Retrograde Transgene Expression via Neuron-Specific Lentiviral Vector Depends on Both Species and Input Projections
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Viral Vector Production
2.3. Surgical Procedures
2.4. Immunohistochemistry
2.5. Image Acquisition and Histological Analyses
3. Results
3.1. Injections of HiRet and NeuRet Vectors into Motor Cortex
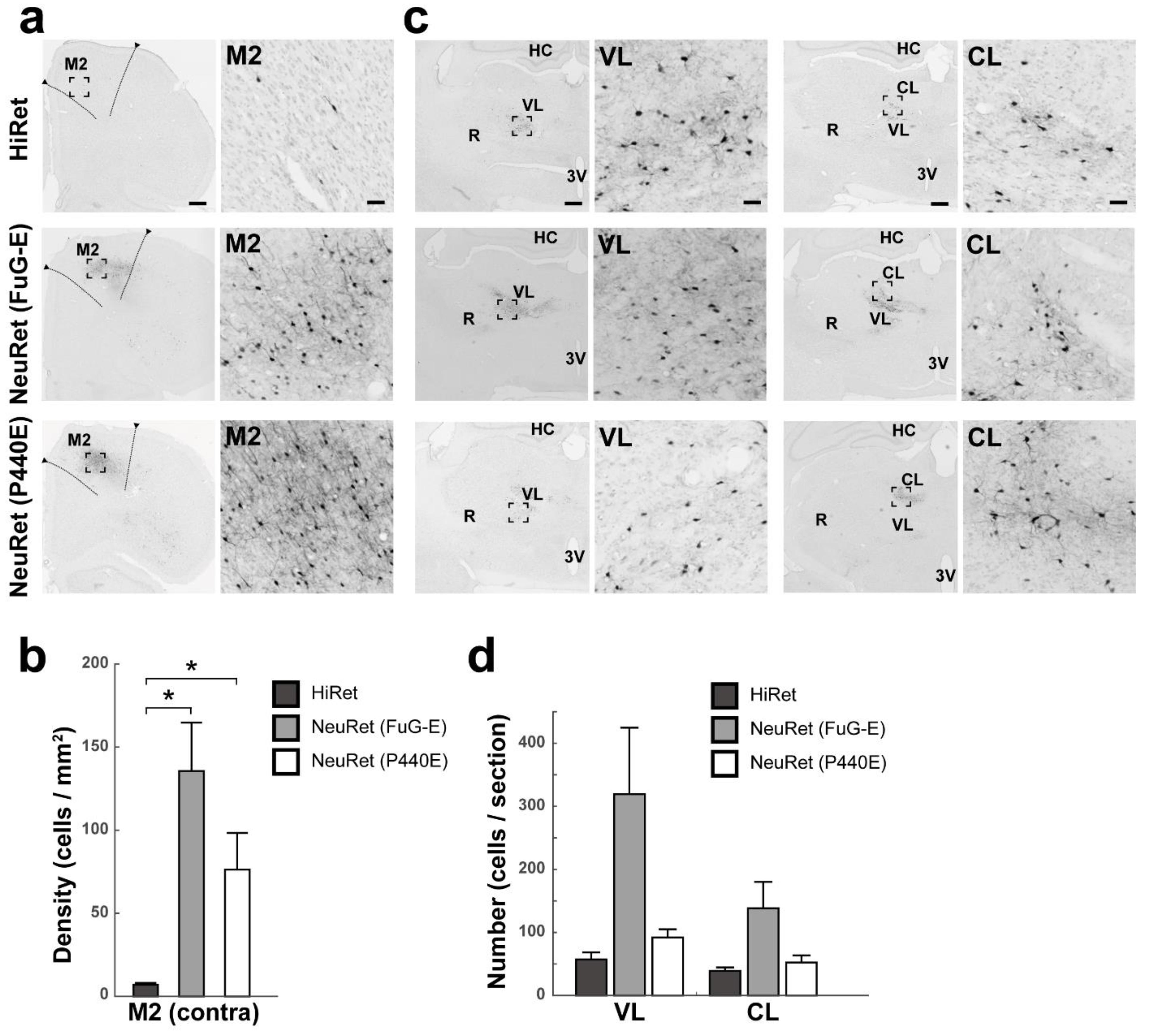
3.2. Retrograde Transgene Expression via HiRet and NeuRet Vectors in Rats
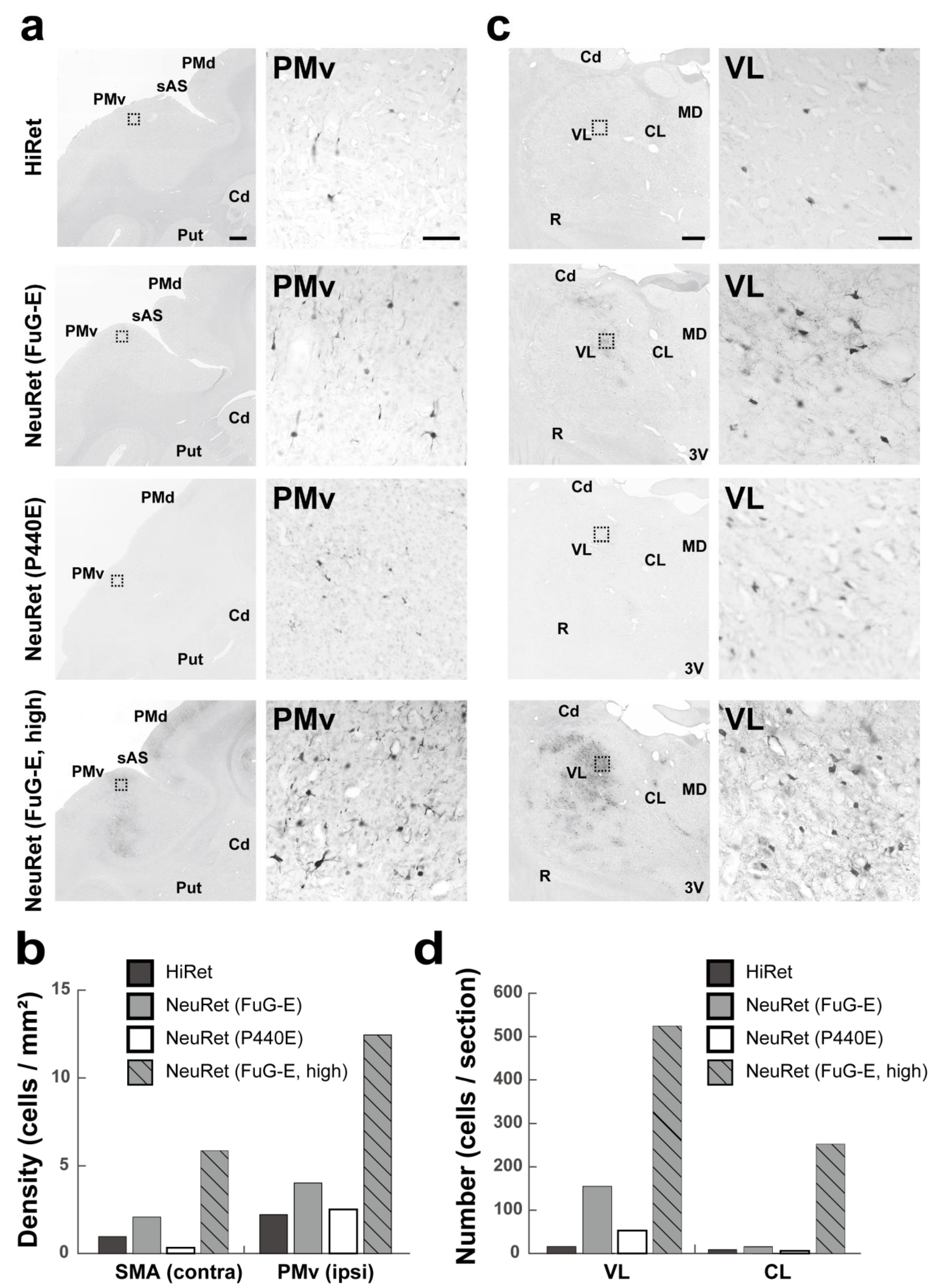
3.3. Retrograde Transgene Expression via HiRet and NeuRet Vectors in Marmosets
3.4. Retrograde Transgene Expression via HiRet and NeuRet Vectors in Macaques
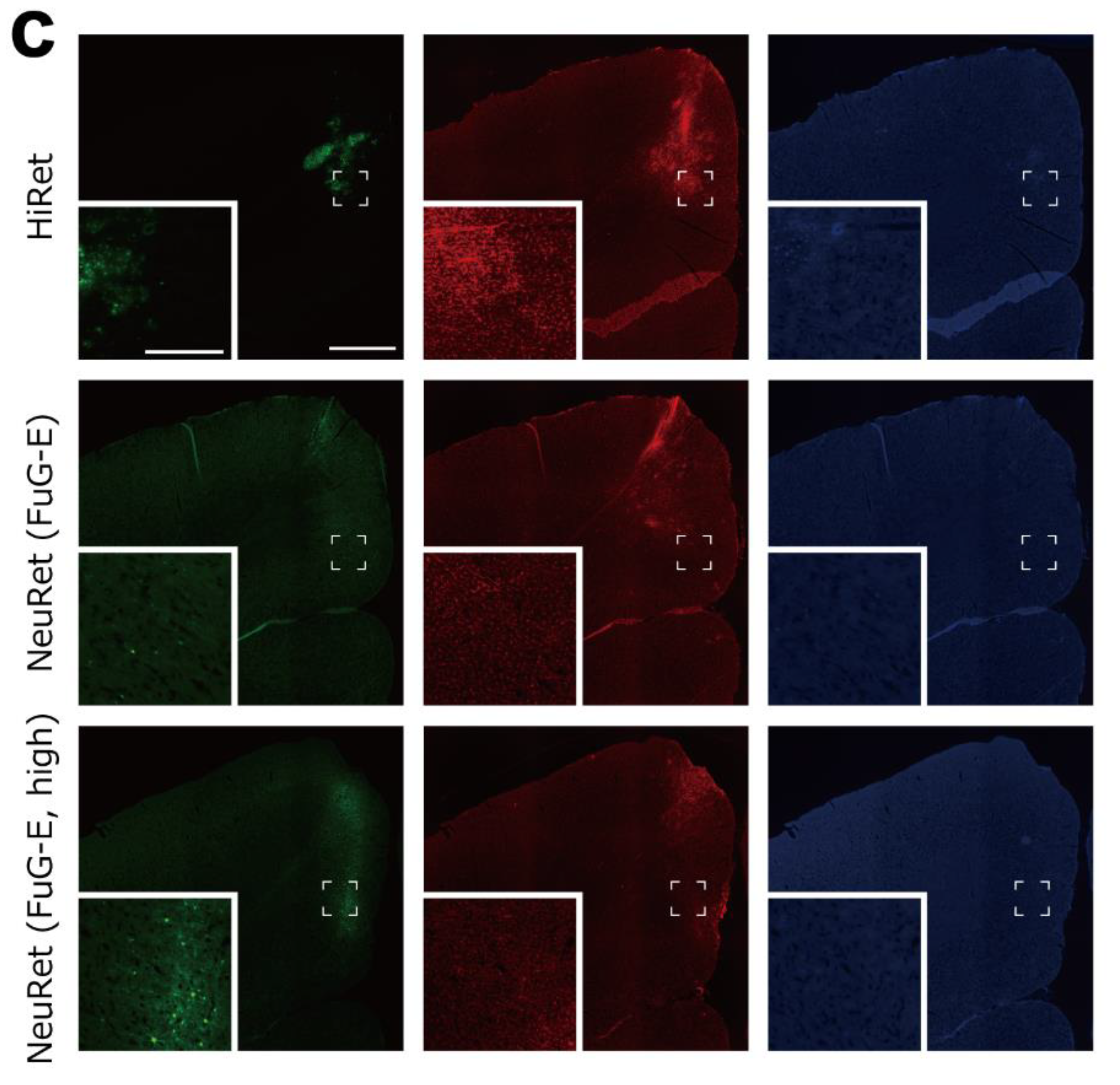
3.5. Inflammatory/Immune Responses of HiRet and NeuRet Vectors
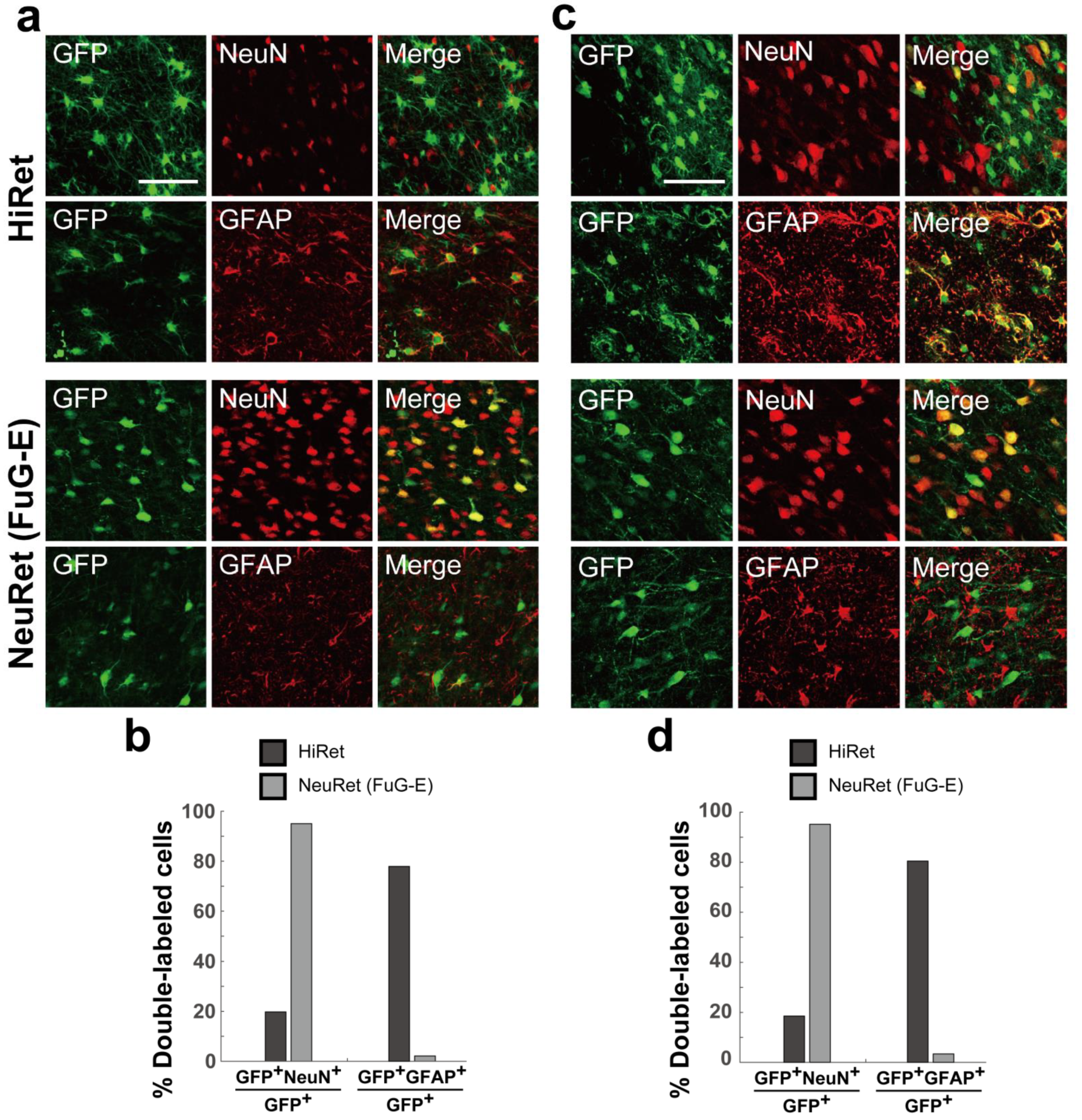
3.6. Neuron Specificity of HiRet and NeuRet Vectors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blömer, U.; Naldini, L.; Kafri, T.; Trono, D.; Verma, I.M.; Gage, F.H. Highly Efficient and Sustained Gene Transfer in Adult Neurons with a Lentivirus Vector. J. Virol. 1997, 71, 6641–6649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockrell, A.S.; Kafri, T. Gene Delivery by Lentivirus Vectors. Mol. Biotechnol. 2007, 36, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Blömer, U.; Gage, F.H.; Trono, D.; Verma, I.M. Efficient Transfer, Integration, and Sustained Long-Term Expression of the Transgene in Adult Rat Brains Injected with a Lentiviral Vector. Proc. Natl. Acad. Sci. USA 1996, 93, 11382–11388. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Kato, S.; Inoue, K.; Takada, M.; Kobayashi, K. Altering Entry Site Preference of Lentiviral Vectors into Neuronal Cells by Pseudotyping with Envelope Glycoproteins; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 175–186. [Google Scholar] [CrossRef]
- Mazarakis, N.D.; Azzouz, M.; Rohll, J.B.; Ellard, F.M.; Wilkes, F.J.; Olsen, A.L.; Carter, E.E.; Barber, R.D.; Baban, D.F.; Kingsman, S.M.; et al. Rabies Virus Glycoprotein Pseudotyping of Lentiviral Vectors Enables Retrograde Axonal Transport and Access to the Nervous System after Peripheral Delivery. Hum. Mol. Genet. 2001, 10, 2109–2121. [Google Scholar] [CrossRef]
- Kato, S.; Inoue, K.; Kobayashi, K.; Yasoshima, Y.; Miyachi, S.; Inoue, S.; Hanawa, H.; Shimada, T.; Takada, M.; Kobayashi, K. Efficient Gene Transfer via Retrograde Transport in Rodent and Primate Brains Using a Human Immunodeficiency Virus Type 1-Based Vector Pseudotyped with Rabies Virus Glycoprotein. Hum. Gene Ther. 2007, 18, 1141–1151. [Google Scholar] [CrossRef]
- Carpentier, D.C.J.; Vevis, K.; Trabalza, A.; Georgiadis, C.; Ellison, S.M.; Asfahani, R.I.; Mazarakis, N.D. Enhanced Pseudotyping Efficiency of HIV-1 Lentiviral Vectors by a Rabies/Vesicular Stomatitis Virus Chimeric Envelope Glycoprotein. Gene Ther. 2012, 19, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Schoderboeck, L.; Riad, S.; Bokor, A.M.; Wicky, H.E.; Strauss, M.; Bostina, M.; Oswald, M.J.; Empson, R.M.; Hughes, S.M. Chimeric Rabies SADB19-VSVg-Pseudotyped Lentiviral Vectors Mediate Long-Range Retrograde Transduction from the Mouse Spinal Cord. Gene Ther. 2015, 22, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Kobayashi, K.; Inoue, K.; Kuramochi, M.; Okada, T.; Yaginuma, H.; Morimoto, K.; Shimada, T.; Takada, M.; Kobayashi, K. A Lentiviral Strategy for Highly Efficient Retrograde Gene Transfer by Pseudotyping with Fusion Envelope Glycoprotein. Hum. Gene Ther. 2011, 22, 197–206. [Google Scholar] [CrossRef]
- Kato, S.; Kuramochi, M.; Takasumi, K.; Kobayashi, K.; Inoue, K.; Takahara, D.; Hitoshi, S.; Ikenaka, K.; Shimada, T.; Takada, M.; et al. Neuron-Specific Gene Transfer through Retrograde Transport of Lentiviral Vector Pseudotyped with a Novel Type of Fusion Envelope Glycoprotein. Hum. Gene Ther. 2011, 22, 1511–1523. [Google Scholar] [CrossRef]
- Kato, S.; Kuramochi, M.; Kobayashi, K.; Fukabori, R.; Okada, K.; Uchigashima, M.; Watanabe, M.; Tsutsui, Y.; Kobayashi, K. Selective Neural Pathway Targeting Reveals Key Roles of Thalamostriatal Projection in the Control of Visual Discrimination. J. Neurosci. 2011, 31, 17169–17179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Kobayashi, K.; Kobayashi, K. Improved Transduction Efficiency of a Lentiviral Vector for Neuron-Specific Retrograde Gene Transfer by Optimizing the Junction of Fusion Envelope Glycoprotein. J. Neurosci. Methods 2014, 227, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, S.; Inoue, K.; Tsuge, H.; Uezono, S.; Nagaya, K.; Fujiwara, M.; Kato, S.; Kobayashi, K.; Takada, M. The Use of an Optimized Chimeric Envelope Glycoprotein Enhances the Efficiency of Retrograde Gene Transfer of a Pseudotyped Lentiviral Vector in the Primate Brain. Neurosci. Res. 2017, 120, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Uezono, S.; Tsuge, H.; Fujiwara, M.; Miwa, M.; Kato, S.; Nakamura, K.; Kobayashi, K.; Inoue, K.; Takada, M. A Note on Retrograde Gene Transfer Efficiency and Inflammatory Response of Lentiviral Vectors Pseudotyped with FuG-E vs. FuG-B2 Glycoproteins. Sci. Rep. 2019, 9, 3567. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Sugawara, M.; Kobayashi, K.; Kimura, K.; Inoue, K.; Takada, M.; Kobayashi, K. Enhancement of the Transduction Efficiency of a Lentiviral Vector for Neuron-Specific Retrograde Gene Delivery through the Point Mutation of Fusion Glycoprotein Type E. J. Neurosci. Methods 2019, 311, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Majka, P.; Bai, S.; Bakola, S.; Bednarek, S.; Chan, J.M.; Jermakow, N.; Passarelli, L.; Reser, D.H.; Theodoni, P.; Worthy, K.H.; et al. Open Access Resource for Cellular-Resolution Analyses of Corticocortical Connectivity in the Marmoset Monkey. Nat. Commun. 2020, 11, 1133. [Google Scholar] [CrossRef]
- Liu, J.; Morel, A.; Wannier, T.; Rouiller, E.M. Origins of Callosal Projections to the Supplementary Motor Area (SMA): A Direct Comparison between Pre-SMA and SMA-Proper in Macaque Monkeys. J. Comp. Neurol. 2002, 443, 71–85. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. Frontal Lobe Inputs to the Digit Representations of the Motor Areas on the Lateral Surface of the Hemisphere. J. Neurosci. 2005, 25, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesielska, A.; Hadaczek, P.; Mittermeyer, G.; Zhou, S.; Wright, J.F.; Bankiewicz, K.S.; Forsayeth, J. Cerebral Infusion of AAV9 Vector-Encoding Non-Self Proteins Can Elicit Cell-Mediated Immune Responses. Mol. Ther. 2013, 21, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Samaranch, L.; Sebastian, W.S.; Kells, A.P.; Salegio, E.A.; Heller, G.; Bringas, J.R.; Pivirotto, P.; DeArmond, S.; Forsayeth, J.; Bankiewicz, K.S. AAV9-Mediated Expression of a Non-Self Protein in Nonhuman Primate Central Nervous System Triggers Widespread Neuroinflammation Driven by Antigen-Presenting Cell Transduction. Mol. Ther. 2014, 22, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Fukabori, R.; Nishizawa, K.; Okada, K.; Yoshioka, N.; Sugawara, M.; Maejima, Y.; Shimomura, K.; Okamoto, M.; Eifuku, S.; et al. Action Selection and Flexible Switching Controlled by the Intralaminar Thalamic Neurons. Cell Rep. 2018, 22, 2370–2382. [Google Scholar] [CrossRef] [Green Version]
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social Reward Requires Coordinated Activity of Nucleus Accumbens Oxytocin and Serotonin. Nature 2013, 501, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, L.A.; Miyamichi, K.; Gao, X.J.; Beier, K.T.; Weissbourd, B.; DeLoach, K.E.; Ren, J.; Ibanes, S.; Malenka, R.C.; Kremer, E.J.; et al. Viral-Genetic Tracing of the Input–Output Organization of a Central Noradrenaline Circuit. Nature 2015, 524, 88–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, M.L.; Saunders, A.; Huang, K.W.; Philson, A.C.; Goldman, M.; Macosko, E.Z.; McCarroll, S.A.; Sabatini, B.L. Genetically Distinct Parallel Pathways in the Entopeduncular Nucleus for Limbic and Sensorimotor Output of the Basal Ganglia. Neuron 2017, 94, 138–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namburi, P.; Beyeler, A.; Yorozu, S.; Calhoon, G.G.; Halbert, S.A.; Wichmann, R.; Holden, S.S.; Mertens, K.L.; Anahtar, M.; Felix-Ortiz, A.C.; et al. A Circuit Mechanism for Differentiating Positive and Negative Associations. Nature 2015, 520, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Rajasethupathy, P.; Sankaran, S.; Marshel, J.H.; Kim, C.K.; Ferenczi, E.; Lee, S.Y.; Berndt, A.; Ramakrishnan, C.; Jaffe, A.; Lo, M.; et al. Projections from Neocortex Mediate Top-down Control of Memory Retrieval. Nature 2015, 526, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, M.E.; Soden, M.E.; Zweifel, L.S.; Palmiter, R.D. Genetic Identification of a Neural Circuit That Suppresses Appetite. Nature 2013, 503, 111–114. [Google Scholar] [CrossRef]
- Osakada, F.; Mori, T.; Cetin, A.H.; Marshel, J.H.; Virgen, B.; Callaway, E.M. New Rabies Virus Variants for Monitoring and Manipulating Activity and Gene Expression in Defined Neural Circuits. Neuron 2011, 71, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Huang, C.; Li, J.Z.; Grewe, B.F.; Zhang, Y.; Eismann, S.; Schnitzer, M.J. High-Speed Recording of Neural Spikes in Awake Mice and Flies with a Fluorescent Voltage Sensor. Science 2015, 350, 1361–1366. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Hughes, E.G.; Shetty, A.S.; Arlotta, P.; Goff, L.A.; Bergles, D.E.; Brown, S.P. Changes in the Excitability of Neocortical Neurons in a Mouse Model of Amyotrophic Lateral Sclerosis Are Not Specific to Corticospinal Neurons and Are Modulated by Advancing Disease. J. Neurosci. 2017, 37, 9037–9053. [Google Scholar] [CrossRef] [Green Version]
- Ciabatti, E.; González-Rueda, A.; Mariotti, L.; Morgese, F.; Tripodi, M. Life-Long Genetic and Functional Access to Neural Circuits Using Self-Inactivating Rabies Virus. Cell 2017, 170, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Sullivan, H.A.; MacLennan, B.J.; Xu, R.; Hou, Y.; Lavin, T.K.; Lea, N.E.; Michalski, J.E.; Babcock, K.R.; Dietrich, S.; et al. Nontoxic, Double-Deletion-Mutant Rabies Viral Vectors for Retrograde Targeting of Projection Neurons. Nat. Neurosci. 2018, 21, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Tervo, D.G.R.; Huang, B.-Y.; Viswanathan, S.; Gaj, T.; Lavzin, M.; Ritola, K.D.; Lindo, S.; Michael, S.; Kuleshova, E.; Ojala, D.; et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, A.K.; El-Nahal, H.G.; Bohlen, M.O.; May, P.J.; Basso, M.A.; Grimaldi, P.; Wang, M.Z.; de Velasco Ezequiel, M.F.; Sommer, M.A.; Heilbronner, S.R. Using rAAV2-Retro in Rhesus Macaques: Promise and Caveats for Circuit Manipulation. J. Neurosci. Methods 2020, 345, 108859. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and Problems with the Use of Viral Vectors for Gene Therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef]
- Yacoub, N.A.; Romanowska, M.; Haritonova, N.; Foerster, J. Optimized Production and Concentration of Lentiviral Vectors Containing Large Inserts. J. Gene Med. 2007, 9, 579–584. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, Y.; Tsuge, H.; Uezono, S.; Tanabe, S.; Fujiwara, M.; Miwa, M.; Kato, S.; Nakamura, K.; Kobayashi, K.; Inoue, K.-i.; et al. Retrograde Transgene Expression via Neuron-Specific Lentiviral Vector Depends on Both Species and Input Projections. Viruses 2021, 13, 1387. https://doi.org/10.3390/v13071387
Otsuka Y, Tsuge H, Uezono S, Tanabe S, Fujiwara M, Miwa M, Kato S, Nakamura K, Kobayashi K, Inoue K-i, et al. Retrograde Transgene Expression via Neuron-Specific Lentiviral Vector Depends on Both Species and Input Projections. Viruses. 2021; 13(7):1387. https://doi.org/10.3390/v13071387
Chicago/Turabian StyleOtsuka, Yukiko, Hitomi Tsuge, Shiori Uezono, Soshi Tanabe, Maki Fujiwara, Miki Miwa, Shigeki Kato, Katsuki Nakamura, Kazuto Kobayashi, Ken-ichi Inoue, and et al. 2021. "Retrograde Transgene Expression via Neuron-Specific Lentiviral Vector Depends on Both Species and Input Projections" Viruses 13, no. 7: 1387. https://doi.org/10.3390/v13071387





