Abstract
Lentiviral vectors have played a critical role in the emergence of gene-modified cell therapies, specifically T cell therapies. Tisagenlecleucel (Kymriah), axicabtagene ciloleucel (Yescarta) and most recently brexucabtagene autoleucel (Tecartus) are examples of T cell therapies which are now commercially available for distribution after successfully obtaining EMA and FDA approval for the treatment of blood cancers. All three therapies rely on retroviral vectors to transduce the therapeutic chimeric antigen receptor (CAR) into T lymphocytes. Although these innovations represent promising new therapeutic avenues, major obstacles remain in making them readily available tools for medical care. This article reviews the biological principles as well as the bioprocessing of lentiviral (LV) vectors and adoptive T cell therapy. Clinical and engineering successes, shortcomings and future opportunities are also discussed. The development of Good Manufacturing Practice (GMP)-compliant instruments, technologies and protocols will play an essential role in the development of LV-engineered T cell therapies.
1. Introduction
Viruses are infectious agents composed of nucleic acids protected by a protein coat. These microbes cannot self-replicate, relying instead on the cells they infect to produce more copies of themselves by hijacking the host’s replication machinery [1]. Viruses are generally associated with disease, but genetic engineers have repurposed their biology to leverage their natural ability to modify or supplement the host cell genetic code, producing a new class of gene delivery tools known as viral vectors [2]. Gamma retroviral (GRV) and lentiviral (LV) vectors are derived from enveloped RNA viruses of the retroviridae family and have been widely used in research and in the clinic due to their ability to integrate genetic material to the host cell genome and stably express a transgene, making them particularly suited to transduce highly replicating cells such as immune cells [3]. Chimeric Antigen Receptor (CAR) T cell therapy is the most successful type of immunotherapy to date and the only T cell-based therapy to have reached the market. To produce CAR T cells, T lymphocytes are genetically modified to express a CAR receptor, conferring them the ability to identify and destroy cancerous cells [4]. CAR T cell therapy has shown promising results for the treatment of blood cancers, particularly CD19 positive B cell malignancies with three products, Tisagenlecleucel (Kymriah) [5,6], Axicabtagene ciloleucel (Yescarta) [7,8] and most recently Brexucabtagene Autoleucel (Tecartus) [9,10] obtaining market authorisation. The bioprocessing of both vector and cellular elements of these therapies are rarely considered simultaneously and are not necessarily produced by the same manufacturer. The biotechnology and pharmaceutical industry are increasingly involved in T cell engineered therapies and sponsoring clinical tests, attesting to the rapid development of this therapeutic space. In this review, we will present the fundamental biological principles and the bioprocessing of LV vectors for gene-modified T cell therapies, as well as recent clinical and engineering advances.
3. Gene-Modified T Cell Therapy
3.1. Biological Principles
One of the roles of T lymphocytes in immunity is to detect and destroy cells infected by harmful pathogens. During infection, T cells can detect foreign pathogens when presented with an immunogenic portion of a specific infectious agent called antigen [47,48,49]. This activation is mediated through the T cell Receptor (TCR), composed of the CD3 receptor and the CD4 or CD8 co-receptors. The CD4 co-receptor is used by helper T cells when interacting with antigen-presenting cells to activate their cytokine release function, which promotes the recruitment and activation of other immune cells, whereas the CD8 co-receptor is used by cytotoxic T cells to activate their cell-killing function. Other co-receptors, including OX40, CD28 and 4-1BB, enhance cell function such as proliferation, cytokine production and cell survival [50,51,52]. Once activated, T cells will start dividing rapidly, produce proinflammatory factors and activate their cytotoxic function to destroy infected cells. When the infection is resolved, the activated T cell population produced by clonal expansion will become exhausted, senescent and ultimately undergo apoptosis [53]. This process leaves only a small, long-lasting subset of memory cells capable of detecting the same antigen and undergoing rapid clonal expansion followed by differentiation to mount a strong and specific adaptive immune response [54,55].
During their maturation in the thymus, T cells that recognise self-peptides are removed in order to prevent autoimmune reactions [56]. Genetic and epigenetic modifications present in cancer cells lead to the deregulation of cell functions such as metabolism, proliferation, DNA repair, apoptosis, motility and attachment [57]. The immune system has the ability to detect some of these factors and use its killing function to eradicate cancer cells [58]. Modifications of the tumour microenvironment (TME) can also be a potential target for the immune system, as characteristics of the TME are clearly distinct from those of healthy surrounding tissue [59]. Lymphocytes which are attracted to the TME and infiltrate the tumour are referred to as tumour-infiltrating lymphocytes (TILs). Presence of these immune cells has been associated with better prognosis and chances of remission in cancer patients, and they have been used in clinic for autologous adoptive cell therapy [60]. However, cancer cells originate from damaged or mutated healthy cells and can therefore be difficult to detect for the immune system [61].
The Chimeric Antigen Receptor, or CAR, is an engineered TCR capable of binding to a target antigen. The CAR can be introduced into T lymphocytes to produce a CAR T cell with the ability to detect a specific antigen. The CAR is composed of the single chain variable Fragment (scFv) of the human IgG antibody. Three generations of CARs have been developed: the first generation was an scFv fragment conjugated to a CD3 signal to promote cytotoxic activity of the CAR T cell [62,63]. The second generation also harboured a CD28 signal to promote cell proliferation and cytokine production upon target binding [64]. The third generation of CAR added 4-1BB and OX40 regions to promote cell survival and prolong the CAR T cell’s in vivo persistence [65]. A fourth generation has recently been developed and contains specific cytokine signals which renders CAR T cells resistant to the immune dampening effects of the TME and further improves their in vivo lifespan [66,67].
3.2. T Cell Therapy Bioprocessing
Two main approaches have emerged from T cell adoptive therapy relying on two different source materials. The allogeneic approach relies on cells sourced from a “universal” source in a one-size-fits-all strategy. The autologous approach relies on cells sourced directly from the patient for a personalised medicine-type therapy. All commercially available T cell adoptive therapies have been relying on the autologous principle as the allogeneic approach has proven difficult to develop due to HLA typing and stem cell technology early advancement [68].
For most CAR T cell therapies commercially available or in clinical development, cells are isolated from the patient’s peripheral blood mononuclear cells (PBMCs) by apheresis or leukapheresis. This material contains a host of immune cells, including B cells, macrophages, monocytes, NK cells and T cells. The first manufacturing step is the isolation of the T cell subset from the PBMCs, which can be performed by magnetic bead selection or selective expansion [69,70]. Magnetic selection refers to the attachment of magnetic beads conjugated to antibodies which bind to a target T cell population which can be isolated when passed through a magnetic column. For CAR T cell development, anti-CD3, or a combination of anti-CD4 and anti-CD8, antibodies can be used to select the T lymphocytes [14]. After selection, cells are typically left to rest for several hours in culture medium. The cells can then be transduced using a viral vector containing the CAR cassette. This step allows the introduction of the CAR gene into the cell to produce fully functional CAR T cells [71]. An alternative to the viral transduction is transfection. T cells can be transfected with pDNA containing the CAR gene—by chemical transfection, using a transfection chemical agent such as polyethyleneimine (PEI)—or by electroporation, using an electric current to form pores in the cell membrane and allow the pDNA to enter the cell. These methods require large quantities of pDNA as they are carried out at the end of the expansion phase on much larger cell numbers and thus have had limited use in therapeutic applications [72].
When producing CAR T cells for therapeutic applications, gene delivery can be achieved by four main types of genetic material transfer: stable integration of the gene to the host T cell genomic DNA; non-integrating transient transfer, where the transgenic DNA persists as an episome; viral vector-mediated transduction; and non-viral transduction [4]. To reach the therapeutic dose of cells required to treat a patient, cells are kept in culture and allowed to expand. To stimulate T cell expansion, cells are activated using beads conjugated to monoclonal antibodies targeting CD3 and CD28 T cell receptors [73]. Post-activation cells can be cultured in bioreactors, which offer the possibility to expand cells in a closed system, at medium scale and with sampling and monitoring to control product quality throughout the process [74].
Development of CAR T cell therapy presents a few challenges including poor cell expansion, short in vivo lifespan, and significant side effects in treated patients [75]. Obtaining enough target T cells for delivering a functional therapy is one of the main challenges. Material isolated for preclinical drug development is often obtained from healthy donors, whereas material used in the clinic originates from individuals with advanced forms of cancer that would have undergone harsh therapeutic regimens including chemotherapy, radiotherapy, immunotherapy or a combination of all of these. Consequently, material for clinical use will be of lesser quality and higher variability. Finally, sampling of immune cells from patients in a critical state will have dramatic consequences on their health and chances of survival [69]. Therefore, effective cell expansion technologies are paramount to bringing CAR T cell therapy from “bench to bed” [14,76].
Despite much success, CAR T cell therapy has been shown to pose major safety challenges. This is due to the potential of the CAR T cell triggering an immune response cascade known as cytokine release syndrome (CRS) or “cytokine storm”. CRS is caused by the constitutive overactivation of T cells resulting in a massive release of pro-inflammatory factors, leading to the activation of more immune cells in a positive feedback loop. CRS symptoms include high fever, seizures, organ failure, low blood pressure, and systemic inflammatory response and can lead to death [75,77].
4. Applications of LV Vector Technologies for T Cell Engineering
4.1. Introduction
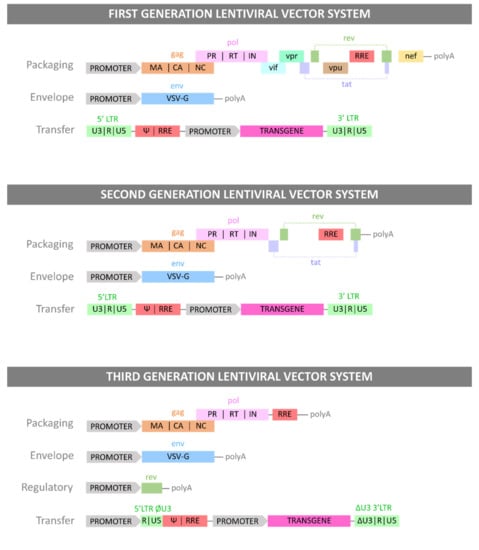
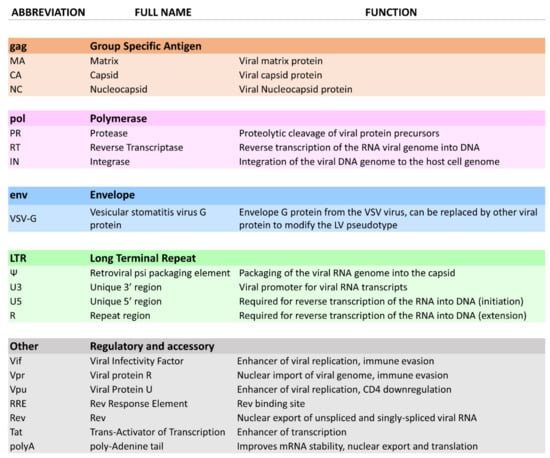
LV vectors are a well-established in vitro technology for research and development, and more recently they have been gaining popularity for clinical applications. Indeed, safety and efficacy of LV clinical applications has been proven for many clinical trials [78] with some products reaching market authorisation. The manufacturing process used for the production of autologous therapies commercially available is described in Figure 3. However, challenges such as CRS, neurologic toxicity, and overall cost need to be addressed to make adoptive T cell therapy a widely accessible therapeutic [75]. The development of Good Manufacturing Practice (GMP) compliant instruments, technologies and techniques are essential for the development of LV-engineered T cell therapies. In this section we will present some applications of LV vector technologies for T cell engineering. The following discusses currently-available CAR T cell therapies, technologies designed to integrate LV applications to T cell engineering, LV-based research and development tools for T cell therapy development, allogeneic approaches using LV modified donor T cells and alternatives to CAR T cell therapy.
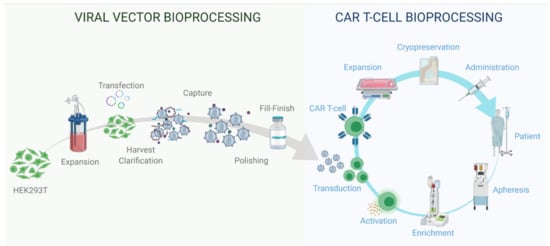
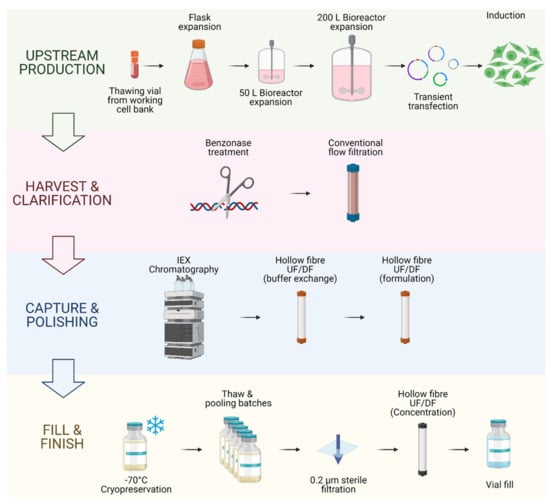
Figure 3.
Viral vector and autologous CAR T cell therapy bioprocessing.
Viral vector bioprocessing begins with the expansion of the producer cell line, e.g., HEK293T cells. Cells are then transfected with plasmid DNA (pDNA) vectors. Post transfection, the viral particles produced are harvested from the cell supernatant. The clarification step is performed to remove large impurities such as aggregates and cell debris; this step may include a nuclease digestion step. The capture step aims at retaining the LV particles and further removes impurities. The polishing step removes impurities which are usually with similar physical properties to the LV vectors. The fill-finish step consists of the final formulation of the LV vector.
Autologous CAR T cell bioprocessing starts with the isolation of peripheral blood mononuclear cells (PBMCs) from the patient by apheresis or leukapheresis. To isolate the T lymphocytes from the PBMCs, an enrichment step can be introduced using antibody-conjugated magnetic beads. T cells are activated using anti-CD3/CD28 antibody-conjugated beads. Post-activation, cells are transduced using lentiviral vectors, and the CAR therapeutic gene is integrated in the target T cell genome to produce fully functional CAR T cells. The activated T cells are then expanded in culture to reach the required cell number for the therapy infusion course. The cell material is then harvested and conditioned for cryopreservation at −120 °C. The material is then shipped back to the clinic to be administered to the patient through intravenous infusion.
4.2. Commercially Available Gene-Modified T Cell Therapies
Of the three CAR T cell therapies with market authorisations, brexucabtagene autoleucel (Tecartus, Kite Pharma Inc., Los Angeles, CA, USA) [9,10] and axicabtagene ciloleucel (Yescarta, Kite Pharma Inc.) [7,8] are transduced using GRV vectors, while tisagenlecleucel (Kymriah®, Novartis International AG, Basel Switzerland) [5,6] is transduced using a lentiviral vector. Brexucabtagene autoleucel and axicabtagene ciloleucel both use the same viral vector for the transduction of the transgene payload under the control of the MSCV promoter [7,9]. Tisagenlecleucel uses a unique LV vector and the transgene is under the control of the EF-1a promoter [5]. Although other gene transfer techniques for adoptive T cell therapy are currently under clinical assessment, none have yet gained market authorization, demonstrating the importance of retroviral vectors as gene transfer tools for cell therapy applications. All EMA and FDA approved therapies have been listed in Table 1.

Table 1.
EMA and FDA approved gene-modified T cell therapies (accessed on 4 January 2021).
The current protocols for autologous CAR T cell therapies have been established and are presented in Figure 3. This autologous approach represents a viable option for commercial products but requires further improvement to address global demand [14]. Due to cost and manufacturing capacity, LV-engineered T cell therapies currently commercially available are only considered as a second-, third- or fourth-line therapeutic option for patients that have failed to respond or relapsed following conventional chemotherapy, radiotherapy and immunotherapy treatments. Improvement of the bioprocesses and technologies required for the production of LV vectors and T cell engineering must both be considered in parallel to improve patient access. Recent advances have been proposed to address process bottlenecks, enhance functionality and improve safety of LV engineered T cell therapies.
Logistics involved in cell and gene therapy which include sourcing of patient material, production, shipping, conditioning, and storage of the drug product are seen as one of the main bottlenecks to making cell and gene therapy a viable therapeutic solution [79]. This 22-day bioprocess is currently being carried out in the United States, posing a major challenge for other markets including the European Union, where patients need to ship their cells across the Atlantic twice before receiving therapy, and outlining the logistics issues posed by the current approach to autologous T cell therapy. To alleviate this, Novartis is increasing its manufacturing capabilities in France and Switzerland [80].
The European Medicines Agency (EMA) PRIority MEdicines scheme (PRIME) and the United States Federal Food and Drug Administration (FDA) breakthrough therapy designation have been developed to facilitate market approval for medicines that demonstrate in pre-clinical studies a significant improvement on current standard of care [81]. All three therapies benefitted from this accelerated evaluation as these therapies have shown high efficacy for B cell malignancies and are addressing an unmet clinical demand [82,83].
Tisagenlecleucel received FDA and EMA approval in 2018 for the treatment of mantle cell lymphoma (MCL) [5,6]. T lymphocytes are modified using a lentiviral vector to transduce them with an anti-CD19 CAR, and modified cells are then infused back into the patient. Clinical investigation is still ongoing in Phase II (ELIANA, NCT02435849), with results available and scheduled to be completed in November 2022 [84,85]. Phase III (BELINDA, NCT03570892) is currently being performed but due to the successful accelerated approval status, tisagenlecleucel can be commercialised before its completion [86]. Encouraging results from the anti-CD19 CAR T cell approach have resulted in increased efforts to standardise protocols for experimental development and anticipate clinical requirements [18,87,88].
4.3. Limitations, Safety and Efficacy
Despite initial clinical successes of CAR T cell therapy in treating blood cancers, safety issues and improving efficacy in solid tumours are still obstacles that need to be addressed.
In addition to separating LV genes into separate plasmid DNA constructs to avoid RCL generation, several modifications to the LV vectors have been implemented to improve safety. One solution to mitigate the development of adverse effects after CAR T cell infusion is the inclusion of transgenes containing a “suicide gene” [89,90]. A “suicide gene” allows the selective killing of CAR T cells by the addition of a specific chemical agent that is otherwise non-toxic to unmodified cells. This technology has been progressed to the pre-clinical and clinical stage [91,92]. For instance, the anti-SLAMF7 therapy against multiple myeloma (MM) included a dimerization domain fused to a caspase-9 domain suicide gene to mitigate the risk of off-target activation, as SLAMF7 is expressed on healthy leukocytes, including NK cells. The authors have shown efficacy in vitro and in vivo, and the modified cells could be eliminated by the addition of the dimerizing agent AP1903 (rimiducid) [93].
Another associated risk to CAR T cell manufacturing is the transduction of other cell types with the transgene. The transduction of a single leukaemia B cell led to the development of a resistant transgenic clone in a patient treated with the anti-CD19 CAR T cell therapy tisagenlecleucel (Kymriah) [94]. This led to the masking of the target CD19 epitope due to the cis-integration of the CAR therapeutic gene during manufacturing. Although a rare event (1 patient out of 369 treated at the time), the use of integrating vectors with broad tropism can represent a serious safety concern. The vector used for this therapy was pseudotyped using a VSV-G envelope protein which confers a broad tropism to the vector [2]. The engineering of more specific chimeric envelope proteins has been suggested as a means to address this issue. Measles envelope-based chimeric protein is capable of targeting the CD3 receptor only present on T cells. In vivo data in a humanised mouse model showed the specificity of such vectors and even allowed in vivo gene delivery to produce functional CAR T cell transduction. Although more work would be required, this method could address potential off-target transduction during ex vivo transduction [95].
The development of resistant lymphoma B cells which do not express the CD19 antigen leading to the relapse of CAR T cell-treated patients has also been reported [96,97]. Transgene engineering has been proposed to address the development of resistant cancers. The introduction of two to three CAR constructs into each engineered cell has been tested in vitro and is under clinical investigation, with encouraging results on preventing the development of resistance to CAR therapy [98,99].
CAR expression level was shown to impact clinical outcome. The careful selection and design of promoters driving the expression of the CAR gene allows the improvement of CAR expression [100]. The different components of the CAR transgene such as the transmembrane and hinge domains has also shown to impact CAR expression. Efforts to optimise the design of intracellular and extracellular domains of the chimeric receptor have been under investigation [101].
Despite initial successes in treating blood cancers, CAR T cell therapy has encountered obstacles and limited success with treating solid tumours [102]. The physical properties of solid tumours as well as the tumour micro-environment (TME) restrict the ability of the CAR T cell to effectively access and kill the cancer cells [103]. The obstacles often cited are the difficulty for the cells to penetrate the tumour bed, the harsh physiological conditions within the tumour and the production of immunosuppressive modulatory proteins by the cancer cells. These barriers are not present in blood cancers as they do not form large tumour beds seen in solid tumour cancer types, thus explaining the success encountered with anti-CD19 CAR therapy.
Finally, alternatives to LV and RV vectors have been suggested to address the limitations and safety issues encountered. Electroporation is one such alternative which consists of the introduction of pDNA by passing an electric current through the cells [104]. The current temporarily generates pores in the cell as well as moving the negatively charge mRNA through the cell suspension and into the cell. Because of the non-integrating nature of this gene transfer technique, safety can be improved, as the expression of the transgene will slowly decrease as the mRNA is degraded and diluted through cell division. Although these techniques present safety advantages, long-term efficacy still needs to be demonstrated in human trials.
4.4. Development of LV-Based Tools for CAR T Cell Therapy Research
In addition to serving as effective and safe tools for CAR transgene transfer into T cells, LV vectors have been employed for developing in vivo and in vitro models. An essential part for developing in vitro and in vivo models is the development of target cells expressing the tumour-associated antigen (TAA) targeted by the CAR T cell therapy. Because patient material is a scarce resource and ethically cannot be used in every research and development study, immortalised cell lines have been a popular cancer model to test the CAR T cell performance in pre-clinical studies [105,106,107]. LV vectors have been utilised to rapidly modify immortalised cell lines to obtain stable expression of the target TAA and to use the modified cells for the development of in vitro killing assays, which are essential to demonstrate the therapy’s potency and specificity [99,106]. The same target cell lines can then be repurposed and engrafted to produce a tumour model in vivo in order to study the safety and efficacy of the therapy [99]. Murine models have played a major role for in vivo testing of CAR T cell therapies, and are used to test different aspects of therapeutic efficacy and safety [108]. Mouse models can be divided into four main classes: syngeneic immunocompetent, human xenograft, immunocompetent transgenic and humanised transgenic [109,110,111]. All these models are infused with human or mouse cancer cell lines to test CAR T cells and are essential to understanding tumour development and systemic immune responses to the therapy [108]. Providing evidence that the therapy developed in pre-clinical phases is both safe and efficacious is also a prerequisite to progressing to in-human clinical phases [112].
4.5. Development of Instruments Integrating LV to Current T Cell Bioprocesses
The autologous approach to CAR T cell therapy has proven to be effective; however, many challenges remain to be addressed. Bioreactor manufacturers have been designing platforms accommodating the requirements of GMP production in closed systems and including specific adaptations with T cell bioprocessing in mind e.g., the ambr 250 (Sartorius, Göttingen, Germany), Replicell™System (Aastrom Biosciences, Cambridge, MA, USA), Cocoon (Lonza, Basel, Switzerland) and Quantum Cell Expansion System (Terumo BCT, Lakewood, CO, USA) [113]. One of the most popular of these systems is the CliniMACS Prodigy bioreactor platform (Miltenyi, Bergisch Gladbach, Germany). It is a single-use, fully closed and GMP-compliant solution for T cell engineering. This bioreactor has a culture chamber which includes a centrifuge for medium exchange and formulation and can be operated in lower grade clean room environments. This platform includes viral vector application lines which allow the T cell therapy manufacturer to fully integrate LV transduction to the manufacturing process [114,115,116]. It is suited for an autologous approach, as the scale is only adapted for the production of a single dose per instrument. Although this solution does not allow scaled-up production, it represents a promising scale-out solution and could be used in a decentralised model where doses are produced closer to, or within, the clinical site [117]. This technology has been used on-site for Phase I clinical trials investigating a dual anti-CD19 anti-CD20 CAR T cell therapy for non-Hodgkin’s B-cell lymphomas. The 14-day manufacturing process fully integrated the transduction of the anti-CD19/CD20 CAR genes using an LV vector. Of the 8 patients treated, all therapeutic doses passed the release criteria, exceeded the cell number required for infusion and were successfully administered [118]. These technologies could provide a solution for improving product quality and production flexibility and could also reduce production costs, logistics complexity and product variability.
4.6. CAR T Cell Allogeneic Approach Using LV Vectors
Autologous CAR T cell therapy has proven to be an effective strategy for current therapeutic applications. The starting cellular material is sourced directly from the patient, mitigating risks associated with graft versus host disease (GvHD) [119]. Furthermore, the autologous nature of the material does not result in the production of anti-HLA donor-specific antibodies (DSAs) which can limit the number of doses and in vivo persistence of the whole organ transplant and allogeneic T cells [120,121]. Although autologous T cell therapies have proven to be easier to develop, major limitations render this approach difficult to scale up for reducing production time and cost. Starting material variability compounded with low cellular expansion potential renders a therapeutic dose difficult to achieve during the expansion of autologous T cells [122]. This is due to the nature of the material sourced from patients who have undergone multiple lines of treatment prior being eligible for cell therapy [123]. In addition, the autologous nature of the material means that each dose is specific to each patient, which rules out any option for large-scale production, leaving only bespoke scale-out options [69]. Due to all these limitations, increasing interest has been generated around the allogeneic approach in a one-size-fits-all model. In this case, the material would originate from a universal source such as genetically modified donor T cells, umbilical cord blood (UCB), placenta, immortalised cell lines, or induced pluripotent stem cell (iPSC)-derived T cells, opening the possibility to large scale production [124,125,126,127,128]. At present, only the genetically modified donor T cell and immortalised cell line approaches have progressed to the clinical stage, as the stem cell-derived approaches still require further pre-clinical optimisation [125,127].
The first proof-of-concept in-human testing of the donor-derived T cell allogeneic CAR T cell therapy was performed in 2015 in two paediatric patients at Great Ormond Street Hospital, London. The UCART19 technology was used in conjunction with lymphodepletion, including an anti-CD52 serotherapy, prior to the CAR T cell infusion. An LV vector was used to introduce the anti-CD19 CAR therapeutic gene, and a transcription activator-like effector nuclease (TALEN), a gene editing tool, was used to knock out the CD52 and TCR genes in the donor T cells to evade lymphodepletion and avoid GvHD. The results of this study showed that following allogeneic CAR T cell infusion, molecular remission could be achieved 28 days after infusion in both patients [125]. UCART19 was exclusively licensed by Cellectis to Servier, who sponsored two Phase I clinical trials started in 2016 and completed in 2020. In total, 7 children and 14 adults with B cell acute lymphoblastic leukaemia were enrolled. Published results from both trials showed that the most common adverse effects were CRS (91%) and neurotoxicity (38%) which resulted in two treatment-related deaths. Overall survival was 55% and progression-free survival 27% [129]. Building on UCART19′s initial success, Cellectis, Allogene and Servier begun investigating the use of TCR and CD52 KO CAR T cells in conjunction with an anti-CD52 recombinant antibody to treat relapsed or refractory large B cell lymphoma or follicular lymphoma. The administration of the allogeneic CAR T cells (ALLO-501) is performed after lymphodepletion of the patient’s own immune cells using fludarabine, cyclophosphamide, and a recombinant anti-CD52 antibody (ALLO-647). This technology is currently under investigation in a phase I, single-arm, open-label trial in 22 patients (NCT03939026) [130]. The preliminary data presented at the American Society of Clinical Oncology in 2020 showed ALLO-501 and ALLO-647 produced an objective response rate (ORR) in 12/19 patients (63%) with complete response (CR) in 37% of responding patients [131].
Although the allogeneic T cell donor approach has shown promising results, it still requires more process steps to knock out or counteract immunogenic T cell biological features that otherwise may result in GvHD. The integration of LV vector technology in the most advanced allogeneic CAR T cell therapy bioprocesses further demonstrate the relevance of this method for gene transfer in T cell engineering. If ongoing clinical trials result in market approval, demand on clinical grade LV vector and stress on global supply will further increase. This outlines the necessity for improved processes and technologies to increase the production and quality of LV vectors for T cell engineering.
4.7. Stable Production of LV Vectors to Address Increasing Demand in Vectors
In an endeavor to address global demand, efforts have been invested in improving current LV bioprocessing protocols and developing technologies to increase production. Large pharmaceutical companies have been investing in research and development of technologies designed to address bottlenecks. One of the main restrictions is the scale at which LV vectors can be produced using the HEK293T cell line. Although this HEK293T adherent cells transiently transfected with plasmid DNA LV systems can achieve high titres, the adherent nature of this cell line represents an obstacle to conventional scale-up solutions such as stir tank bioreactor production [132]. Efforts to address several of the bottlenecks presented by this method of production have been attempted in recent years. Stable production of LV vectors with dedicated packaging cell lines have been suggested as a potential solution to reduce cost associated with the use of large quantities of GMP-grade plasmid DNA required in transient production models [133]. In stable packaging cell lines (PCL), the viral genes required for LV production are stably transduced into the PCL genome. High titre-producing clones can be selected for vector production [33,134,135,136,137,138]. This approach has shown some encouraging results, though low titres compared to transient transfection still remains a major obstacle [139]. In addition to stable PCLs, the development of suspension variants of HEK 293T cells has also been suggested as an alternative [140]. Recently, Chen et al. (2020) combined both suspension and stable aspects in their cell line using a single stably-transfected construct producing comparable LV titres to transient transfection systems. This technology was tested in a stir tank bioreactor which offers potential for up-scaling production; CAR T cell therapy was cited as one of the potential applications [141]. Originally developed by the Telethon Institute for Gene Therapy (TIGET, Milan, Italy) and Cell Genesys (San Francisco, CA, USA), this technology was transferred to GlaxoSmithKline under the rare diseases strategic alliance and patented (patent number PCT/EP2016/078336), outlining the potential for future commercialisation [141,142].
4.8. Dedicated LV Technology for CAR T Cell Therapy Applications
In addition to increasing global production capabilities of LV vectors, development of dedicated LV particles for CAR T cell applications can improve therapy bioprocessing and biological function. When using LV vectors for gene delivery, the activation of T cells is essential to achieving high levels of transduction efficiency [143]. The activation of T cells also leverages the natural ability of immune cells to clonally expand when responding to an antigen. This biological feature of T lymphocytes is used to produce sufficient cell numbers required for treating the patient from the initial sample obtained by leukapheresis. This process is typically carried out using magnetic or polymer beads conjugated to anti-CD3 and anti-CD28 antibodies presented in Table 2 [69]. The LentiSTIM and RetroSTIM technology (patent number PCT/GB2016/050537) has been developed to combine both transduction and activation of CAR T cells. During the LV vector budding from the producer cell, some cellular proteins are carried along on the viral envelope [144]. This LentiSTIM particle is produced in cell lines expressing anti-CD3 and anti-CD28 membrane-bound mitogens, which are subsequently present on the LV envelope as it buds from the producer cell membrane. The same approach has also been employed to express biotin groups on the LV envelope to improve downstream purification [145]. This technology streamlines the CAR T cell production by reducing the reagents and process steps required and improving vector purity [146].

Table 2.
GMP compliant T cell activation technologies.
4.9. Alternative Approaches to CAR T Cell Therapy Using LV Technology
TCR T cell therapy is an alternative to CAR-transduced T cells for targeting malignancies. Instead of relying on a chimeric antigen receptor, this therapy relies on a modified T cell receptor [147]. The T cells are engineered to express a TCR with a specific affinity for a target antigen, and the manufacturing of this type of therapy is identical to CAR T cell therapy. The main advantage of TCR T cells is that the engineered receptor uses the same activation and immune pathways as a non-modified T cell, reducing the risk of CRS and neurotoxicity [148]. Adaptimmune Therapeutics, PLC is the proprietor of the Specific Peptide Enhanced Affinity Receptor (SPEAR) technology and is currently sponsoring or licensing technology in several clinical trials for the treatment of solid tumour cancers. The SPEAR T cells contain optimised TCRs and are manufactured using an LV vector encoding the TCR transgene [149]. The first completed Phase II trial was the “redirected auto T cells for advanced myeloma” trial sponsored by GlaxoSmithKline (NCT01352286), resulting in an ORR in 20/25 patients (80%) at day 42 and 11/25 patients (44%) after 1 year [149,150]. The SPEAR T cells are also under clinical testing in the Phase II SPEAREAD 1 (NCT04044768) and 2 (NCT04408898) trials sponsored by Adaptimmune, investigating the therapy ADP-A2M4 for the treatment of synovial sarcoma, myxoid liposarcoma and head and neck cancer [151]. T cells redirected for universal cytokine-mediated killing or TRUCKs are another type of alternative receptor to CARs. Sometimes referred to as the fourth generation of CAR T cells, TRUCKs have been developed to improve CAR T cell efficacy in solid tumours through cytokine-release. Upon binding of the CAR to its target antigen, specific cytokines are released from the gene-modified T cell, therefore counteracting immunosuppressant effects of the TME [152]. TRUCKs are currently under clinical investigation and can give an answer to improving CAR activity in solid tumours. For example, an anti-EGFR-IL12-CART is currently tested against metastatic colorectal cancer (NCT03542799) [153]. Specific LV technology has been developed to transduce all the elements required to produce a fully functional TRUCK using a single LV construct, further increasing the appeal of this class of T cell-based immunotherapy [154].
In addition to alternatives to chimeric antigen receptors, novel T cell subsets are considered as new potential therapeutic cells. Amongst new candidates, γδ T cells, natural killer T (NKT) and T regulatory (Treg) cells have been suggested as promising alternative cell types for adoptive T cell therapy and LV technology has been frequently employed for their engineering [155,156]. The γδ T cells account for 1–10% of the CD3+ T cell population. They are a highly conserved immune cell type and have shown attractive characteristics for adoptive T cell therapy applications. These include their potent graft versus tumour (GVT) activity as well as their low GVHD potential with allogeneic transplantation [157]. A study tracking the immune profile of 108 cancer patients up to 3 months after having received HSCT demonstrated that high levels of γδ T cells were associated with significantly higher overall survival and relapse-free survival [158]. Protocols and patents for γδ T cell culture and transduction using LV vectors have been published and developed to include adaptations for future manufacturing, such as serum-free medium culture [159,160,161]. Some studies have been progressed to Phase I testing, such as the IN8BIO Inc. technology NC200 clinical trial investigating the use of LV-modified γδ T cells to target glioblastoma multiform (GBM) in adults [162].
Invariant natural killer T cells or iNKT cells have also been suggested as a promising T cell subset for CAR T therapy. As γδ T cells, they are a rare subset of T cells and represent less than 1% of circulating PBMCs. They play an important immunomodulatory role and have shown to have strong anti-tumour activity [163]. A strong advantage of iNKT is the ability to transplant them without causing GVHD, making them a strong candidate for “off the shelf” applications. iNKT cells have been used in multiple clinical trials including therapy against solid tumours such as neuroblastoma. An anti-GD2 CAR construct has been transduced using a GRV vector into iNKT cells to target neuroblastoma. Preclinical data against showed in vivo efficacy with no significant toxicity, which led to progression to clinical phase [164,165]. The ongoing Phase I clinical study investigates the efficacy of CAR iNKTs called GINAKIT cells (NCT03294954) in combination with antibody therapy against relapsed or refractory high-risk neuroblastoma in patients between 1 and 21 years old [166]. iNKTs are also being tested against blood cancers, e.g., an anti-CD19 CAR-iNKT therapy for relapsed, refractory, high-risk B cell tumours is currently in phase I trials (NCT04814004) [167].
Regulatory T cells, or Tregs, are a subset of CD4-positive T cells, of which the principal role is controlling immune responses [168]. Rather than carrying out pro-inflammatory functions, Tregs have an inhibitory effect on other immune cell types, controlling immune responses to foreign pathogens and preventing autoimmune diseases. Tregs have been suggested for new promising therapeutic applications and expand adoptive T cell therapy beyond oncology [169]. Because of their natural ability to dampen immune responses, these cells are particularly suited for the treatment of autoimmune diseases and the prevention of GvHD in graft recipients [120,170]. The first in-human Treg clinical trial testing Sangamo CAR Treg product TX200-KT02 has been approved for Phase I/IIa (2019-001730-34). The Tregs are transduced using a LV vector to express a CAR receptor against the recipient’s HLA A*2 and prevent immune-mediated allograft rejection in patients with end-stage renal disease (ESRD) [171].
5. Conclusions
LV vectors have been instrumental in the development of gene modified T cell therapies. These retroviral vectors have represented a safe and effective tool for gene transfer and have been applied to many of the development steps required for the recent progression of CAR T cell therapies to the market. Not only have LV vectors been directly applied for the transfer of the CAR therapeutic gene, they have also been employed to develop target cells required for in vitro and in vivo testing. The importance of LV vectors in T cell therapy development has been reflected by not only their integration into T cell therapy protocols but also instrument design used in their manufacturing. The advancement of therapies through later phase clinical trial and access to fast-track schemes set up by regulators, as well as active involvement from the biotechnology and pharmaceutical industry, foretells the future importance of T cell therapies in the oncology therapeutic arsenal. Although alternatives for gene transfer have been proposed, LV and GRV vectors are currently the most advanced methods for gene-modified T cell therapy manufacturing.
Author Contributions
Conceptualization, R.P.L.; investigation, R.P.L.; writing—original draft preparation, R.P.L.; writing—review and editing, R.P.L., S.V. and Q.A.R.; supervision, S.V. and Q.A.R.; project administration, S.V. and Q.A.R.; funding acquisition, Q.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was secured from the UK Engineering and Physical Sciences Research Council (EPSRC) through the Future Targeted Healthcare Manufacturing Hub hosted at University College London with UK university partners (Grant Reference: EP/P006485/1), including financial and in-kind support from the consortium of industrial users and sector organisations.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This review article did not include original data, all material cited has been referenced in text and in the bibliography.
Acknowledgments
The authors would like to acknowledge the funding and support of the UK Engineering and Physical Sciences Research Council (EPSRC) through the Future Targeted Healthcare Manufacturing Hub hosted at University College London with UK university partners (Grant Reference: EP/P006485/1), including financial and in-kind support from the consortium of industrial users and sector organisations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- David, R. Wessner Origin of Viruses. Nat. Educ. 2010, 3, 37. [Google Scholar]
- Milone, M.C.; O’Doherty, U. Clinical Use of Lentiviral Vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Miller, A.D. Retroviral Vectors: From Cancer Viruses to Therapeutic Tools. Hum. Gene Ther. 2014, 25, 989–994. [Google Scholar] [CrossRef]
- Mohanty, R.; Chowdhury, C.R.; Arega, S.; Sen, P.; Ganguly, P.; Ganguly, N. CAR T Cell Therapy: A New Era for Cancer Treatment (Review). Oncol. Rep. 2019, 42, 2183–2195. [Google Scholar] [CrossRef]
- European Medicines Agency; Committee for Medicinal Products for Human Use; Committee for Medicinal Products for Human Use (CHMP). Summary of Positive Opinion Kymriah. 2018. [Google Scholar]
- Food and Drug Administration; Center for Biologics Evaluation and Research. August 30, 2017 Approval Letter—KYMRIAH; Food and Drug Administration: Silver Spring, MD, USA, 2017. [Google Scholar]
- European Medicines Agency; Committee for Medicinal Products for Human Use; Committee for Medicinal Products for Human Use (CHMP). Summary of Positive Opinion for Yescarta. 2018. [Google Scholar]
- Food and Drug Administration; Center for Biologics Evaluation and Research. October 18, 2017 Approval Letter—YESCARTA; Food and Drug Administration: Silver Spring, MD, USA, 2017. [Google Scholar]
- European Medicines Agency; Committee for Medicinal Products for Human Use; Committee for Medicinal Products for Human Use (CHMP). Summary of Positive Opinion for Tecartus. 2020. [Google Scholar]
- Food and Drug Administration; Center for Biologics Evaluation and Research. July 24, 2020 Approval Letter—TECARTUS; Food and Drug Administration: Silver Spring, MD, USA, 2020. [Google Scholar]
- Elsner, C.; Bohne, J. The Retroviral Vector Family: Something for Everyone. Virus Genes 2017, 53, 714–722. [Google Scholar] [CrossRef]
- Cantore, A.; Ranzani, M.; Bartholomae, C.C.; Volpin, M.; Valle, P.D.; Sanvito, F.; Sergi, L.S.; Gallina, P.; Benedicenti, F.; Bellinger, D.; et al. Liver-Directed Lentiviral Gene Therapy in a Dog Model of Hemophilia B. Sci. Transl. Med. 2015, 12, 7. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Lauer, A.K.; Sohn, E.H.; Mir, T.A.; Naylor, S.; Anderton, M.C.; Kelleher, M.; Harrop, R.; Ellis, S.; Mitrophanous, K.A. Lentiviral Vector Gene Transfer of Endostatin/Angiostatin for Macular Degeneration (GEM) Study. Hum. Gene Ther. 2017, 28, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.L.; Miskin, J.; Wonnacott, K.; Keir, C. Global Manufacturing of CAR T Cell Therapy. Mol. Ther. Methods Clin. Dev. 2017, 4, 92–101. [Google Scholar] [CrossRef]
- Munis, A.M. Gene Therapy Applications of Non-Human Lentiviral Vectors. Viruses 2020, 12, 1106. [Google Scholar] [CrossRef]
- Shaw, A.M.; Joseph, G.L.; Jasti, A.C.; Sastry-Dent, L.; Witting, S.; Cornetta, K. Differences in Vector-Genome Processing and Illegitimate Integration of Non-Integrating Lentiviral Vectors. Gene Ther. 2017, 24, 12–20. [Google Scholar] [CrossRef]
- Powell, S.K.; Rivera-Soto, R.; Gray, S.J. Viral Expression Cassette Elements to Enhance Transgene Target Specificity and Expression in Gene Therapy. Discov. Med. 2015, 19, 49–57. [Google Scholar]
- Gándara, C.; Affleck, V.; Stoll, E.A. Manufacture of Third-Generation Lentivirus for Preclinical Use, with Process Development Considerations for Translation to Good Manufacturing Practice. Hum. Gene Ther. Methods 2018, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Barajas, B.C.; Tanaka, M.; Robinson, B.A.; Phuong, D.J.; Chutiraka, K.; Reed, J.C.; Lingappa, J.R. Identifying the Assembly Intermediate in Which Gag First Associates with Unspliced HIV-1 RNA Suggests a Novel Model for HIV-1 RNA Packaging. PLoS Pathog. 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.H.; Huang, K.J.; Wang, C.T. HIV-1 Mutant Assembly, Processing and Infectivity Expresses Pol Independent of Gag. Viruses 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Bandres, J.C.; Wang, Q.F.; O’Leary, J.; Baleaux, F.; Amara, A.; Hoxie, J.A.; Zolla-Pazner, S.; Gorny, M.K. Human Immunodeficiency Virus (HIV) Envelope Binds to CXCR4 Independently of CD4, and Binding Can Be Enhanced by Interaction with Soluble CD4 or by HIV Envelope Deglycosylation. J. Virol. 1998, 72, 2500–2504. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; Wong, R.; Stoilov, P.; Pan, S.; Blencowe, B.; Cheung, P.; Harrigan, P.R.; Cochrane, A. Identification of Small Molecule Modulators of HIV-1 Tat and Rev Protein Accumulation. Retrovirology 2017, 14, 7. [Google Scholar] [CrossRef]
- Zotova, A.; Atemasova, A.; Pichugin, A.; Filatov, A.; Mazurov, D. Distinct Requirements for HIV-1 Accessory Proteins during Cell Coculture and Cell-Free Infection. Viruses 2019, 11, 390. [Google Scholar] [CrossRef]
- Daniels, S.M.; Sinck, L.; Ward, N.J.; Melendez-Pe~ Na, C.E.; Scarborough, R.J.; Azar, I.; Rance, E.; Icha Daher, A.; Pang, K.-M.; Rossi, J.J.; et al. HIV-1 RRE RNA Acts as an RNA Silencing Suppressor by Competing with TRBP-Bound SiRNAs. RNA Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Datta, G.; Geiger, J.D.; Chen, X. Apolipoprotein E Isoform Dependently Affects Tat-Mediated HIV-1 LTR Transactivation. J. Neuroinflammation 2018, 15. [Google Scholar] [CrossRef]
- Kay, M.A.; Glorioso, J.C.; Naldini, L. Viral Vectors for Gene Therapy: The Art of Turning Infectious Agents into Vehicles of Therapeutics. Nat. Med. 2001, 7, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral Vectors: A Look Back and Ahead on Gene Transfer Technology. New Microbiol. 2013, 36, 1–22. [Google Scholar]
- Rothe, M.; Modlich, U.; Schambach, A. Biosafety Challenges for Use of Lentiviral Vectors in Gene Therapy. Curr. Gene Ther. 2014, 13, 453–468. [Google Scholar] [CrossRef]
- Dull, T.; Zufferey, R.; Kelly, M.; Mandel, R.J.; Nguyen, M.; Trono, D.; Naldini, L. A Third-Generation Lentivirus Vector with a Conditional Packaging System. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.L.; Case, S.S.; Crooks, G.M.; Kohn, D.B. Critical Factors Influencing Stable Transduction of Human CD34+ Cells with HIV-1-Derived Lentiviral Vectors. Mol. Ther. 2000, 2, 71–80. [Google Scholar] [CrossRef]
- Vink, C.A.; Counsell, J.R.; Perocheau, D.P.; Karda, R.; Buckley, S.M.K.; Brugman, M.H.; Galla, M.; Schambach, A.; Mckay, T.R.; Waddington, S.N.; et al. Eliminating HIV-1 Packaging Sequences from Lentiviral Vector Proviruses Enhances Safety and Expedites Gene Transfer for Gene Therapy. Mol. Ther. 2017, 25, 1790–1804. [Google Scholar] [CrossRef]
- Albrecht, C.; Hosiner, S.; Tichy, B.; Aldrian, S.; Hajdu, S.; Nürnberger, S. Comparison of Lentiviral Packaging Mixes and Producer Cell Lines for RNAi Applications. Mol. Biotechnol. 2015, 57, 499–505. [Google Scholar] [CrossRef]
- Valkama, A.J.; Leinonen, H.M.; Lipponen, E.M.; Turkki, V.; Malinen, J.; Heikura, T.; Ylä-Herttuala, S.; Lesch, H.P. Optimization of Lentiviral Vector Production for Scale-up in Fixed-Bed Bioreactor. Gene Ther. 2018, 25, 39–46. [Google Scholar] [CrossRef]
- Strobel, B.; Zuckschwerdt, K.; Zimmermann, G.; Mayer, C.; Eytner, R.; Rechtsteiner, P.; Kreuz, S.; Lamla, T. Standardized, Scalable, and Timely Flexible Adeno-Associated Virus Vector Production Using Frozen High-Density HEK-293 Cell Stocks and CELLdiscs. Hum. Gene Ther. Methods 2019, 30, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rout-Pitt, N.; McCarron, A.; McIntyre, C.; Parsons, D.; Donnelley, M. Large-Scale Production of Lentiviral Vectors Using Multilayer Cell Factories. J. Biol. Methods 2018, 5, 90. [Google Scholar] [CrossRef]
- McCarron, A.; Donnelley, M.; McIntyre, C.; Parsons, D. Transient Lentiviral Vector Production Using a Packed-Bed Bioreactor System. Hum. Gene Ther. Methods 2019, 30, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, I.; Ballard, B.; Zhang, M.; Chen, Y.; West, J.; Dotti, G.; Savoldo, B. Inducible Caspase-9 Selectively Modulates the Toxicities of CD19-Specific Chimeric Antigen Receptor-Modified T Cells. Mol. Ther. 2017, 25, 580–592. [Google Scholar] [CrossRef]
- Blessing, D.; Déglon, N.; Schneider, B.L. Scalable production and purification of adeno-associated viral vectors (AAV). In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1850, pp. 259–274. [Google Scholar]
- Labisch, J.J.; Bollmann, F.; Wolff, M.W.; Pflanz, K. A New Simplified Clarification Approach for Lentiviral Vectors Using Diatomaceous Earth Improves Throughput and Safe Handling. J. Biotechnol. 2021, 326, 11–20. [Google Scholar] [CrossRef]
- Sastry, L.; Xu, Y.; Cooper, R.; Pollok, K.; Cornetta, K. Evaluation of Plasmid DNA Removal from Lentiviral Vectors by Benzonase Treatment. Hum. Gene Ther. 2004, 15, 221–226. [Google Scholar] [CrossRef]
- Merten, O.W.; Hebben, M.; Bovolenta, C. Production of Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 2016, 3, 16017. [Google Scholar] [CrossRef]
- Ruscic, J.; Perry, C.; Mukhopadhyay, T.; Takeuchi, Y.; Bracewell, D.G. Lentiviral Vector Purification Using Nanofiber Ion-Exchange Chromatography. Mol. Ther. Methods Clin. Dev. 2019, 15, 52–62. [Google Scholar] [CrossRef]
- Kumru, O.S.; Wang, Y.; Gombotz, C.W.R.; Kelley-Clarke, B.; Cieplak, W.; Kim, T.; Joshi, S.B.; Volkin, D.B. Physical Characterization and Stabilization of a Lentiviral Vector Against Adsorption and Freeze-Thaw. J. Pharm. Sci. 2018, 107, 2764–2774. [Google Scholar] [CrossRef]
- Valkama, A.J.; Oruetxebarria, I.; Lipponen, E.M.; Leinonen, H.M.; Käyhty, P.; Hynynen, H.; Turkki, V.; Malinen, J.; Miinalainen, T.; Heikura, T.; et al. Development of Large-Scale Downstream Processing for Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 2020, 17, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Jones, P. Lentiviral Vector CMC Considerations for Clinical and Commercial Use. In Proceedings of the ATMP Manufacturing Community 19th Technical Meeting: Manufacturing ATMPs at Scale, Dublin, Ireland, 10 October 2019. [Google Scholar]
- Truran, R.; Buckley, R.; Radcliffe, P.; Miskin, J.; Mitrophanous, K. Virus Purification; United States Patent and Trademark Office: Washington, DC, USA, 2015. [Google Scholar]
- Newell, E.W.; Davis, M.M. Beyond Model Antigens: High-Dimensional Methods for the Analysis of Antigen-Specific T Cells. Nat. Biotechnol. 2014, 32, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Ma, K.Y.; Schonnesen, A.A.; Zhang, M.; He, C.; Sun, E.; Williams, C.M.; Jia, W.; Jiang, N. High-Throughput Determination of the Antigen Specificities of T Cell Receptors in Single Cells. Nat. Biotechnol. 2018, 36, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Spottiswoode, C.N.; Busch, R. Vive La Difference! Self/Non-Self Recognition and the Evolution of Signatures of Identity in Arms Races with Parasites. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 1–12. [Google Scholar] [CrossRef]
- Eun, S.-Y.; Lee, S.-W.; Xu, Y.; Croft, M. 4-1BB Ligand Signaling to T Cells Limits T Cell Activation. J. Immunol. 2015, 194, 134–141. [Google Scholar] [CrossRef]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lin, Q.; Zhang, Z.; Zhang, L. Therapeutic Strategies for the Costimulatory Molecule OX40 in T-Cell-Mediated Immunity. Acta Pharm. Sin. B 2020, 10, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T Cell Responses: Naïve to Memory and Everything in Between. Am. J. Physiol. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Clénet, M.L.; Gagnon, F.; Moratalla, A.C.; Viel, E.C.; Arbour, N. Peripheral Human CD4+CD8+ T Lymphocytes Exhibit a Memory Phenotype and Enhanced Responses to IL-2, IL-7 and IL-15. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Baliu-Piqué, M.; Verheij, M.W.; Drylewicz, J.; Ravesloot, L.; de Boer, R.J.; Koets, A.; Tesselaar, K.; Borghans, J.A.M. Short Lifespans of Memory T-Cells in Bone Marrow, Blood, and Lymph Nodes Suggest That T-Cell Memory Is Maintained by Continuous Self-Renewal of Recirculating Cells. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.N. T-Cell Development and the CD4-CD8 Lineage Decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Candeias, S.M.; Gaipl, U.S. The Immune System in Cancer Prevention, Development and Therapy. Anticancer. Agents Med. Chem. 2015, 16, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Aguilera, J.V.; Palermo, B.; Markovic, S.N.; Nisticò, P.; Signore, A. Relevance of Immune Cell and Tumor Microenvironment Imaging in the New Era of Immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 1–21. [Google Scholar] [CrossRef]
- Forget, M.A.; Haymaker, C.; Hess, K.R.; Meng, Y.J.; Creasy, C.; Karpinets, T.; Fulbright, O.J.; Roszik, J.; Woodman, S.E.; Kim, Y.U.; et al. Prospective Analysis of Adoptive TIL Therapy in Patients with Metastatic Melanoma: Response, Impact of Anti-CTLA4, and Biomarkers to Predict Clinical Outcome. Clin. Cancer Res. 2018, 24, 4416–4428. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune Evasion in Cancer: Mechanistic Basis and Therapeutic Strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Gorochov, G.; Waks, T.; Eshhar, Z. Generation of Effector T Cells Expressing Chimeric T Cell Receptor with Antibody Type-Specificity. Transplant. Proc. 1989, 21, 127–130. [Google Scholar]
- Kuwana, Y.; Asakura, Y.; Utsunomiya, N.; Nakanishi, M.; Arata, Y.; Itoh, S.; Nagase, F.; Kurosawa, Y. Expression of Chimeric Receptor Composed of Immunoglobulin-Derived V Resions and T-Cell Receptor-Derived C Regions. Biochem. Biophys. Res. Commun. 1987, 149, 960–968. [Google Scholar] [CrossRef]
- Blaeschke, F.; Stenger, D.; Kaeuferle, T.; Willier, S.; Lotfi, R.; Kaiser, A.D.; Assenmacher, M.; Döring, M.; Feucht, J.; Feuchtinger, T. Induction of a Central Memory and Stem Cell Memory Phenotype in Functionally Active CD4+ and CD8+ CAR T Cells Produced in an Automated Good Manufacturing Practice System for the Treatment of CD19+ Acute Lymphoblastic Leukemia. Cancer Immunol. Immunother. 2018, 67, 1053–1066. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef]
- Hurton, L.V.; Singh, H.; Najjar, A.M.; Switzer, K.C.; Mi, T.; Maiti, S.; Olivares, S.; Rabinovich, B.; Huls, H.; Forget, M.A.; et al. Tethered IL-15 Augments Antitumor Activity and Promotes a Stem-Cell Memory Subset in Tumor-Specific T Cells. Proc. Natl. Acad. Sci. USA 2016, 113, E7788–E7797. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.T.; Krenciute, G. Next Generation CAR T Cells for the Immunotherapy of High-Grade Glioma. Front. Oncol. 2019, 9, 69. [Google Scholar] [CrossRef]
- Townsend, M.H.; Bennion, K.; Robison, R.A.; O’Neill, K.L. Paving the Way towards Universal Treatment with Allogenic T Cells. Immunol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rivière, I. Clinical Manufacturing of CAR T Cells: Foundation of a Promising Therapy. Mol. Ther. Oncolytics 2016, 3, 16015. [Google Scholar] [CrossRef]
- Tyagarajan, S.; Spencer, T.; Smith, J. Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials. Mol. Ther. Methods Clin. Dev. 2020, 16, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, M.; März, J.; Kummer, M.; Schuler, G.; Dörrie, J.; Schuler-Thurner, B.; Schaft, N. Clinical-Scale Production of Car-t Cells for the Treatment of Melanoma Patients by Mrna Transfection of a Cspg4-Specific Car under Full Gmp Compliance. Cancers 2019, 11, 1198. [Google Scholar] [CrossRef] [PubMed]
- Ghorashian, S.; Kramer, A.M.; Onuoha, S.; Wright, G.; Bartram, J.; Richardson, R.; Albon, S.J.; Casanovas-Company, J.; Castro, F.; Popova, B.; et al. Enhanced CAR T Cell Expansion and Prolonged Persistence in Pediatric Patients with ALL Treated with a Low-Affinity CD19 CAR. Nat. Med. 2019, 25, 1408–1414. [Google Scholar] [CrossRef]
- Xhangolli, I.; Dura, B.; Lee, G.H.; Kim, D.; Xiao, Y.; Fan, R. Single-Cell Analysis of CAR-T Cell Activation Reveals A Mixed TH1/TH2 Response Independent of Differentiation. Genom. Proteom. Bioinforma. 2019, 17, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.; Bachier, C.; Westin, J.; Rezvani, K.; Shpall, E.J. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden. Am. Soc. Clin. Oncol. Educ. B. 2019, 39, 433–444. [Google Scholar] [CrossRef]
- Fesnak, A.D. The Challenge of Variability in Chimeric Antigen Receptor T Cell Manufacturing. Regen. Eng. Transl. Med. 2019, 1–8. [Google Scholar] [CrossRef]
- Bonifant, C.L.; Jackson, H.J.; Brentjens, R.J.; Curran, K.J. Toxicity and Management in CAR T-Cell Therapy. Mol. Ther. Oncolytics 2016, 3, 16011. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, K.T.; Jadlowsky, J.K.; Hwang, W.T.; Suhoski-Davis, M.; Gonzalez, V.E.; Kulikovskaya, I.; Gupta, M.; Lacey, S.F.; Plesa, G.; Chew, A.; et al. Retroviral and Lentiviral Safety Analysis of Gene-Modified T Cell Products and Infused HIV and Oncology Patients. Mol. Ther. 2018, 26, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.; Stanton, J.; Powers, D.; Karnieli, O.; Nahum, S.; Abraham, E.; Parisse, J.S.; Oh, S. Managing Particulates in Cell Therapy: Guidance for Best Practice. Cytotherapy 2016, 18, 1063–1076. [Google Scholar] [CrossRef]
- Maziarz, R.T.; Waller, E.K.; Jaeger, U.; Fleury, I.; McGuirk, J.; Holte, H.; Jaglowski, S.; Schuster, S.J.; Bishop, M.R.; Westin, J.R.; et al. Patient-Reported Long-Term Quality of Life after Tisagenlecleucel in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood Adv. 2020, 4, 629–637. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Meeting Report: Workshop with Stakeholders on Support to Quality Development in Early Access Approaches (i.e., PRIME, Breakthrough Therapies). 2018.
- Stirrups, R. CAR T-Cell Therapy for Relapsed or Refractory Mantle-Cell Lymphoma. Lancet. Oncol. 2020, 21, e239. [Google Scholar] [CrossRef]
- Papadouli, I.; Mueller-Berghaus, J.; Beuneu, C.; Ali, S.; Hofner, B.; Petavy, F.; Tzogani, K.; Miermont, A.; Norga, K.; Kholmanskikh, O.; et al. EMA Review of Axicabtagene Ciloleucel (Yescarta) for the Treatment of Diffuse Large B-Cell Lymphoma. Oncologist 2020, 25, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Laetsch, T.W.; Myers, G.D.; Baruchel, A.; Dietz, A.C.; Pulsipher, M.A.; Bittencourt, H.; Buechner, J.; De Moerloose, B.; Davis, K.L.; Nemecek, E.; et al. Patient-Reported Quality of Life after Tisagenlecleucel Infusion in Children and Young Adults with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukaemia: A Global, Single-Arm, Phase 2 Trial. Lancet Oncol. 2019, 20, 1710–1718. [Google Scholar] [CrossRef]
- Detela, G.; Lodge, A. EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation. Mol. Ther. Methods Clin. Dev. 2019, 13, 205–232. [Google Scholar] [CrossRef]
- Sena-Esteves, M.; Gao, G. Titration of Lentivirus Vectors. Cold Spring Harb. Protoc. 2018, 2018, 281–285. [Google Scholar] [CrossRef]
- Lana, M.G.; Strauss, B.E. Production of Lentivirus for the Establishment of CAR-T Cells. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2020; Volume 2086, pp. 61–67. [Google Scholar]
- Yu, S.; Yi, M.; Qin, S.; Wu, K. Next Generation Chimeric Antigen Receptor T Cells: Safety Strategies to Overcome Toxicity. Mol. Cancer 2019, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, K.; Al-Obaidi, M.; Di Stasi, A. Generation of suicide gene-modified chimeric antigen receptor-redirected T-cells for cancer immunotherapy. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1895, pp. 57–73. [Google Scholar]
- ClinicalTrials.gov. 3rd Generation GD-2 Chimeric Antigen Receptor and ICaspase Suicide Safety Switch. Available online: https://clinicaltrials.gov/ct2/show/NCT01822652?term=CAR+suicide&draw=2&rank=1 (accessed on 17 June 2021).
- ClinicalTrials.gov. A Phase I Trial of T Cells Expressing an Anti-GD2 Chimeric Antigen Receptor in Children and Young Adults With GD2+ Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT02107963?term=CAR+suicide&draw=2&rank=5 (accessed on 17 June 2021).
- Amatya, C.; Pegues, M.A.; Lam, N.; Vanasse, D.; Geldres, C.; Choi, S.; Hewitt, S.M.; Feldman, S.A.; Kochenderfer, J.N. Development of CAR T Cells Expressing a Suicide Gene Plus a Chimeric Antigen Receptor Targeting Signaling Lymphocytic-Activation Molecule F7. Mol. Ther. 2021, 29, 702–717. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Xu, J.; Barrett, D.M.; Fraietta, J.A.; Reich, T.J.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; et al. Induction of Resistance to Chimeric Antigen Receptor T Cell Therapy by Transduction of a Single Leukemic B Cell. Nat. Med. 2018, 24, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.M.; Braun, A.H.; Scheib, L.; Agarwal, S.; Schneider, I.C.; Fusil, F.; Perian, S.; Sahin, U.; Thalheimer, F.B.; Verhoeyen, E.; et al. Combining T-Cell-Specific Activation and in Vivo Gene Delivery through CD3-Targeted Lentiviral Vectors. Blood Adv. 2020, 4, 5702–5715. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, Q.; Liang, X.; Chen, Z.; Zhang, X.; Zhou, X.; Li, M.; Tu, H.; Liu, Y.; Tu, S.; et al. Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Front. Immunol. 2019, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.Q.; Zhang, R.L.; Liu, F.; Wang, Y.; Yan, Z.L.; Song, Y.P.; Yang, T.; Li, P.; Wang, Z.; et al. Donor-Derived CD19 CAR-T Cell Therapy of Relapse of CD19-Positive B-ALL Post Allotransplant. Leukemia 2021, 35, 1563–1570. [Google Scholar] [CrossRef]
- Tong, C.; Zhang, Y.; Liu, Y.; Ji, X.; Zhang, W.; Guo, Y.; Han, X.; Ti, D.; Dai, H.; Wang, C.; et al. Optimized Tandem CD19/CD20 CAR-Engineered T Cells in Refractory/Relapsed B-Cell Lymphoma. Blood 2020, 136, 1632–1644. [Google Scholar] [CrossRef]
- Fousek, K.; Watanabe, J.; Joseph, S.K.; George, A.; An, X.; Byrd, T.T.; Morris, J.S.; Luong, A.; Martínez-Paniagua, M.A.; Sanber, K.; et al. CAR T-Cells That Target Acute B-Lineage Leukemia Irrespective of CD19 Expression. Leukemia 2020. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering Strategies to Overcome the Current Roadblocks in CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef]
- Fujiwara, K.; Tsunei, A.; Kusabuka, H.; Ogaki, E.; Tachibana, M.; Okada, N. Hinge and Transmembrane Domains of Chimeric Antigen Receptor Regulate Receptor Expression and Signaling Threshold. Cells 2020, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. Driving T-Cell Immunotherapy to Solid Tumors. Nat. Biotechnol. 2018, 36, 215–219. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Palazon, A.; Noguera-Ortega, E.; Powell, D.J.; Guedan, S. CAR-T Cells Hit the Tumor Microenvironment: Strategies to Overcome Tumor Escape. Front. Immunol. 2020, 11, 1109. [Google Scholar] [CrossRef]
- Ang, W.X.; Ng, Y.Y.; Xiao, L.; Chen, C.; Li, Z.; Chi, Z.; Tay, J.C.K.; Tan, W.K.; Zeng, J.; Toh, H.C.; et al. Electroporation of NKG2D RNA CAR Improves Vγ9Vδ2 T Cell Responses against Human Solid Tumor Xenografts. Mol. Ther. Oncolytics 2020, 17, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, K.; Rengstl, B.; Oehm, P.; Michel, K.; Billmeier, A.; Hayduk, N.; Klein, O.; Kuna, K.; Ouchan, Y.; Wöll, S.; et al. An RNA Vaccine Drives Expansion and Efficacy of Claudin-CAR-T Cells against Solid Tumors. Science 2020, 367, 446–453. [Google Scholar] [CrossRef]
- Andersch, L.; Radke, J.; Klaus, A.; Schwiebert, S.; Winkler, A.; Schumann, E.; Grunewald, L.; Zirngibl, F.; Flemmig, C.; Jensen, M.C.; et al. CD171- and GD2-Specific CAR-T Cells Potently Target Retinoblastoma Cells in Preclinical in Vitro Testing. BMC Cancer 2019, 19. [Google Scholar] [CrossRef]
- Marcinkowski, B.; Stevanović, S.; Helman, S.R.; Norberg, S.M.; Serna, C.; Jin, B.; Gkitsas, N.; Kadakia, T.; Warner, A.; Davis, J.L.; et al. Cancer Targeting by TCR Gene-Engineered T Cells Directed against Kita-Kyushu Lung Cancer Antigen-1. J. Immunother. Cancer 2019, 7, 229. [Google Scholar] [CrossRef]
- Siegler, E.L.; Wang, P. Preclinical Models in Chimeric Antigen Receptor-Engineered T-Cell Therapy. Hum. Gene Ther. 2018, 29, 534–546. [Google Scholar] [CrossRef]
- Hu, B.; Ren, J.; Luo, Y.; Keith, B.; Young, R.M.; Scholler, J.; Zhao, Y.; June, C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017, 20, 3025–3033. [Google Scholar] [CrossRef]
- Davila, M.L.; Kloss, C.C.; Gunset, G.; Sadelain, M. CD19 CAR-Targeted T Cells Induce Long-Term Remission and B Cell Aplasia in an Immunocompetent Mouse Model of B Cell Acute Lymphoblastic Leukemia. PLoS ONE 2013, 8, e61338. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; Xia, J.; Rafiq, S.; Huang, X.; Hu, Z.; Zhou, X.; Brentjens, R.J.; Yang, Y.G. Modeling Anti-CD19 CAR T Cell Therapy in Humanized Mice with Human Immunity and Autologous Leukemia. EBioMedicine 2019, 39, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Seimetz, D.; Heller, K.; Richter, J. Approval of First CAR-Ts: Have We Solved All Hurdles for ATMPs? Cell Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.P.; Zylberberg, E.; Ellison, S.; Levine, B.L. Chimeric Antigen Receptor–T Cell Therapy Manufacturing: Modelling the Effect of Offshore Production on Aggregate Cost of Goods. Cytotherapy 2019, 21, 224–233. [Google Scholar] [CrossRef]
- Fernández, L.; Fernández, A.; Mirones, I.; Escudero, A.; Cardoso, L.; Vela, M.; Lanzarot, D.; de Paz, R.; Leivas, A.; Gallardo, M.; et al. GMP-Compliant Manufacturing of NKG2D CAR Memory T Cells Using CliniMACS Prodigy. Front. Immunol. 2019, 10, 2361. [Google Scholar] [CrossRef]
- Zhang, W.; Jordan, K.R.; Schulte, B.; Purev, E. Characterization of Clinical Grade CD19 Chimeric Antigen Receptor T Cells Produced Using Automated CliniMACS Prodigy System. Drug Des. Devel. Ther. 2018, 12, 3343. [Google Scholar] [CrossRef]
- Marín Morales, J.M.; Münch, N.; Peter, K.; Freund, D.; Oelschlägel, U.; Hölig, K.; Böhm, T.; Flach, A.C.; Keßler, J.; Bonifacio, E.; et al. Automated Clinical Grade Expansion of Regulatory T Cells in a Fully Closed System. Front. Immunol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Ran, T.; Eichmüller, S.B.; Schmidt, P.; Schlander, M. Cost of Decentralized CAR T-cell Production in an Academic Nonprofit Setting. Int. J. Cancer 2020, 147, 3438–3445. [Google Scholar] [CrossRef]
- Zhu, F.; Shah, N.N.; Schneider, D.; Xu, H.; Chaney, K.; Luib, L.; Keever-Taylor, C.; Dropulic, B.; Orentas, R.; Hari, P.; et al. Automated Manufacturing of CD20.19 Bi-Specific Chimeric Antigen Receptor T (CAR-T) Cells at an Academic Center for a Phase I Clinical Trial in Relapsed, Refractory NHL. Biol. Blood Marrow Transplant. 2019, 25, S62. [Google Scholar] [CrossRef]
- Ghosh, A.; Smith, M.; James, S.E.; Davila, M.L.; Velardi, E.; Argyropoulos, K.V.; Gunset, G.; Perna, F.; Kreines, F.M.; Levy, E.R.; et al. Donor CD19 CAR T Cells Exert Potent Graft-versus-Lymphoma Activity with Diminished Graft-versus-Host Activity. Nat. Med. 2017, 23, 242–249. [Google Scholar] [CrossRef]
- Sicard, A.; Lamarche, C.; Speck, M.; Wong, M.; Rosado-Sánchez, I.; Blois, M.; Glaichenhaus, N.; Mojibian, M.; Levings, M.K. Donor-specific Chimeric Antigen Receptor Tregs Limit Rejection in Naive but Not Sensitized Allograft Recipients. Am. J. Transplant. 2020, 20, 1562–1573. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zhang, R.; Tsao, S.T.; Liu, Y.C.; Chen, X.; Lu, D.P.; Castillo, P.; Chang, L.J. Sequential Allogeneic and Autologous CAR-T-Cell Therapy to Treat an Immune-Compromised Leukemic Patient. Blood Adv. 2018, 2, 1691–1695. [Google Scholar] [CrossRef]
- Fesnak, A.D. The Challenge of Variability in Chimeric Antigen Receptor T Cell Manufacturing. Regen. Eng. Transl. Med. 2020, 6, 322–329. [Google Scholar] [CrossRef]
- Smith, S.D.; Reddy, P.; Sokolova, A.; Chow, V.A.; Lynch, R.C.; Shadman, M.A.; Till, B.G.; Shustov, A.R.; Warren, E.H.; Ujjani, C.S.; et al. Eligibility for CAR T-cell Therapy: An Analysis of Selection Criteria and Survival Outcomes in Chemorefractory DLBCL. Am. J. Hematol. 2019, 94, E117–E116. [Google Scholar] [CrossRef]
- Herrera, L.; Santos, S.; Vesga, M.A.; Anguita, J.; Martin-Ruiz, I.; Carrascosa, T.; Juan, M.; Eguizabal, C. Adult Peripheral Blood and Umbilical Cord Blood NK Cells Are Good Sources for Effective CAR Therapy against CD19 Positive Leukemic Cells. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Qasim, W.; Zhan, H.; Samarasinghe, S.; Adams, S.; Amrolia, P.; Stafford, S.; Butler, K.; Rivat, C.; Wright, G.; Somana, K.; et al. Molecular Remission of Infant B-ALL after Infusion of Universal TALEN Gene-Edited CAR T Cells. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Karasiewicz, K.; He, S.; Ng, M.; Tess, K.; Ling, W.; Kaufmann, G.F.; Zeldis, J.B.; Ji, H.; Hariri, R.; Zhang, X. Preclinical Evaluation of Human Placental-Derived Allogeneic CD19 CAR-T Cells Against B Cell Malignancies. Blood 2019, 134, 3222. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-Man Clinical Trial of CAR NK-92 Cells: Safety Test of CD33-CAR NK-92 Cells in Patients with Relapsed and Refractory Acute Myeloid Leukemia. Am. J. Cancer Res. 2018, 8, 1083–1089. [Google Scholar]
- Nianias, A.; Themeli, M. Induced Pluripotent Stem Cell (IPSC)–Derived Lymphocytes for Adoptive Cell Immunotherapy: Recent Advances and Challenges. Curr. Hematol. Malig. Rep. 2019, 14, 261–268. [Google Scholar] [CrossRef]
- Benjamin, R.; Graham, C.; Yallop, D.; Jozwik, A.; Mirci-Danicar, O.C.; Lucchini, G.; Pinner, D.; Jain, N.; Kantarjian, H.; Boissel, N.; et al. Genome-Edited, Donor-Derived Allogeneic Anti-CD19 Chimeric Antigen Receptor T Cells in Paediatric and Adult B-Cell Acute Lymphoblastic Leukaemia: Results of Two Phase 1 Studies. Lancet 2020, 396, 1885–1894. [Google Scholar] [CrossRef]
- Clinical Trial: Safety and Efficacy of ALLO-501 Anti-CD19 Allogeneic CAR T Cells in Adults with Relapsed/Refractory Large B Cell or Follicular Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03939026 (accessed on 23 January 2021).
- Neelapu, S.S.; Munoz, J.; Locke, F.L.; Miklos, D.B.; Brown, R.; McDevitt, J.T.; Mardiros, A.; Demirhan, E.; Konto, C.; Tees, M.T. First-in-Human Data of ALLO-501 and ALLO-647 in Relapsed/Refractory Large B-Cell or Follicular Lymphoma (R/R LBCL/FL): ALPHA Study. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Martínez-Molina, E.; Chocarro-Wrona, C.; Martínez-Moreno, D.; Marchal, J.A.; Boulaiz, H. Large-Scale Production of Lentiviral Vectors: Current Perspectives and Challenges. Pharmaceutics 2020, 12, 1051. [Google Scholar] [CrossRef]
- Karda, R.; Counsell, J.R.; Karbowniczek, K.; Caproni, L.J.; Tite, J.P.; Waddington, S.N. Production of Lentiviral Vectors Using Novel, Enzymatically Produced, Linear DNA. Gene Ther. 2019, 26, 86–92. [Google Scholar] [CrossRef]
- Ikeda, Y.; Goto, Y.; Yonemitsu, Y.; Miyazaki, M.; Sakamoto, T.; Ishibashi, T.; Tabata, T.; Ueda, Y.; Hasegawa, M.; Tobimatsu, S.; et al. Simian Immunodeficiency Virus-Based Lentivirus Vector for Retinal Gene Transfer: A Preclinical Safety Study in Adult Rats. Gene Ther. 2003, 10, 1161–1169. [Google Scholar] [CrossRef][Green Version]
- Throm, R.E.; Ouma, A.A.; Zhou, S.; Chandrasekaran, A.; Lockey, T.; Greene, M.; De Ravin, S.S.; Moayeri, M.; Malech, H.L.; Sorrentino, B.P.; et al. Efficient Construction of Producer Cell Lines for a SIN Lentiviral Vector for SCID-X1 Gene Therapy by Concatemeric Array Transfection. Blood 2009, 113, 5104–5110. [Google Scholar] [CrossRef]
- Stornaiuolo, A.; Piovani, B.M.; Bossi, S.; Zucchelli, E.; Corna, S.; Salvatori, F.; Mavilio, F.; Bordignon, C.; Rizzardi, G.P.; Bovolenta, C. RD2-Molpack-Chim3, a Packaging Cell Line for Stable Production of Lentiviral Vectors for Anti-HIV Gene Therapy. Hum. Gene Ther. Methods 2013, 24, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Sanber, K.S.; Knight, S.B.; Stephen, S.L.; Bailey, R.; Escors, D.; Minshull, J.; Santilli, G.; Thrasher, A.J.; Collins, M.K.; Takeuchi, Y. Construction of Stable Packaging Cell Lines for Clinical Lentiviral Vector Production. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Manceur, A.P.; Kim, H.; Misic, V.; Andreev, N.; Dorion-Thibaudeau, J.; Lanthier, S.; Bernier, A.; Tremblay, S.; Gélinas, A.M.; Broussau, S.; et al. Scalable Lentiviral Vector Production Using Stable HEK293SF Producer Cell Lines. Hum. Gene Ther. Methods 2017, 28, 330–339. [Google Scholar] [CrossRef]
- Jiang, W.; Hua, R.; Wei, M.; Li, C.; Qiu, Z.; Yang, X.; Zhang, C. An Optimized Method for High-Titer Lentivirus Preparations without Ultracentrifugation. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Bauler, M.; Roberts, J.K.; Wu, C.-C.; Fan, B.; Ferrara, F.; Yip, B.H.; Diao, S.; Kim, Y.-I.; Moore, J.; Zhou, S.; et al. Production of Lentiviral Vectors Using Suspension Cells Grown in Serum-Free Media. Mol. Ther. Methods Clin. Dev. 2020, 17, 58–68. [Google Scholar] [CrossRef]
- Chen, Y.H.; Pallant, C.; Sampson, C.J.; Boiti, A.; Johnson, S.; Brazauskas, P.; Hardwicke, P.; Marongiu, M.; Marinova, V.M.; Carmo, M.; et al. Rapid Lentiviral Vector Producer Cell Line Generation Using a Single DNA Construct. Mol. Ther. Methods Clin. Dev. 2020, 19, 47–57. [Google Scholar] [CrossRef]
- Johnson, S.; Pallant, C.; Vamva, E.; Vink, C. Stable Cell Lines for Retroviral Production; World Intellectual Property Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Verhoeyen, E.; Costa, C.; Cosset, F.L. Lentiviral Vector Gene Transfer into Human T Cells. Methods Mol. Biol. 2009, 506, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Wheeler, J.X.; Thorpe, R.; Collins, M.; Takeuchi, Y.; Zhao, Y. Mass Spectrometry Analysis Reveals Differences in the Host Cell Protein Species Found in Pseudotyped Lentiviral Vectors. Biologicals 2018, 52, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mekkaoui, L.; Parekh, F.; Kotsopoulou, E.; Darling, D.; Dickson, G.; Cheung, G.W.; Chan, L.; MacLellan-Gibson, K.; Mattiuzzo, G.; Farzaneh, F.; et al. Lentiviral Vector Purification Using Genetically Encoded Biotin Mimic in Packaging Cell. Mol. Ther. Methods Clin. Dev. 2018, 11, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Pule, M.; Mekkaoui, L. Retroviral and Lentiviral Vectors; World Intellectual Property Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Rath, J.A.; Arber, C. Engineering Strategies to Enhance TCR-Based Adoptive T Cell Therapy. Cells 2020, 9, 1485. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, L.; Wang, F.; Zhang, Y.; Liu, B.; Zhao, T. Chimeric Antigen Receptor T (CAR-T) Cells Expanded with IL-7/IL-15 Mediate Superior Antitumor Effects. Protein Cell 2019, 10, 764–769. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Faitg, T.H.; Lowther, D.E.; Badros, A.Z.; Chagin, K.; Dengel, K.; Iyengar, M.; Melchiori, L.; Navenot, J.M.; Norry, E.; et al. Long-term Safety and Activity of NY-ESO-1 SPEAR T Cells after Autologous Stem Cell Transplant for Myeloma. Blood Adv. 2019, 3, 2022–2034. [Google Scholar] [CrossRef]
- Clinical Trial: Redirected Auto T Cells for Advanced Myeloma. Available online: https://clinicaltrials.gov/ct2/show/NCT01352286 (accessed on 27 January 2021).
- Sanderson, J.P.; Crowley, D.J.; Wiedermann, G.E.; Quinn, L.L.; Crossland, K.L.; Tunbridge, H.M.; Cornforth, T.V.; Barnes, C.S.; Ahmed, T.; Howe, K.; et al. Preclinical Evaluation of an Affinity-Enhanced MAGE-A4-Specific T-Cell Receptor for Adoptive T-Cell Therapy. Oncoimmunology 2020, 9. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKS, the Fourth-generation CAR T Cells: Current Developments and Clinical Translation. Adv. Cell Gene Ther. 2020, 3, e84. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. EGFR-IL12-CART Cells for Patients With Metastatic Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03542799 (accessed on 20 June 2021).
- Zimmermann, K.; Kuehle, J.; Dragon, A.C.; Galla, M.; Kloth, C.; Rudek, L.S.; Sandalcioglu, I.E.; Neyazi, B.; Moritz, T.; Meyer, J.; et al. Design and Characterization of an “All-in-One” Lentiviral Vector System Combining Constitutive Anti-Gd2 Car Expression and Inducible Cytokines. Cancers 2020, 12, 375. [Google Scholar] [CrossRef]
- Patel, S.; Burga, R.A.; Powell, A.B.; Chorvinsky, E.A.; Hoq, N.; McCormack, S.E.; Van Pelt, S.N.; Hanley, P.J.; Cruz, C.R.Y. Beyond CAR T Cells: Other Cell-Based Immunotherapeutic Strategies against Cancer. Front. Oncol. 2019, 9, 196. [Google Scholar] [CrossRef]
- Heczey, A.; Liu, D.; Tian, G.; Courtney, A.N.; Wei, J.; Marinova, E.; Gao, X.; Guo, L.; Yvon, E.; Hicks, J.; et al. Invariant NKT Cells with Chimeric Antigen Receptor Provide a Novel Platform for Safe and Effective Cancer Immunotherapy. Blood 2014, 124, 2824–2833. [Google Scholar] [CrossRef]
- Vantourout, P.; Hayday, A. Six-of-the-Best: Unique Contributions of Γδ T Cells to Immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef]
- Minculescu, L.; Marquart, H.V.; Ryder, L.P.; Andersen, N.S.; Schjoedt, I.; Friis, L.S.; Kornblit, B.T.; Petersen, S.L.; Haastrup, E.; Fischer-Nielsen, A.; et al. Improved Overall Survival, Relapse-Free-Survival, and Less Graft-vs.-Host-Disease in Patients with High Immune Reconstitution of TCR Gamma Delta Cells 2 Months after Allogeneic Stem Cell Transplantation. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Wang, R.N.; Wen, Q.; He, W.T.; Yang, J.H.; Zhou, C.Y.; Xiong, W.J.; Ma, L. Optimized Protocols for Γδ T Cell Expansion and Lentiviral Transduction. Mol. Med. Rep. 2019, 19, 1471–1480. [Google Scholar] [CrossRef]
- Sutton, K.; Dasgupta, A.; David, M.; Doering, C.; Spencer, H.T. 402. Bioengineering of Peripheral Blood Derived Gamma Delta T Cells in a Serum-Free Expansion Medium. Mol. Ther. 2016, 24, S159. [Google Scholar] [CrossRef]
- Charles David, P.; Haishan, L.; Lahusen, T.; Liou, M. Methods and Compositions for the Activation of Gamma-Delta T-Cells; World Intellectual Property Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Clinical Trial: Novel Gamma-Delta (Γδ) T Cell Therapy for Treatment of Patients With Newly Diagnosed Glioblastoma. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04165941 (accessed on 22 January 2021).
- Altman, J.B.; Benavides, A.D.; Das, R.; Bassiri, H. Antitumor Responses of Invariant Natural Killer T Cells. J. Immunol. Res. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Heczey, A.; Courtney, A.N.; Montalbano, A.; Robinson, S.; Liu, K.; Li, M.; Ghatwai, N.; Dakhova, O.; Liu, B.; Raveh-Sadka, T.; et al. Anti-GD2 CAR-NKT Cells in Patients with Relapsed or Refractory Neuroblastoma: An Interim Analysis. Nat. Med. 2020, 26, 1686–1690. [Google Scholar] [CrossRef]
- Xu, X.; Huang, W.; Heczey, A.; Liu, D.; Guo, L.; Wood, M.; Jin, J.; Courtney, A.N.; Liu, B.; Di Pierro, E.J.; et al. NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced in Vivo Persistence and Antitumor Activity against Neuroblastoma. Clin. Cancer Res. 2019, 25, 7126–7138. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. GD2 Specific CAR and Interleukin-15 Expressing Autologous NKT Cells to Treat Children With Neuroblastoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03294954?cond=CAR+NKT&draw=2&rank=1 (accessed on 20 June 2021).
- ClinicalTrials.gov. Clinical Study of CAR-INKT Cells in the Treatment of Relapsed/Refractory/High-Risk B-Cell Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04814004?cond=iNKT&draw=2&rank=2 (accessed on 20 June 2021).
- Zhao, H.; Liao, X.; Kang, Y. Tregs: Where We Are and What Comes Next? Front. Immunol. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, W.; Liang, C.-L.; Chen, Y.; Liu, H.; Qiu, F.; Dai, Z. Chimeric Antigen Receptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance. Front. Immunol. 2018, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, E.; Volk, H.D.; Reinke, P.; Abou-El-Enein, M. Toward an Optimized Process for Clinical Manufacturing of CAR-Treg Cell Therapy. Trends Biotechnol. 2020, 38, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-001730-34/NL (accessed on 23 January 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).