Phytohormone Signaling of the Resistance to Plum pox virus (PPV, Sharka Disease) Induced by Almond (Prunus dulcis (Miller) Webb) Grafting to Peach (P. persica L. Batsch)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plum pox virus (PPV) Isolate
2.3. Evaluation of Sharka Symptoms and PPV Detection
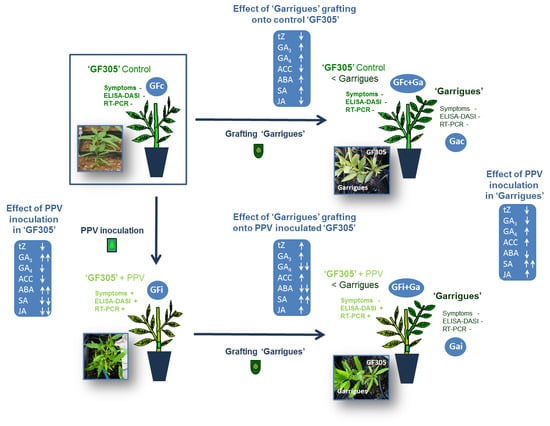
2.4. Experimental Design
2.5. Phytohormone Analysis
3. Results
3.1. Evaluation of Sharka Symptoms and PPV Detection
3.2. Growth-Related Phytohormones
3.3. Stress-Related Phytohormones
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- García, J.A.; Glasa, M.; Cambra, M.; Candresse, T. Plum pox virus and sharka: A model potyvirus and a major disease. Mol. Plant Pathol. 2014, 15, 226–241. [Google Scholar] [CrossRef] [PubMed]
- European and Mediterranean Plant Protection Organization (EPPO). Available online: https://www.eppo.int/ (accessed on 20 February 2018).
- Sihelská, N.; Glasa, M.; Šubr, Z.W. Host preference of the major strains of Plum pox virus—Opinions based on regional and world-wide sequence data. J. Integr. Agric. 2017, 16, 510–515. [Google Scholar] [CrossRef]
- Rubio, M.; Martínez-Gómez, P.; Pascal, T.; Dicenta, F. Sensitivity of peach cultivars against a Dideron isolate of Plum pox virus. Sci. Hortic. 2012, 144, 81–86. [Google Scholar] [CrossRef]
- Cirilli, M.; Geuna, F.; Babini, A.O.; Bozhkova, V.; Catalano, L.; Cavagna, B.; Dallot, S.; Decroocq, V.; Dondini, L.; Foschi, S.; et al. Fighting sharka in peach: Current limitations and future prospects. Front. Plant Sci. 2016, 7, 1290. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, M.; Rossini, L.; Geuna, F.; Palmisano, F.; Minafra, A.; Castrignanò, T.; Gattolin, S.; Ciacciulli, A.; Babini, A.R.; Liverani, A.; et al. Genetic dissection of sharka disease tolerance in peach (P. persica L. Batsch). BMC Plant Biol. 2017, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; Martínez-Gómez, P.; Dicenta, F. Resistance of almond cultivars to Plum pox virus (sharka). Plant Breed. 2003, 122, 462–464. [Google Scholar] [CrossRef]
- Rubio, M.; Martínez-Gómez, P.; García, J.A.; Dicenta, F. Interspecific transfer of resistance to Plum pox virus from almond to peach by grafting. Ann. Appl. Biol. 2013, 163, 466–474. [Google Scholar]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Studhan, M.E.; Maclntosh, G.C. Phytohormone signaling pathway analysis method for comparing hormone responses in plant-pest interactions. BMC Res. Notes 2012, 5, 392. [Google Scholar]
- O’Brien, J.A.; Benková, E. Cytokinincross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; García-Marcos, A.; Manzano, A.; García de Lacoba, M.; Camañes, G.; García-Agustín, P.; Díaz-Ruiz, J.R.; Tenllado, F. Comparative Analysis of Transcriptomic and Hormonal Responses to Compatible and Incompatible Plant-Virus Interactions that Lead to Cell Death. Mol. Plant-Microbe Int. 2012, 25, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N.S. Roles of plant hormones in the regulation of host–virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Volko, S.M.; Ledford, H.; Ausubel, F.M.; Dong, X. Roles of Salicylic Acid, Jasmonic Acid, and Ethylene in CPR-Induced Resistance in Arabidopsis. Plant Cell 2014, 12, 2175–2190. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone Crosstalk in Plant Disease and Defense: More Than Just JASMONATE-SALICYLATE Antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Yand, Y.X.; Ahammed, G.J.; Wu, C.; Fan, S.; Zhou, Y.H. Crosstalk among Jasmonate, Salicylate and Ethylene Signaling Pathways in Plant Disease and Immune Responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar]
- Pieterse, C.M.J.; van der Does, D.; Zamioudis, C.; León-Reyes, A.; van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Collum, T.D.; Culver, J. The impact of phytohormones on virus infection and disease. Curr. Opin. Virol. 2016, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pustovoitova, T.N.; Eremin, G.V.; Rassvetaeva, E.G.; Zhdanova, N.E.; Zholkevich, V.N. Drought resistance, recovery capacity, and phytohormone content in polyploid plum leaves. Russ. J. Plant Physiol. 1996, 43, 232–235. [Google Scholar]
- Pérez-Jiménez, M.; Cantero-Navarro, E.; Pérez-Alfocea, F.; Cos-Terrer, J. Endogenous hormones response to cytokinins with regard to organogenesis in explants of peach (Prunus persica L. Batsch) cultivars and rootstocks (P. persica x Prunus dulcis). Plant Physiol. Biochem. 2014, 84, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, M.; Cantero-Navarro, E.; Pérez-Alfocea, F.; Le-Disqet, I.; Guivar’h, A.; Cos-Terrer, J. Relationship between endogenous hormonal content and somatic organogenesis in callus of peach (Prunus persica L. Batsch) cultivars and Prunus persica x Prunus dulcis rootstocks. J. Plant Physiol. 2014, 171, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, I.A.; López-Ortega, G.; Burow, M.; Bayo-Canha, A.; Junge, A.; Gericke, O.; Moller, B.L.; Sánchez-Pérez, R. Transcriptome and Metabolite Changes during Hydrogen Cyanamide-Induced Floral Bud Break in Sweet Cherry. Front. Plant Sci. 2017, 8, 1233. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Manfra, M.; De Nisco, M.; Tenore, G.; Troisi, J.; Di Fiori, R.; Novellino, E. Trichoderma harzianum strain T-22 induces changes in phytohormone levels in cherry rootstocks (Prunus cerasus x P. canescens). Plant Growth Regul. 2011, 65, 421–425. [Google Scholar] [CrossRef]
- Bernhard, R.; Marénaud, C.; Sutic, D. Le pêcher GF305 indicateur polyvalent des virus des espèces à noyaux. Ann. Phytopathol. 1969, 1, 603–617. [Google Scholar]
- Rubio, M.; García-Ibarra, A.; Martínez-Gómez, P.; Dicenta, F. Analysis of the main factors involved in the evaluation of Prunus resistance to Plum pox virus (Sharka) in control greenhouse conditions. Sci. Hortic. 2009, 123, 46–50. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andujar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Martínez-Andújar, M.E.; Ghanem, M.E.; Acosta, M.; Sánchez-Bravo, J.; Asins, M.J.; Cuarter, J.; Lutts, I.C.; Dodd, I.C.; Pérez-Alfocea, F. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant Cell Environ. 2009, 32, 958–938. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Dong, X. How do plants achieved immunity? Defense without specialized immune cells. Nat. Rev. 2012, 12, 89–100. [Google Scholar]
- Vallad, G.E.; Goodman, R.M. Systemic acquired resistance and Induced systemic resistance in conventional agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Kegler, H.; Grüntzig, M. The resistance of the plum hybrid k4 and its progenies to Plum pox virus. Acta Hort. 1992, 309, 229–234. [Google Scholar] [CrossRef]
- Hartmann, W.; Petruschke, M. Sharka resistant plums and prunes by utilization of hypersensitivity. Acta Hort. 2000, 538, 391–395. [Google Scholar]
- Hartmann, W.; Neumüller, M. Plum Breeding. In Breeding Plantation Tree Crops: Temperate Species Plum Breeding; Springer: New York, NY, USA, 2009. [Google Scholar]
- Zuriaga, E.; Soriano, J.M.; Zhebentyayeva, T.; Romero, C.; Dardick, C.; Cañizares, J.; Badenes, M.L. Genomic analysis reveals MATH gene(s) as candidate(s) for plum pox virus (PPV) resistance in apricot (Prunus armeniaca L.). Mol. Plant Pathol. 2013, 14, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; Ballester, A.R.; Olivares, P.M.; Castro, M.; Dicenta, F.; Martínez-Gómez, P. Gene expression analysis of Plum pox virus (Sharka) susceptibility/resistance in apricot (Prunus armeniaca L.). PLoS ONE 2015, 10, e014e4670. [Google Scholar] [CrossRef] [PubMed]
- Zuriaga, E.; Romero, C.; Blanca, J.M.; Badenes, M.L. Resistance to Plum pox virus (PPV) in apricot (Prunus armeniaca L.) is associated with down-regulation of two MATHd genes. BMC Plant Biol. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a multifaceted hormone to combat disease. Ann. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Baebler, Š.; Witek, K.; Petek, M.; Stare, K.; Tušek-Žnidarič, M.; Pompe-Novak, M.; Morgiewicz, K. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J. Exp. Bot. 2014, 65, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Muñoz, N.; Velázquez, K.; Vives, M.C.; Ruiz-Ruiz, S.; Pina, J.A.; Flores, R.; Moreno, P.; Guerri, J. The resistance of sour orange to Citrus tristeza virus is mediated by both the salicylic acid and RNA silencing defense pathways. Mol. Plant Pathol. 2016, 18, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Lewsey, M.G.; Carr, J.P. Effects of DICER-like proteins 2, 3 and 4 on cucumber mosaic virus and tobacco mosaic virus infections in salicylic acid-treated plants. J. Gen. Virol. 2009, 90, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Chern, M.; Fitzgerald, H.A.; Canlas, P.E.; Navarre, D.A.; Ronald, P.C. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol. Plant-Microbe Int 2005, 18, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Sansregret, R.; Dufour, V.; Langlois, M.; Daayf, F.; Dunoyer, P.; Voinnet, O. Extreme resistance as a host counter-counter defense against viral suppression of RNA silencing. PLoS Pathog. 2013, 9, e1003435. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, J.M.; Saenz, P.; García, J.A. Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of plum pox virus in tobacco. Plant J. 2006, 48, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.; Granell, P.; Tarraga, S.; López-Gresa, P.; Conejero, V.; Belles, J.M. Salicylic acid and gentisic acid induce RNA silencing-related genes and plant resistance to RNA pathogens. Plant Physiol. Biochem. 2014, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cueto-Ginzo, I.A.; Serrano, L.; Sin, E.; Rodríguez, R.; Morales, J.C.; Lade, S.B.; Medina, V.; Achon, M.A. Exogenous salicylic acid treatment delays initial infection and counteracts alterations induced by Maize dwarf mosaic virus in the maize proteome. Physiol. Mol. Plant Pathol. 2016, 96, 47–59. [Google Scholar] [CrossRef]
- Ma, K.W.; Ma, W. Phytohormone pathways as targets of pathogens to facilitate infection. Plant Mol. Biol. 2016, 91, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Ton, J. Long-distance signaling in plant deference. Trends Plant Sci. 2008, 13, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Zhao, X.; Hassell, R.; Thies, J. Defense mechanism involved in disease resistance of grafted vegetables. HortScience 2012, 47, 164–170. [Google Scholar]
- Argueso, C.T.; Ferreira, F.J.; Epple, P.; To, J.P.; Hutchison, C.E.; Schalle, G.E. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012, 8, e1002448. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.H.; Hwang, I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Großkinsky, D.K.; Naseem, M.; Abdelmohsen, U.R.; Plickert, N.; Engelke, T.; Griebel, T.; Zeier, J.; Novák, O.; Strnad, M.; Pfeifhofer, H.; et al. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 2011, 157, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Giron, D.; Frago, E.; Glevarec, G.; Pieterse, C.M.J.; Dicke, M. Cytokininsas key regulators in plant–microbe–insect interactions: Connecting plant growth and defense. Funct. Ecol. 2013, 27, 599–609. [Google Scholar] [CrossRef]
- Kovac, M.; Muller, A.; Milovanovic, D.; Milavec, M.; Duchting, P.; Ravnikar, M. Multiple hormone analysis indicates involvement of jasmonate signaling in the early defence of potato to potato virus YNTN. Biol. Plant. 2009, 53, 195–199. [Google Scholar] [CrossRef]
- García-Marcos, A.; Pacheco, R.; Manzano, A.; Aguilar, E.; Tenllado, F. Oxylipin biosynthesis genes positively regulate programmed cell death during compatible infections with the synergistic pair Potato virus X–Potato virus Y and Tomato spotted wilt virus. J. Virol. 2013, 87, 5769–5783. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xi, D.H.; Yuan, S.; Xu, F.; Zhang, D.W.; Lin, H.H. Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana. Mol. Plant-Microbe Interact. 2014, 27, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Kobayashi, M.; Mitsuhara, I.; Seo, S. Jasmonic acid negatively regulates resistance to Tobacco mosaic virus in tobacco. Plant Cell Physiol. 2013, 54, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.E.; Schippers, J.H.M.; Dijkwel, P.P.; Wagstaff, C. Ethylene and Senescence Processes. Ann. Plant Rev. 2012, 5, 305–341. [Google Scholar]
- Marco, S.; Levy, D. Involvement of Ethylene in the Development of Cucumber Mosaic Virus-Induced Chlorotic Lesions in Cucumber Cotyledons. Physiol. Plant Pathol. 1979, 14, 235–244. [Google Scholar] [CrossRef]
- De Laat, A.M.M.; Van Loon, L.C. Regulation of Ethylene Biosynthesis in Virus-Infected Tobacco-Leaves. 3. The Relationship between Stimulated Ethylene Production and Symptom Expression in Virus-Infected Tobacco-Leaves. Physiol. Plant Pathol. 1983, 22, 261–273. [Google Scholar] [CrossRef]
- Helliwell, C.A.; Sheldon, C.C.; Olive, M.R.; Walker, A.R.; Zeevaart, J.A.; Peacock, W. Cloning of the Arabidopsis ent-kaurene oxidase gene GA3. Proc. Natl. Acad. Sci. USA 1998, 95, 9019–9024. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.F.; Gao, F.; Cao, X.S.; Chen, M.; Ye, G.Y.; Wei, C.H. The rice dwarf virus P2 protein interacts with ent-kaurene oxidases in vivo, leading to reduced biosynthesis of gibberellins and rice dwarf. Plant Physiol. 2005, 139, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Kusajima, M.; Yasuda, M.; Kawashima, A.; Nojiri, H.; Yamane, H.; Nakajima, M.; Akutsu, K.; Nakashita, H. Suppressive effect of abscisic acid on systemic acquired resistance in tobacco plants. J. Gen. Plant Pathol. 2010, 76, 161–167. [Google Scholar] [CrossRef]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Martínez-Andujar, C.; Martínez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unravelling rootstock×scion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef] [PubMed]



| Cycle 1 | Cycle 2 | |||||
|---|---|---|---|---|---|---|
| Treatments | Samples | Symptoms 1 | ELISA 2 | Symptoms 1 | ELISA 2 | RT-PCR 3 |
| GF305 Control | GFc | 0 (0.0) | 0 (0.052) | 0 (0.0) | 0 (0.067) | 0 |
| GF305 + PPV | GFi | 8 (3.3) | 8 (3.529) | 8 (4.2) | 8 (3.187) | 8 |
| GF305 Control < Garrigues | Gac | 0 (0.0) | 0 (0.061) | 0 (0.0) | 0 (0.059) | 0 |
| GF305 + PPV < Garrigues | Gai | 0 (0.0) | 0 (0.052) | 0 (0.0) | 0 (0.052) | 0 |
| GF305 Control < Garrigues | GFc+Ga | 0 (0.0) | 0 (0.059) | 0 (0.0) | 0 (0.058) | 0 |
| GF305 + PPV < Garrigues | GFi+Ga | 2 (1.0) | 2 (1.222) | 1 (1.0) | 4 (1.487) | 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehkordi, A.N.; Rubio, M.; Babaeian, N.; Albacete, A.; Martínez-Gómez, P. Phytohormone Signaling of the Resistance to Plum pox virus (PPV, Sharka Disease) Induced by Almond (Prunus dulcis (Miller) Webb) Grafting to Peach (P. persica L. Batsch). Viruses 2018, 10, 238. https://doi.org/10.3390/v10050238
Dehkordi AN, Rubio M, Babaeian N, Albacete A, Martínez-Gómez P. Phytohormone Signaling of the Resistance to Plum pox virus (PPV, Sharka Disease) Induced by Almond (Prunus dulcis (Miller) Webb) Grafting to Peach (P. persica L. Batsch). Viruses. 2018; 10(5):238. https://doi.org/10.3390/v10050238
Chicago/Turabian StyleDehkordi, Azam Nikbakht, Manuel Rubio, Nadali Babaeian, Alfonso Albacete, and Pedro Martínez-Gómez. 2018. "Phytohormone Signaling of the Resistance to Plum pox virus (PPV, Sharka Disease) Induced by Almond (Prunus dulcis (Miller) Webb) Grafting to Peach (P. persica L. Batsch)" Viruses 10, no. 5: 238. https://doi.org/10.3390/v10050238