Essbauer, S.; Ahne, W.; Blahak, S. Occurrence of an invertebrate iridescent-like virus (Iridoviridae) in reptiles. J. Vet. Med. B 2001, 48, 685–694. [Google Scholar] [CrossRef]
- Weinmann, N.; Papp, T.; Pedro Alves de Matos, A.; Teifke, J.P.; Marschang, R.E. Experimental infection of crickets (Gryllus bimaculatus) with an invertebrate iridovirus isolated from a high-casqued chameleon (Chamaeleo hoehnelii). J. Vet. Diagn. Investig. 2007, 19, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Papp, T.; Spann, D.; Marschang, R.E. Development and use of a real-time polymerase chain reaction for the detection of group II invertebrate iridoviruses in pet lizards and prey insects. J. Zoo Wildl. Med. 2014, 45, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E. Viruses infecting reptiles. Viruses 2011, 3, 2087–2126. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, A.C.; Papp, T.; Marschang, R.E. Repeated detection of an invertebrate iridovirus in amphibians. J. Herpetol. Med. Surg. 2016, 26, 54–58. [Google Scholar] [CrossRef]
- McIntosh, A.; Kimura, M. Replication of the insect Chilo iridescent virus (CIV) in a poikilothermic vertebrate cell line. Intervirology 1974, 4, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Ohba, M.; Aizawa, K. Lethal toxicity of arthropod iridoviruses to an amphibian Rana limnocharis. Arch. Virol. 1981, 68, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Ohba, M.; Aizawa, K. Mammalian toxicity of an insect iridovirus. Acta Virol. 1982, 26, 165–168. [Google Scholar] [PubMed]
- Boucias, D.G.; Maruniak, J.E.; Pendland, J.C. Characterization of an iridovirus from the southern mole cricket, Scapteriscus vicinus. J. Invertebr. Pathol. 1987, 50, 238–245. [Google Scholar] [CrossRef]
- Fowler, H.G. Natural microbial control of cricket populations (Orthoptera: Gryllotalpidae: Scapteriscus borelli): Regulation of populations aggregated in time and space. Rev. Brasil. Biol. 1989, 49, 1039–1051. [Google Scholar] [PubMed]
- Kleespies, R.G.; Tidona, C.A.; Darai, G. Characterization of a new iridovirus isolated from crickets and investigations on the host range. J. Invertebr. Pathol. 1999, 73, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Just, F.; Essbauer, S. Characterization of an iridescent virus isolated from Gryllus bimaculatus (Orthoptera: Gryllidae). J. Invertebr. Pathol. 2001, 77, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.W.; van Cleef, K.W.; Venselaar, H.; van Rij, R.P. A dsRNA-binding protein of a complex invertebrate DNA virus suppresses the Drosophila RNAi response. Nucleic Acids Res. 2014, 42, 12237–12248. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.W.; van Cleef, K.W.; Vodovar, N.; İnce, İ.A.; Blanc, H.; Vlak, J.M.; Saleh, M.C.; van Rij, R.P. The DNA virus Invertebrate iridescent virus 6 is a target of the Drosophila RNAi machinery. Proc. Natl. Acad. Sci. USA 2012, 109, E3604–E3613. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.; Mueller, S.; Goto, A.; Barbier, V.; Paro, S.; Bonnay, F.; Dostert, C.; Troxler, L.; Hetru, C.; Meignin, C.; et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J. Immunol. 2013, 190, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.M.; Kochinke, K.; Oortveld, M.A.; Marks, H.; Kramer, D.; de Jong, E.K.; Asztalos, Z.; Westwood, J.T.; Stunnenberg, H.G.; Sokolowski, M.B.; et al. Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol. 2011, 9, e1000569. [Google Scholar] [CrossRef] [PubMed]
- Merkling, S.H.; Bronkhorst, A.W.; Kramer, J.M.; Overheul, G.J.; Schenck, A.; Van Rij, R.P. The epigenetic regulator G9a mediates tolerance to RNA virus infection in Drosophila. PLoS Pathog. 2015, 11, e1004692. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, O.; Arnold, J.; de Faria, I.J.D.S.; Olmo, R.P.; Bergami, F.; Meignin, C.; Hoffmann, J.A.; Marques, J.T.; Imler, J.L. Analysis of the contribution of hemocytes and autophagy to Drosophila antiviral immunity. J. Virol. 2016, 90, 5415–5426. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, L.R.; Bastos, R.G.; Hiroyasu, A.; Goodman, A.G. Invertebrate iridescent virus 6, a DNA virus, stimulates a mammalian innate immune response through RIG-I-like receptors. PLoS ONE 2016, 11, e0166088. [Google Scholar] [CrossRef] [PubMed]
- Delius, H.; Darai, G.; Flügel, R. DNA analysis of Insect iridescent virus 6: Evidence for circular permutation and terminal redundancy. J. Virol. 1984, 49, 609–614. [Google Scholar] [PubMed]
- Fischer, M.; Schnitzler, P.; Delius, H.; Rösen-Wolff, A.; Darai, G. Molecular biology of insect iridescent virus type 6. In Molecular Biology of Iridoviruses; Springer: Boston, MA, USA, 1990; pp. 47–80. [Google Scholar]
- İnce, İ.A.; Özcan, K.; Vlak, J.M.; van Oers, M.M. Temporal classification and mapping of non-polyadenylated transcripts of an invertebrate iridovirus. J. Gen. Virol. 2013, 94, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.B.; Granoff, A. Frog virus 3 DNA is heavily methylated at CpG sequences. Virology 1980, 107, 250–257. [Google Scholar] [CrossRef]
- İnce, İ.A.; Boeren, S.; van Oers, M.M.; Vlak, J.M. Temporal proteomic analysis and label-free quantification of viral proteins of an invertebrate iridovirus. J. Gen. Virol. 2015, 96, 196–205. [Google Scholar] [CrossRef] [PubMed]
- İnce, İ.A.; Boeren, S.A.; van Oers, M.M.; Vervoort, J.J.; Vlak, J.M. Proteomic analysis of Chilo iridescent virus. Virology 2010, 405, 253–258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nalçacıoğlu, R.; Ince, I.A.; Demirbağ, Z. The biology of Chilo iridescent virus. Virol. Sin. 2009, 24, 285–294. [Google Scholar] [CrossRef]
- Jacob, T.; Van den Broeke, C.; Favoreel, H.W. Viral serine/threonine protein kinases. J. Virol. 2011, 85, 1158–1173. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Seligy, V.L. Characterization of iridovirus IV1 polypeptides: Mapping by surface labelling. Res. Virol. 1997, 148, 239–250. [Google Scholar] [CrossRef]
- Barray, S.; Devauchelle, G. Study of the structural polypeptides of Chilo suppressalis iridescent virus (Iridovirus type 6). Can. J. Microbiol. 1979, 25, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Barray, S.; Devauchelle, G. Protein synthesis in cells infected by Chilo iridescent virus (iridovirus, type 6). Arch. Virol. 1985, 86, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.C.; Tinsley, T.W. The proteins of Iridescent virus type 2 and 6. J. Invertebr. Pathol. 1972, 19, 273–274. [Google Scholar] [CrossRef]
- Elliott, R.M.; Lescott, T.; Kelly, D.C. Serological relationships of an iridescent virus (type 25) recently isolated from Tipula sp. with two other iridescent viruses (types 2 and 22). Virology 1977, 81, 309–316. [Google Scholar] [CrossRef]
- Petit, F.; Devauchelle, G. Modifications in the phosphorylation of ribosomal proteins and ribosome-associated proteins in invertebrate cells infected with iridovirus type 6. Ann. Inst. Pasteur Virol. 1986, 137, 205–214. [Google Scholar] [CrossRef]
- Chitnis, N.S.; Paul, E.R.; Lawrence, P.K.; Henderson, C.W.; Ganapathy, S.; Taylor, P.V.; Virdi, K.S.; D’Costa, S.M.; May, A.R.; Bilimoria, S.L. A virion associated protein kinase induces apoptosis. J. Virol. 2011, 85, 13144–13152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Granoff, A. Frog virus 3: A DNA virus with an unusual life-style. Prog. Med. Virol. 1984, 30, 187–198. [Google Scholar] [PubMed]
- Wang, S.; Huang, X.; Huang, Y.; Hao, X.; Xu, H.; Cai, M.; Wang, H.; Qin, Q. Entry of a novel marine DNA virus, Singapore grouper iridovirus, into host cells occurs via clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner. J. Virol. 2014, 88, 13047–13063. [Google Scholar] [CrossRef] [PubMed]
- Clout, N.J.; Tisi, D.; Hohenester, E. Novel fold revealed by the structure of a FAS1 domain pair from the insect cell adhesion molecule fasciclin I. Structure 2003, 11, 197–203. [Google Scholar] [CrossRef]
- Braunwald, J.; Nonnenmacher, H.; Tripier-Darcy, F. Ultrastructural and biochemical study of frog virus 3 uptake by BHK-21 cells. J. Gen. Virol. 1985, 66, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Barray, S.; Devauchelle, G. Protein synthesis in cells infected by Chilo iridescent virus: Evidence for temporal control of three classes of induced polypeptides. Ann. l’Inst. Pasteur/Virol. 1987, 138, 253–261. [Google Scholar] [CrossRef]
- D’Costa, S.M.; Yao, H.J.; Bilimoria, S.L. Transcription and temporal cascade in Chilo iridescent virus infected cells. Arch. Virol. 2001, 146, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, S.M.; Yao, H.J.; Bilimoria, S.L. Transcriptional mapping in Chilo iridescent virus infections. Arch. Virol. 2004, 149, 723–742. [Google Scholar] [CrossRef] [PubMed]
- Goorha, R. Frog virus 3 DNA replication occurs in two stages. J. Virol. 1982, 43, 519–528. [Google Scholar] [PubMed]
- Wang, F.; Bi, X.; Chen, L.M.; Hew, C.L. ORF018R, a highly abundant virion protein from Singapore grouper iridovirus, is involved in serine/threonine phosphorylation and virion assembly. J. Gen. Virol. 2008, 89, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tran, B.N.; Wang, F.; Ounjai, P.; Wu, J.; Hew, C.L. Visualization of assembly intermediates and budding vacuoles of Singapore grouper iridovirus in Grouper embryonic cells. Sci. Rep. 2016, 6, 18696. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 2008, 320, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Orange-Balangé, N.; Devauchelle, G. Effect of infection with iridovirus type 6 on invertebrate cell phospholipids. Ann. Inst. Pasteur Virol. 1982, 133, 205–214. [Google Scholar] [CrossRef]
- Cerutti, M.; Cerutti, P.; Devauchelle, G. Infectivity of vesicles prepared from Chilo iridescent virus inner membrane: Evidence for recombination between associated DNA fragments. Virus Res. 1989, 12, 299–313. [Google Scholar] [CrossRef]
- Nalcacioglu, R.; Ince, I.A.; Vlak, J.M.; Demirbag, Z.; van Oers, M.M. The Chilo iridescent virus DNA polymerase promoter contains an essential AAAAT motif. J. Gen. Virol. 2007, 88, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Nalçacioğlu, R.; Marks, H.; Vlak, J.M.; Demirbağ, Z.; van Oers, M.M. Promoter analysis of the Chilo iridescent virus DNA polymerase and major capsid protein genes. Virology 2003, 317, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Dizman, Y.A.; Demirbag, Z.; Ince, I.A.; Nalcacioglu, R. Transcriptomic analysis of Chilo iridescent virus immediate early promoter. Virus Res. 2012, 167, 353–357. [Google Scholar] [CrossRef] [PubMed]
- İnce, İ.A.; Pijlman, G.P.; Vlak, J.M.; van Oers, M.M. Hairpin structures with conserved sequence motifs determine the 3’ ends of non-polyadenylated invertebrate iridovirus transcripts. Virology 2017, 511, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. Host defense, viruses and apoptosis. Cell Death Differ. 2001, 8, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, N.S.; D’Costa, S.M.; Paul, E.R.; Bilimoria, S.L. Modulation of iridovirus-induced apoptosis by endocytosis, early expression, JNK, and apical caspase. Virology 2008, 370, 333–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Everett, H.; McFadden, G. Viruses and apoptosis: Meddling with mitochondria. Virology 2001, 288, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Razvi, E.S.; Welsh, R.M. Apoptosis in viral infections. Adv. Virus Res. 1995, 45, 1–60. [Google Scholar] [PubMed]
- İnce, İ.A.; Westenberg, M.; Vlak, J.M.; Demirbağ, Z.; Nalçacioğlu, R.; van Oers, M.M. Open reading frame 193R of Chilo iridescent virus encodes a functional inhibitor of apoptosis (IAP). Virology 2008, 376, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.R.; Chitnis, N.S.; Henderson, C.W.; Kaul, R.J.; D’Costa, S.M.; Bilimoria, S.L. Induction of apoptosis by iridovirus virion protein extract. Arch. Virol. 2007, 152, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, A.; Muratoglu, H.; Demirbag, Z.; Vlak, J.M.; van Oers, M.M.; Nalcacioglu, R. Construction and characterization of a recombinant invertebrate iridovirus. Virus Res. 2014, 189, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Nalcacioğlu, R.; Muratoglu, H.; Yeşilyurt, A.; van Oers, M.; Vlak, J.M.; Demirbağ, Z. Enhanced insecticidal activity of Chilo iridescent virus expressing an insect specific neurotoxin. J. Invertebr. Pathol. 2016, 138, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Barbosa-Solomieu, V.; Chinchar, V.G. A decade of advances in iridovirus research. Adv. Virus Res. 2005, 65, 173–248. [Google Scholar] [PubMed]
- Ricou, G. Production de Tipula paludosa Meig. en prarie en function de l’humiditié du sol. Revue d’Ecologie et de Biologie du Sol. 1975, 12, 69–89. [Google Scholar]
- Fukuda, T. Per os transmission of Chilo iridescent virus to Mosquitoes. J. Invertebr. Pathol. 1971, 18, 152–153. [Google Scholar] [CrossRef]
- Ohba, M.; Aizawa, K. Multiplication of Chilo iridescent virus in noninsect arthropods. J. Invertebr. Pathol. 1979, 33, 278–283. [Google Scholar] [CrossRef]
- Andino, R.; Domingo, E. Viral quasispecies. Virology 2015, 479–480, 46–51. [Google Scholar] [CrossRef] [PubMed]


| Sub-Qualification | Genus | Virion Size | Hosts | GC Content | DNA Methylation |
|---|---|---|---|---|---|
| Betairidovirinae | Iridovirus | 120–130 nm | arthropods, particularly insects | 29–32% | absent |
| Betairidovirinae | Chloriridovirus | 180 nm | Diptera with aquatic larval stages, mainly mosquitoes | 48% | absent |
| Alphairidovirinae | Ranavirus | 150 nm | Reptilia, Amphibia and Osteichthyes | 54% | present |
| Alphairidovirinae | Lymphocystivirus | 198–227 nm | flounder, plaice, and dab | 29.1–33% | present |
| Alphairidovirinae | Megalocytivirus | 140–200 nm | sea fish | 54.8% | present |
| Genus (Subfamily) | Species, Strains, and Isolates | Size (bp) | ORFs | G + C % | GenBank Accession Number | References |
|---|---|---|---|---|---|---|
| Iridovirus | IIV-6 | 212,482 | 215 * | 28.63 | AF303741 | [7] |
| IIV-31 | 220,222 | 203 | 35.09 | HF920637 | [11] | |
| Chloriridovirus | IIV-3 | 191,132 | 126 | 48 | DQ643392 | [12] |
| IIV-9 | 205,791 | 191 | 31 | GQ918152 | [13] | |
| IIV-22 | 197,693 | 167 | 28.05 | HF920633 | [14] | |
| IIV-22A | 196,456 | 174 | 28 | HF920634 | [15] | |
| IIV-25 | 204,815 | 177 | 30.32 | HF920635 | [16] | |
| IIV-30 | 198,533 | 177 | 28.1 | HF920636 | [17] | |
| IIV-3 | 191,132 | 126 | 48 | DQ643392 | [12] | |
| Ranavirus | ADRV | 106,734 | 101 | 55 | KC865735 | [18] |
| ATV | 106,332 | 96 | 54 | AY150217 | [19] | |
| BIV-ME | 103,531 | 100 | 55.2 | KX185156 | [20] | |
| CGSI–HN1104 | 105,375 | 111 | 55.2 | KF512820 | Unpublished | |
| CH8/96 | 105,811 | 75 | 55 | KP266741 | [21] | |
| CMTV/2008/E | 106,878 | 104 | 55.3 | JQ231222 | [22] | |
| CMTV/2013/NL | 107,772 | 104 | 55.3 | KP056312 | [23] | |
| CodV | 114,865 | 98 | 54.9 | KX574342 | [24] | |
| DFV | ND | ND | ND | ND | Unpublished | |
| ECV-13051/2012 | 127,751 | 135 | 54.2 | KT989884 | Unpublished | |
| ECV-14612/12 | 127,549 | 136 | 54 | KT989885 | Unpublished | |
| EHNV | 127,011 | 100 | 54.05 | NC028461 | [25] | |
| ESV | 127,732 | 136 | 54.23 | JQ724856 | [26] | |
| FV3 | 105,903 | 98 | 55 | AY548484 | [27] | |
| GGRV | 103,681 | 73 | 55 | KP266742 | [21] | |
| GIV | 139,793 | 120 | 49 | AY666015 | [28] | |
| ATV-GUFFY | 106,437 | 99 | 54 | KR075882 | [29] | |
| GV6 | ND | ND | ND | ND | Unpublished | |
| LMBV | ND | ND | ND | ND | Unpublished | |
| PPIV | 108,041 | 109 | 55.3 | KX574341 | [30] | |
| RCV-Z | 106,890 | 98 | 55 | MF187210 | [31] | |
| REV 282/102 | 107,444 | 101 | 55.2 | MF538628 | [32] | |
| RGV | 105,791 | 106 | 55 | JQ654586 | [33] | |
| Rmax | 115,510 | 100 | 54.9 | KX574343 | [24] | |
| ATV-RRV | 106,971 | 102 | 54.1 | KR075879 | [34] | |
| SERV | 126,965 | 111 | 54.7 | KX353311 | [35] | |
| SGIV | 140,131 | 162 | 48.64 | AY521625 | [36] | |
| SSME | 105,070 | 95 | 55 | KJ175144 | [37] | |
| STIV | 105,890 | 105 | 55.1 | EU627010 | [38] | |
| TFV | 105,057 | 105 | 55.01 | AF389451 | [39] | |
| ToRV-1 | 103,876 | 76 | 55 | KP266743 | [21] | |
| Lymphocystivirus | LCDV-1 | 102,653 | 108 | 29.1 | NC_00182 | [40] |
| LCDV-C | 186,250 | 239 | 27.2 | AY380826 | [41] | |
| LCDV-Sa | 208,501 | 183 | 33 | KX643370 | [42] | |
| Megalocytivirus | GSIV-K1 | 112,565 | 135 | 53.02 | KT804738 | [43] |
| ISKNV | 111,362 | 125 | 54.8 | AF371960 | [44] | |
| LYCIV | 111,760 | ND | 53.92 | AY779031 | [45] | |
| OSGIV | 112,636 | 126 | 54 | AY894343 | [46] | |
| RBIV-KOR-TY1 | 112,080 | 116 | 53 | AY532606 | [47] | |
| RSIV | 112,415 | 93 | 54 | AB104413 | [48] | |
| SDDV | 124,244 | 129 | 37 | KR139659 | [49] | |
| TRBIV | 110,104 | 115 | 55 | GQ273492 | [50] | |
| Unclassified | AMIV | 163,023 | 148 | 39 | KF938901 | [51] |
| SHIV | 165,809 | 170 | 34.6 | MF599468 | [52] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
İnce, İ.A.; Özcan, O.; Ilter-Akulke, A.Z.; Scully, E.D.; Özgen, A. Invertebrate Iridoviruses: A Glance over the Last Decade. Viruses 2018, 10, 161. https://doi.org/10.3390/v10040161
İnce İA, Özcan O, Ilter-Akulke AZ, Scully ED, Özgen A. Invertebrate Iridoviruses: A Glance over the Last Decade. Viruses. 2018; 10(4):161. https://doi.org/10.3390/v10040161
Chicago/Turabian Styleİnce, İkbal Agah, Orhan Özcan, Ayca Zeynep Ilter-Akulke, Erin D. Scully, and Arzu Özgen. 2018. "Invertebrate Iridoviruses: A Glance over the Last Decade" Viruses 10, no. 4: 161. https://doi.org/10.3390/v10040161
APA Styleİnce, İ. A., Özcan, O., Ilter-Akulke, A. Z., Scully, E. D., & Özgen, A. (2018). Invertebrate Iridoviruses: A Glance over the Last Decade. Viruses, 10(4), 161. https://doi.org/10.3390/v10040161







