Activity of Galidesivir in a Hamster Model of SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Evaluation of Antiviral Activity
2.1.1. Cell Lines
2.1.2. Test Article
2.1.3. Viral Yield Reduction Assay and Cytopathic Effect Assay
2.1.4. In Vitro Imaging Assay
2.2. In Vivo Evaluation of Galidesivr in the Hamster Model of SARS-CoV-2
2.2.1. Animals
2.2.2. Test Article and Vehicle Control
2.2.3. Virus
2.2.4. Experimental Infection and Evaluation of Syrian Golden Hamsters
2.2.5. Virus Plaque Assay
2.2.6. Statistical Analysis
3. Results
3.1. Evaluation of the In Vitro Antiviral Activity of Galidesivir against SARS-CoV-2
3.2. Evaluation of the In Vivo Activity of Galidesivr in the Hamster Model of SARS-CoV-2
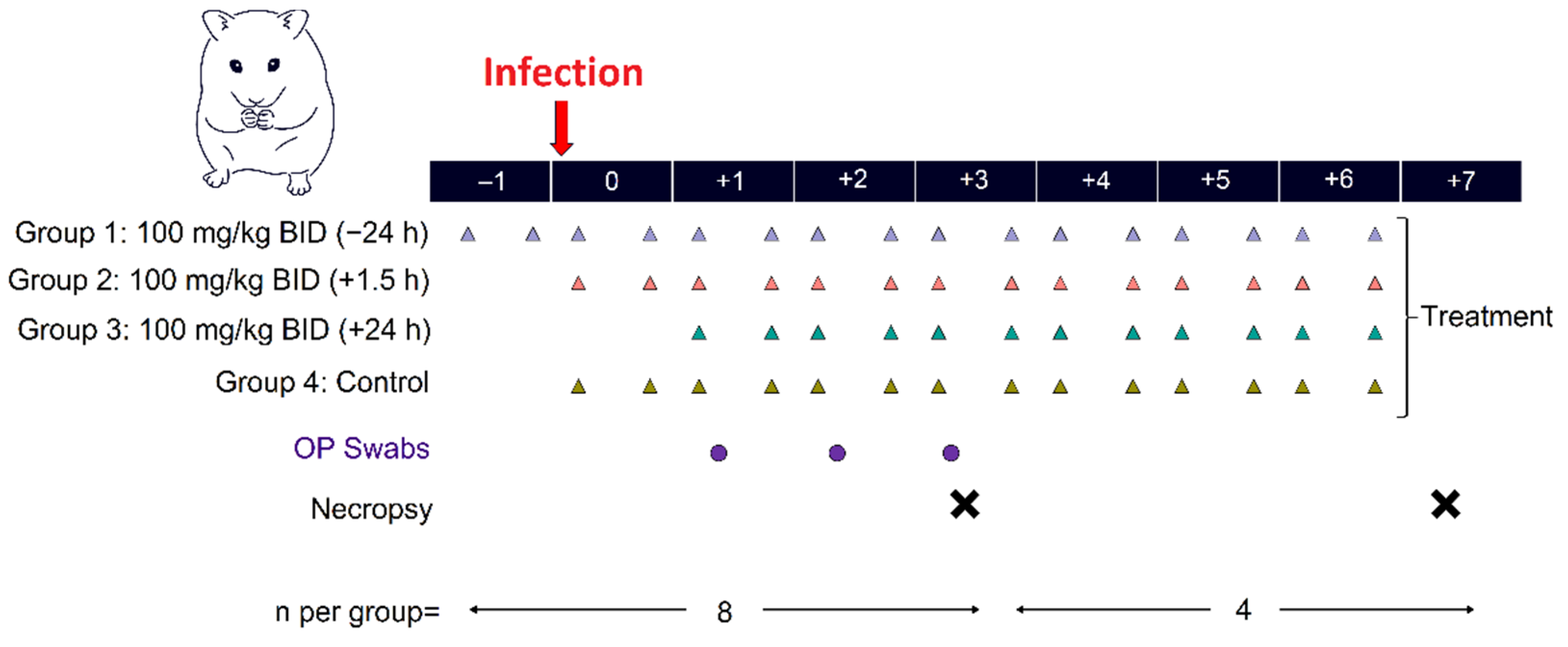
3.2.1. Study Design
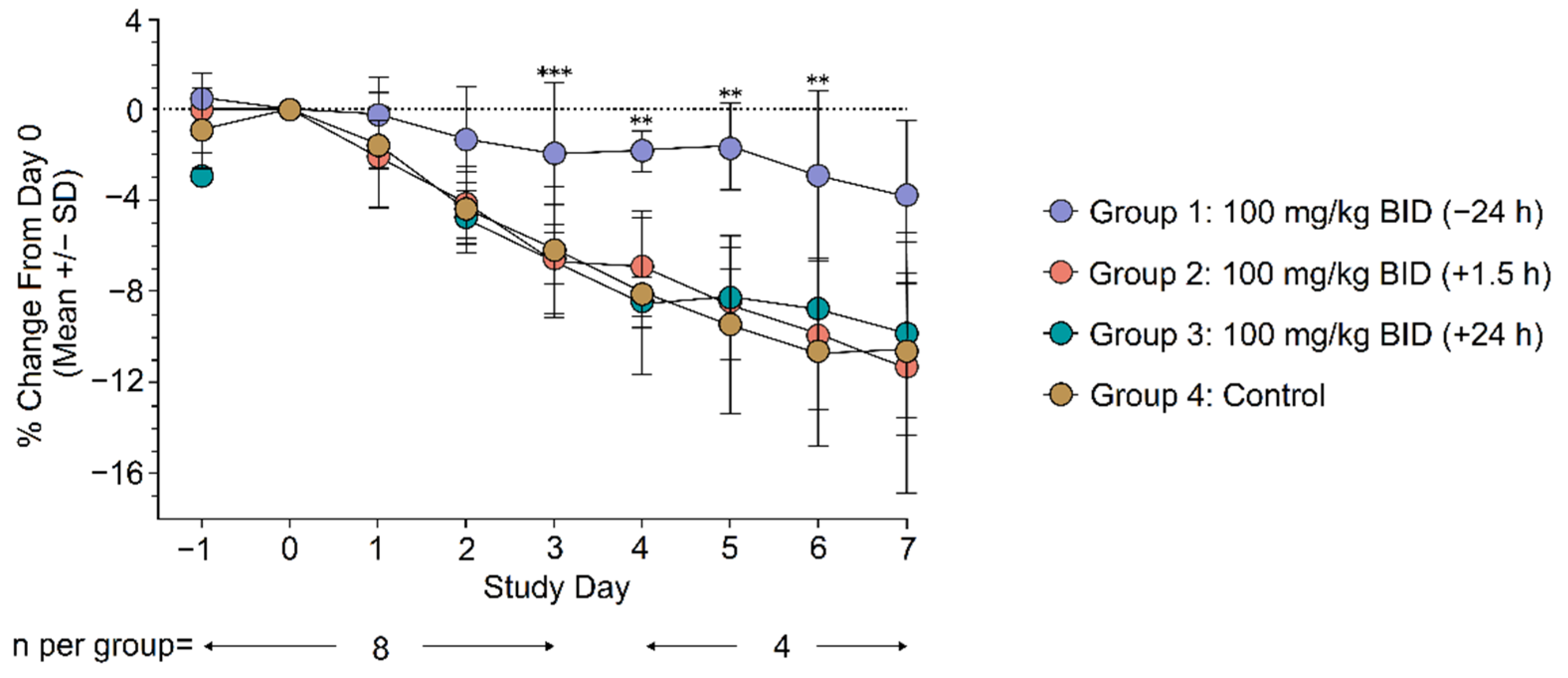
3.2.2. Clinical Evaluation
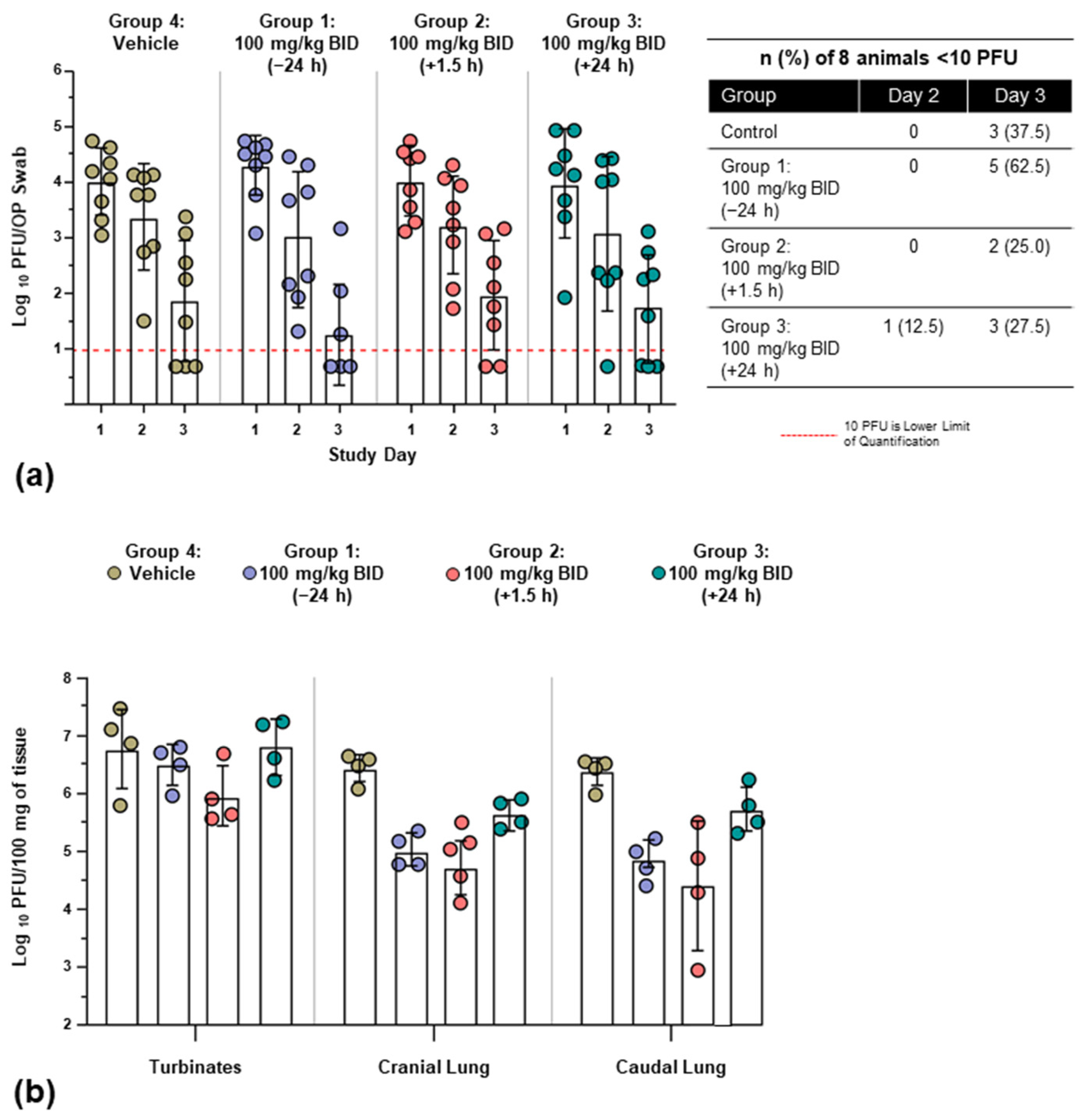
3.2.3. Viral Burden
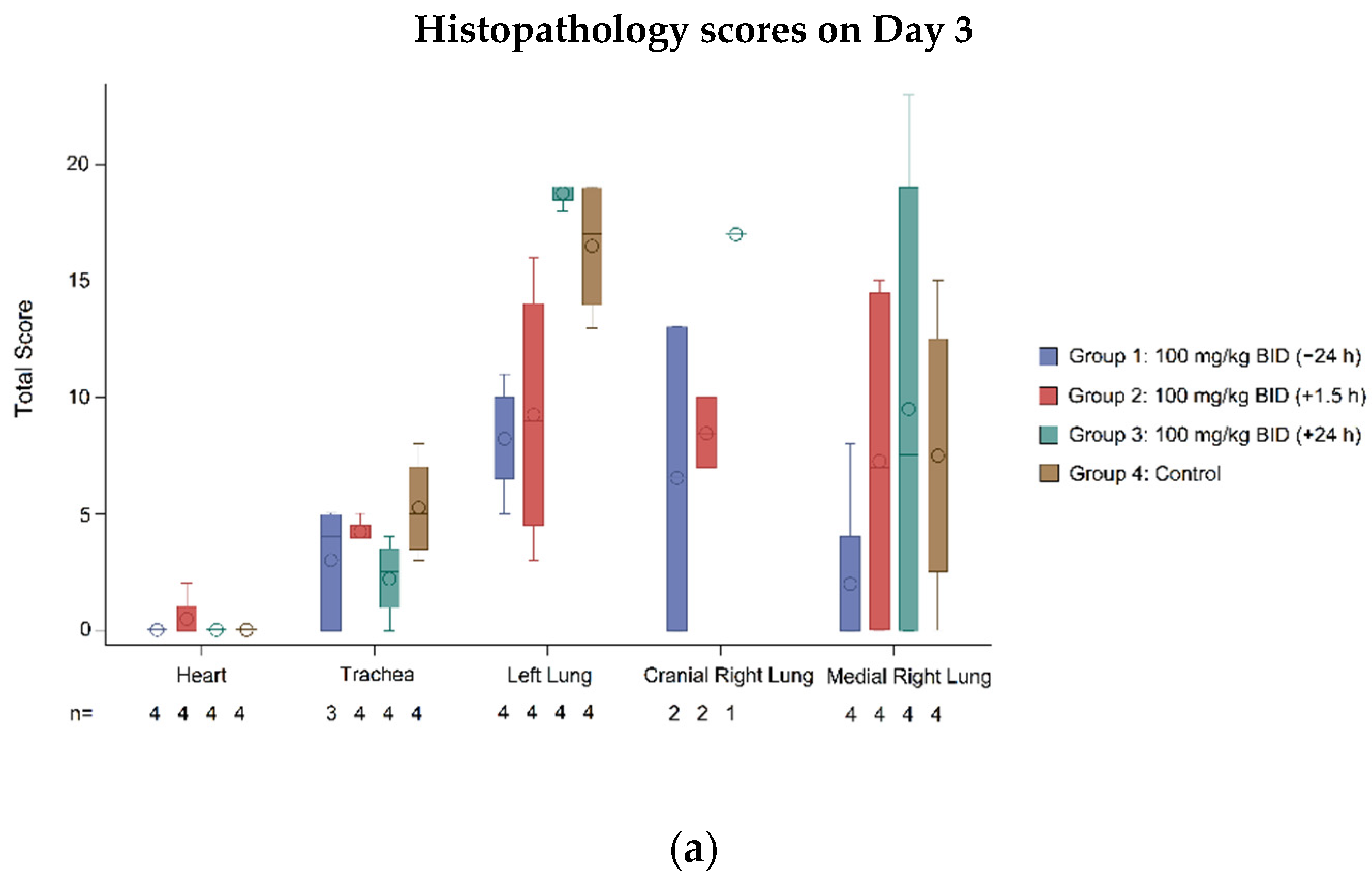
3.2.4. Histopathology
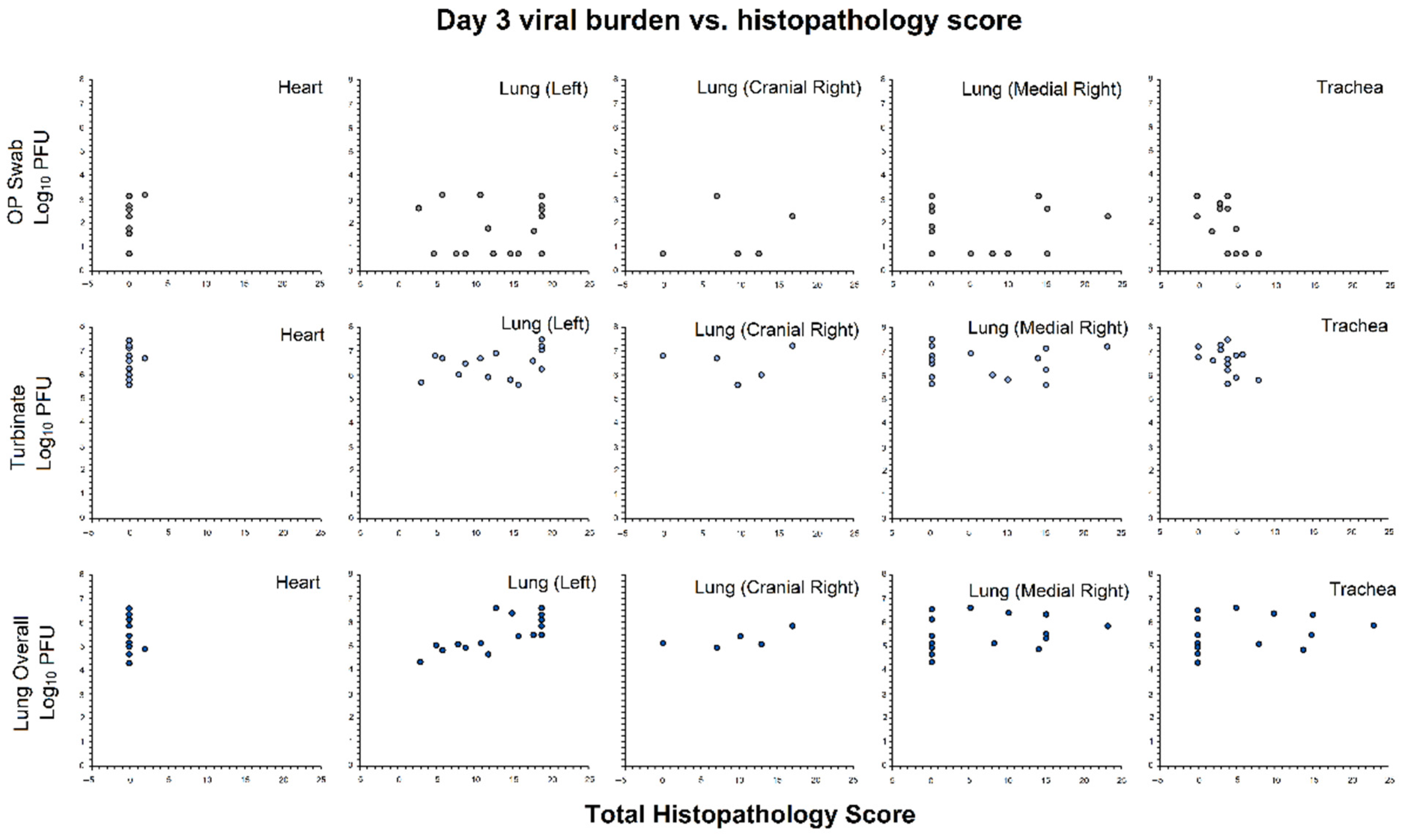
3.2.5. Association of Viral Burden and Histopathology Scores
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Map: Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 8 December 2021).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Center for Drug Evaluation Research Application Number: 214787Orig1s000: Summary Review. Available online: Accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000Sumr.pdf (accessed on 22 May 2021).
- Emergency Use Authorization for Bamlanivimab 700 mg and Etesevimab 1400 mg IV Administered Together, Center for Drug Evaluation and Research Review. Available online: https://www.fda.gov/media/146255/download (accessed on 10 September 2021).
- Emergency Use Authorization for Casirivimab and Imdevimab, Center for Drug Evaluation Research Review. Available online: https://www.fda.gov/media/144468/download (accessed on 10 September 2021).
- Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate (accessed on 9 December 2021).
- Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Available online: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ (accessed on 9 December 2021).
- Warren, T.K.; Wells, J.; Panchal, R.G.; Stuthman, K.S.; Garza, N.L.; van Tongeren, S.A.; Dong, L.; Retterer, C.J.; Eaton, B.P.; Pegoraro, G.; et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 2014, 508, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Demarest, J.F.; Taylor, R.; Gowen, B.B.; Walling, D.M.; Mathis, A.; Babu, Y.S. An update on the progress of galidesivir (BCX4430), a broad -spectrum antiviral. Antivir. Res. 2021, 195, 105180. [Google Scholar] [CrossRef]
- Julander, J.G.; Siddharthan, V.; Evans, J.; Taylor, R.; Tolbert, K.; Apuli, C.; Stewart, J.; Collins, P.; Gebre, M.; Neilson, S.; et al. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir. Res. 2017, 137, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Osuna, C.E.; Best, K.; Taylor, R.; Chen, E.; Yoon, G.; Kublin, J.L.; Schalk, D.; Schultz-Darken, N.; Capuano, S.; et al. A direct-acting antiviral drug abrogates viremia in Zika virus-infected rhesus macaques. Sci. Transl. Med. 2020, 12, eaau9135. [Google Scholar] [CrossRef] [PubMed]
- Westover, J.B.; Mathis, A.; Taylor, R.; Wandersee, L.; Bailey, K.W.; Sefing, E.J.; Hickerson, B.T.; Jung, K.H.; Sheridan, W.P.; Gowen, B.B. Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters. Antivir. Res. 2018, 156, 38–45. [Google Scholar] [CrossRef]
- Taylor, R.; Kotian, P.; Warren, T.; Panchal, R.; Bavari, S.; Julander, J.; Dobo, S.; Rose, A.; El-Kattan, Y.; Taubenheim, B.; et al. BCX4430—A broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J. Infect. Public Health 2016, 9, 220–226. [Google Scholar] [CrossRef]
- Choy, K.T.; Wong, A.Y.; Kaewpreedee, P.; Sia, S.F.; Chen, D.; Hui, K.P.Y.; Chu, D.K.W.; Chan, M.C.W.; Cheung, P.P.; Huang, X.; et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020, 178, 104786. [Google Scholar] [CrossRef]
- Aftab, S.O.; Ghouri, M.Z.; Masood, M.U.; Haider, Z.; Khan, Z.; Ahmad, A.; Munawar, N. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020, 18, 275. [Google Scholar] [CrossRef]
- Elfiky, A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020, 253, 117592. [Google Scholar] [CrossRef]
- Chan, J.F.; Zhang, A.J.; Yuan, S.; Poon, V.K.; Chan, C.C.; Lee, A.C.; Chan, W.M.; Fan, Z.; Tsoi, H.W.; Wen, L.; et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in a golden Syrian hamster model: Implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020, 71, 2428–2446. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.M.; Chin, A.W.H.; Fung, K.; Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.A.P.M.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R. (BioCryst Pharmaceuticals, Inc., Durham, NC, USA). Personal communication, 2020.
- Smee, D.F.; Evans, W.J.; Nicolaou, K.C.; Tarbet, E.B.; Day, C.W. Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antivir. Res. 2016, 131, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Hurst, B.L.; Evans, W.J.; Clyde, N.; Wrigth, S.; Peterson, C.; Jung, K.H.; Day, C.W. Evaluation of cell viability dyes in antiviral assays with RNA viruses that exhibit different cytopathogenic properties. J. Virol. Methods 2017, 246, 51–57. [Google Scholar] [CrossRef]
- Dittmar, M.; Lee, J.S.; Whig, K.; Segrist, E.; Li, M.; Kamalia, B.; Castellana, L.; Ayyanathan, K.; Cardenas-Diaz, F.L.; Morrisey, E.E.; et al. Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-CoV02. Cell Rep. 2021, 35, 108959. [Google Scholar] [CrossRef]
- Jia, Q.; Bielefeldt-Ohmann, H.; Maison, R.M.; Masleša-Galić, S.; Cooper, S.K.; Bowen, R.A.; Horowitz, M.A. Replicating bacterium-vectored vaccine expression SARS-CoV-2 membrane and nucleocapsid proteins protects against sever COVID-19-like disease in hamsters. NPJ Vaccines 2021, 6, 47. [Google Scholar] [CrossRef]
- Ragan, I.; Hartson, L.; Pidcoke, H.; Bowen, R.; Goodrich, R.P. Pathogen reduction of SARS-CoV-2 in plasma and whole blood using riboflavin and UV light. PLoS ONE 2020, 15, e0233947. [Google Scholar] [CrossRef]
- Vandyck, K.; Abdelnabi, R.; Gupta, K.; Jochmans, D.; Jekle, A.; Deval, J.; Misner, D.; Bardiot, D.; Foo, C.S.; Liu, C.; et al. ALG-097111, a potent and selective SARS-CoV-2 3-chymotrypsin-like cysteine protease inhibitor exhibits in vivo efficacy in a Syrian Hamster model. Biochem. Biophys. Res. Commun. 2021, 555, 134–139. [Google Scholar] [CrossRef]
- Williamson, B.N.; Feldmann, F.; Schwarz, B.; Meade-White, K.; Porter, D.P.; Schulz, J.; Doremalen, N.; Leighton, I.; Yinda, C.K.; Pérez-Pérez, L.; et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020, 585, 273–276. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of Covid-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S. Treating influenza infection, from now and into the future. Front. Immunol. 2018, 9, 1946. [Google Scholar] [CrossRef]
- Warren, T.; MacLennan, S.; Mathis, A.; Giuliano, E.; Taylor, R.; Sheridan, W. Efficacy of galidesivir against Ebola virus disease in rhesus monkeys. Open Forum Infect. Dis. 2017, 4, S302. [Google Scholar] [CrossRef][Green Version]
- Julander, J.G.; Bantia, S.; Taubenheim, B.R.; Minning, D.M.; Kotian, P.; Morrey, J.D.; Smee, D.F.; Sheridan, W.P.; Babu, Y.S. BCX4430, a novel nucleoside analog, effectively treats yellow fever in a Hamster model. Antimicrob. Agents Chemother. 2014, 58, 6607–6614. [Google Scholar] [CrossRef] [PubMed]


| Cell Line | SARS-CoV-2 Strain | Assay | Compound Pre-Incubation Period | EC50 (μM) | EC90 (μM) | CC50 (μM) | SI |
|---|---|---|---|---|---|---|---|
| Caco-2 | WA1/2020 | VYR | 24 h | n.d. | 14.19 | 82.8 | 5.8 * |
| Vero-76 | WA1/2020 | VYR | 24 h | n.d. | 10.94 | >295.7 | >27 * |
| CPE | 50.3 | 5.8 * | |||||
| Calu-3 | WA1/2020 | Imaging | 2 h ‡ | 14.15 | n.d. | >50 | >3.5 † |
| Group | n | Mean (PFU/100 mg, log10) | Difference vs. Control | 95% CI for Treatment Difference | p-Value 1 | |
|---|---|---|---|---|---|---|
| Caudal lung | Group 1: 100 mg/kg BID (−24 h) | 4 | 4.86 | −1.53 | (−2.68, −0.38) | <0.05 |
| Group 2: 100 mg/kg BID (+1.5 h) | 4 | 4.42 | −1.97 | (−3.12, −0.81) | <0.05 | |
| Group 3: 100 mg/kg BID (+24 h) | 4 | 5.74 | −0.65 | (−1.80, 0.51) | NS | |
| Group 4: control | 4 | 6.39 | NA | NA | NA | |
| Cranial lung | Group 1: 100 mg/kg BID (−24 h) | 4 | 5.03 | −1.42 | (−2.22, −0.61) | <0.05 |
| Group 2: 100 mg/kg BID (+1.5 h) | 4 | 4.75 | −1.70 | (−2.50, −0.89) | <0.05 | |
| Group 3: 100 mg/kg BID (+24 h) | 4 | 5.68 | −0.77 | (−1.57, 0.04) | NS | |
| Group 4: control | 4 | 6.45 | NA | NA | NA | |
| Turbinates | Group 1: 100 mg/kg BID (−24 h) | 4 | 6.51 | −0.31 | (−1.47, 0.86) | NS |
| Group 2: 100 mg/kg BID (+1.5 h) | 4 | 5.96 | −0.85 | (−2.02, 0.31) | NS | |
| Group 3: 100 mg/kg BID (+24 h) | 4 | 6.83 | 0.01 | (−1.15, 1.18) | NS | |
| Group 4: control | 4 | 6.81 | NA | NA | NA |
| Tissue | Statistic | Group 1: 100 mg/kg BID (−24 h) | Group 2: 100 mg/kg BID (+1.5 h) | Group 3: 100 mg/kg BID (+24 h) | Group 4: Control |
|---|---|---|---|---|---|
| Heart | Animals with score of zero, n (%) | 8 (100) | 7 (87.5) | 7 (87.5) | 8 (100) |
| p-value 1 vs. control | NA | 0.3017 | 0.3017 | ||
| Trachea | Animals with score of zero, n (%) | 4 (50.0) | 2 (25.0) | 3 (37.5) | 0 |
| p-value 1 vs. control | 0.0209 | 0.1306 | 0.0547 | ||
| Left lung | Animals with score of zero, n (%) | 1 (12.5) | 0 | 0 | 0 |
| p-value 1 vs. control | 0.3017 | NA | NA | ||
| Cranial right lung | Animals with score of zero, n (%) | 3 (37.5) | 0 | 0 | 0 |
| p-value 1 vs. control | 0.0547 | NA | NA | ||
| Medial right lung | Animals with score of zero, n (%) | 5 (62.5) | 3 (37.5) | 2 (25.0) | 1 (12.5) |
| p-value 1 vs. control | 0.0389 | 0.2482 | 0.5218 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, R.; Bowen, R.; Demarest, J.F.; DeSpirito, M.; Hartwig, A.; Bielefeldt-Ohmann, H.; Walling, D.M.; Mathis, A.; Babu, Y.S. Activity of Galidesivir in a Hamster Model of SARS-CoV-2. Viruses 2022, 14, 8. https://doi.org/10.3390/v14010008
Taylor R, Bowen R, Demarest JF, DeSpirito M, Hartwig A, Bielefeldt-Ohmann H, Walling DM, Mathis A, Babu YS. Activity of Galidesivir in a Hamster Model of SARS-CoV-2. Viruses. 2022; 14(1):8. https://doi.org/10.3390/v14010008
Chicago/Turabian StyleTaylor, Ray, Richard Bowen, James F. Demarest, Michael DeSpirito, Airn Hartwig, Helle Bielefeldt-Ohmann, Dennis M. Walling, Amanda Mathis, and Yarlagadda S. Babu. 2022. "Activity of Galidesivir in a Hamster Model of SARS-CoV-2" Viruses 14, no. 1: 8. https://doi.org/10.3390/v14010008
APA StyleTaylor, R., Bowen, R., Demarest, J. F., DeSpirito, M., Hartwig, A., Bielefeldt-Ohmann, H., Walling, D. M., Mathis, A., & Babu, Y. S. (2022). Activity of Galidesivir in a Hamster Model of SARS-CoV-2. Viruses, 14(1), 8. https://doi.org/10.3390/v14010008







