Full Genome Sequencing of Three Sedoreoviridae Viruses Isolated from Culicoides spp. (Diptera, Ceratopogonidae) in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus Isolates
2.2. Cells
2.3. Extracting Viral Nucleic Acid for Test
2.4. RT-qPCR
2.5. Preparing Viral Genomic RNA
2.6. Amplifying Viral Genome
2.7. DNA Electrophoresis
2.8. Complete Sequencing of Viral Genomes
2.9. Sequence Data and Phylogenetic Analysis
3. Results
3.1. Primary Identification
3.2. Complete Genome Sequences
3.3. Phylogenetic Analysis to Confirm Virus Status
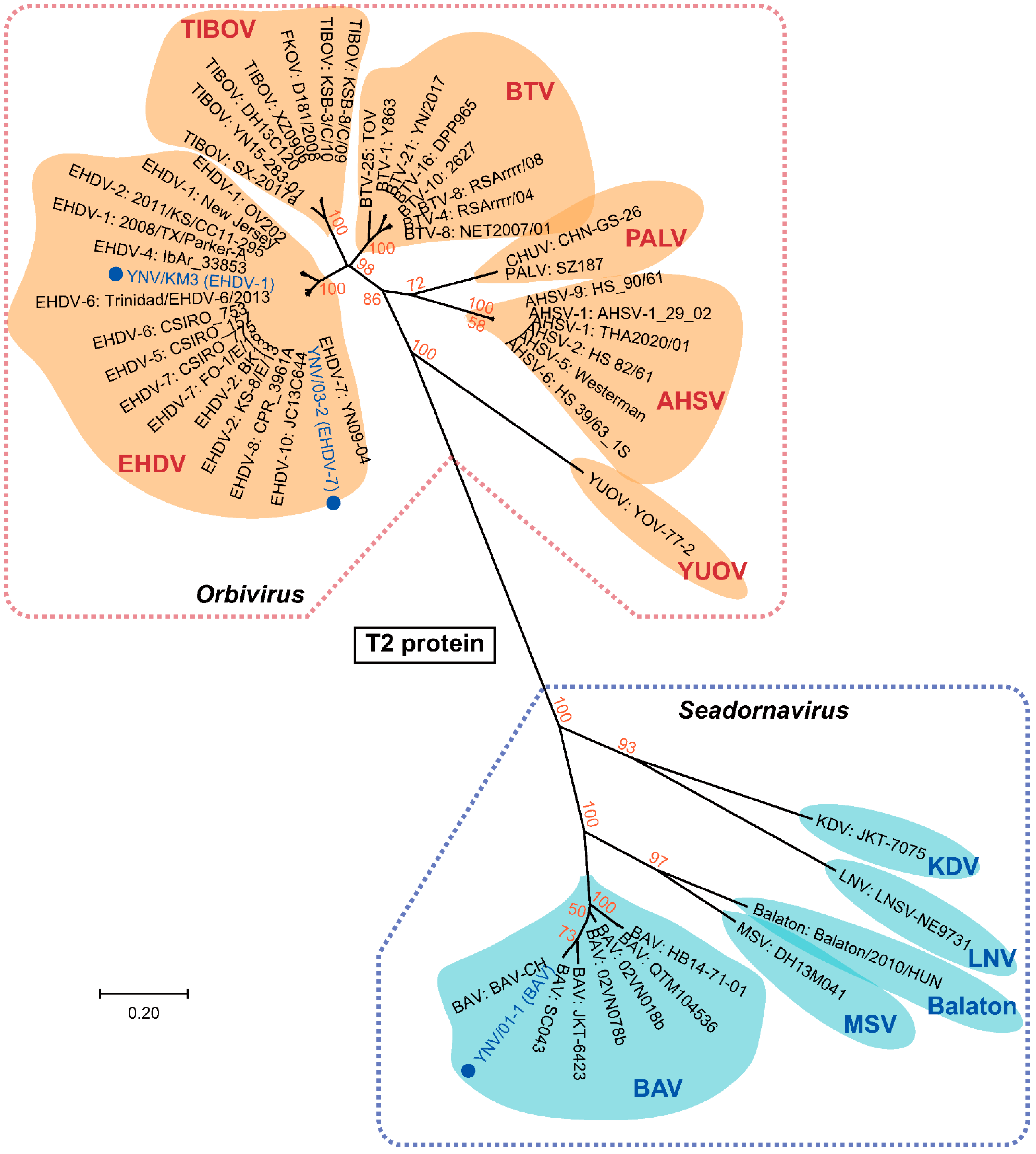
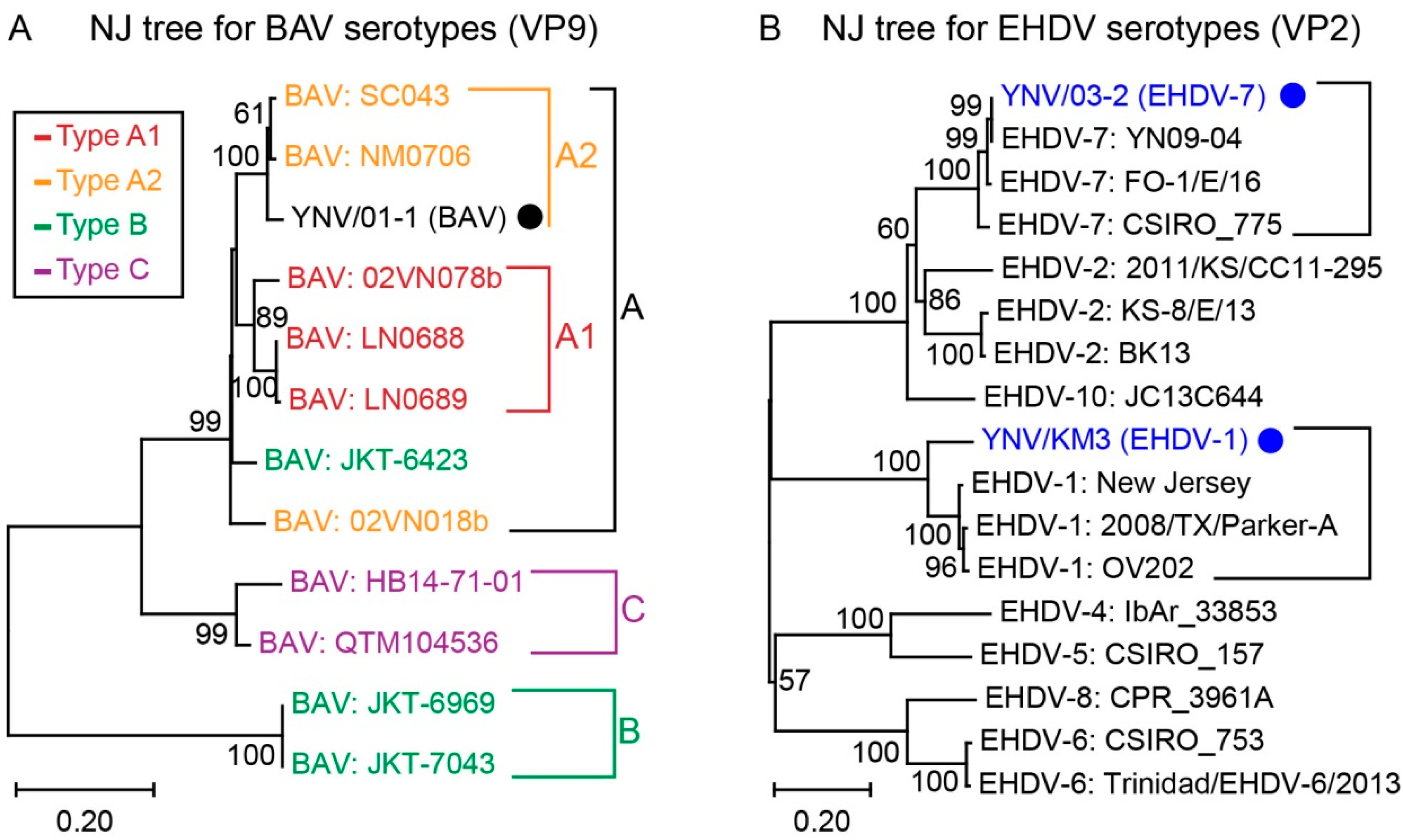
3.4. Phylogenetic Analysis for Viral Serotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiff, L.A.; Nibert, M.L.; Tyler, K.L. Orthoreoviruses and Their Replication. In Fields Virology, 5th ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 2, pp. 2388–2471. [Google Scholar]
- ICTV Virus Taxonomy: 2019 Release. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 9 May 2020).
- Hassan, S.S.; Roy, P. Expression and functional characterization of bluetongue virus VP2 protein: Role in cell entry. J. Virol. 1999, 73, 9832–9842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.-H.; Nason, E.; Staeuber, N.; Jiang, W.; Monastryrskaya, K.; Roy, P. RGD Tripeptide of Bluetongue Virus VP7 Protein Is Responsible for Core Attachment to Culicoides Cells. J. Virol. 2001, 75, 3352–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, P. Orbiviruses. In Fields Virology, 5th ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2007; Volume 2, pp. 2541–2568. [Google Scholar]
- Mills, M.; Michel, K.; Pfannenstiel, R.S.; Ruder, M.G.; Veronesi, E.; Nayduch, D. Culicoides–virus interactions: Infection barriers and possible factors underlying vector competence. Curr. Opin. Insect Sci. 2017, 22, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mertens, P.; Burroughs, J.; Walton, A.; Wellby, M.; Fu, H.; O’Hara, R.; Brookes, S.; Mellor, P. Enhanced Infectivity of Modified Bluetongue Virus Particles for Two Insect Cell Lines and for Two Culicoides Vector Species. Virology 1996, 217, 582–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attoui, H.; De Micco, P.; De Lamballerie, X.; Billoir, F.; Biagini, P. Complete sequence determination and genetic analysis of Banna virus and Kadipiro virus: Proposal for assignment to a new genus (Seadornavirus) within the family Reoviridae. J. Gen. Virol. 2000, 81, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Attoui, H.; Jaafar, F.M.; Belhouchet, M.; Tao, S.; Chen, B.; Liang, G.; Tesh, R.B.; De Micco, P.; De Lamballerie, X. Liao ning virus, a new Chinese Seadornavirus that replicates in transformed and embryonic mammalian cells. J. Gen. Virol. 2006, 87, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; He, Y.; Zhou, Y.; Meng, J.; Zhu, W.; Chen, H.; Liao, D.; Man, Y. Isolation and Genetic Characterization of Mangshi Virus: A Newly Discovered Seadornavirus of the Reoviridae Family Found in Yunnan Province, China. PLoS ONE 2015, 10, e0143601. [Google Scholar] [CrossRef]
- ICTV ICTV Master Species List 2020.v1. Available online: https://talk.ictvonline.org/files/master-species-lists/m/msl/12314 (accessed on 21 February 2022).
- Xu, P.T.; Wang, Y.M.; Zuo, J.M.; Lin, J.W.; Xu, P.M. New Orbiviruses isolated from patients with unknown fever and encephalitis in Yunnan Province Chin. J. Virol. 1990, 6, 27–33. [Google Scholar]
- Liu, H.; Li, M.H.; Zhai, Y.G.; Meng, W.S.; Sun, X.H.; Cao, Y.X.; Fu, S.H.; Wang, H.Y.; Xu, L.H.; Tang, Q.; et al. Banna virus, China, 1987–2007. Emerg. Infect. Dis. 2010, 16, 514–517. [Google Scholar] [CrossRef]
- Nabeshima, T.; Nga, P.T.; Guillermo, P.; Parquet MD, C.; Yu, F.; Thuy, N.T.; Trang, B.M.; Hien, N.T.; Nam, V.S.; Inoue, S.; et al. Isolation and Molecular Characterization of Banna Virus from Mosquitoes, Vietnam. Emerg. Infect. Dis. 2008, 14, 1276–1279. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Gao, X.-Y.; Fu, S.-H.; Li, M.-H.; Zhai, Y.-G.; Meng, W.-S.; Sun, X.-H.; Lv, Z.; Wang, H.-Y.; Shen, X.-X.; et al. Molecular evolution of emerging Banna virus. Infect. Genet. Evol. 2016, 45, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, Y.; Fu, S.; Lei, W.; Guo, X.; Feng, Y.; Gao, X.; Li, X.; Yang, Z.; Xu, Z.; et al. Genome sequencing and phylogenetic analysis of Banna virus (genus Seadornavirus, family Reoviridae) isolated from Culicoides. Sci. China Life Sci. 2017, 60, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.P.; Xie, X.C.; Zhi, Q.; Ma, L.; Liu, Y.D.; Dangzheng, W.; Abdureciti, A.; Hashan, Y.; Zhang, Y.J.; Wang, C.; et al. First isolation of new Orbivirus (Banna) from ticks and infected cattle sera in Xinjiang. Endem. Dis. Bull. 1992, 7, 64–69. [Google Scholar]
- Xia, H.; Liu, H.; Zhao, L.; Atoni, E.; Wang, Y.; Yuan, Z. First Isolation and Characterization of a Group C Banna Virus (BAV) from Anopheles sinensis Mosquitoes in Hubei, China. Viruses 2018, 10, 555. [Google Scholar] [CrossRef] [Green Version]
- Shope, R.E.; MacNamara, L.G.; Mangold, R. A virus-induced epizootic hemorrhagic disease of the virginia white-tailed deer (Odocoileus virginianus). J. Exp. Med. 1960, 111, 155–170. [Google Scholar] [CrossRef]
- MacLachlan, N.; Zientara, S.; Savini, G.; Daniels, P. Epizootic haemorrhagic disease. Rev. Sci. Et Tech. l’OIE 2015, 34, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Casey, C.; Rathbun, S.; Stallknecht, D.; Ruder, M. Spatial Analysis of the 2017 Outbreak of Hemorrhagic Disease and Physiographic Region in the Eastern United States. Viruses 2021, 13, 550. [Google Scholar] [CrossRef]
- Cêtre-Sossah, C.; Roger, F.; Sailleau, C.; Rieau, L.; Zientara, S.; Bréard, E.; Viarouge, C.; Beral, M.; Esnault, O.; Cardinale, E. Epizootic haemorrhagic disease virus in Reunion Island: Evidence for the circulation of a new serotype and associated risk factors. Veter. Microbiol. 2014, 170, 383–390. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides Biting Midges: Their Role as Arbovirus Vectors. Annu. Rev. Èntomol. 2000, 45, 307–340. [Google Scholar] [CrossRef]
- Savini, G.; Afonso, A.; Mellor, P.; Aradaib, I.; Yadin, H.; Sanaa, M.; Wilson, W.; Monaco, F.; Domingo, M. Epizootic heamorragic disease. Res. Vet. Sci. 2011, 91, 1–17. [Google Scholar] [CrossRef]
- OIE Epizootic Haemorrhagic Disease (Infection with Epizootic Hemorrhagic Disease Virus). Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.07_EHD.pdf (accessed on 30 November 2021).
- Shirafuji, H.; Kato, T.; Yamakawa, M.; Tanaka, T.; Minemori, Y.; Yanase, T. Characterization of genome segments 2, 3 and 6 of epizootic hemorrhagic disease virus strains isolated in Japan in 1985–2013: Identification of their serotypes and geographical genetic types. Infect. Genet. Evol. 2017, 53, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Hiromatsu, R.; Kaida, M.; Kato, T.; Yanase, T.; Shirafuji, H. Isolation of epizootic hemorrhagic disease virus serotype 7 from cattle showing fever in Japan in 2016 and improvement of a reverse transcription-polymerase chain reaction assay to detect epizootic hemorrhagic disease virus. J. Veter. Med. Sci. 2021, 83, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Z.; Wang, J.; Li, Z.; Yang, Z.; Liao, D.; Zhu, J.; Li, H. Novel Serotype of Epizootic Hemorrhagic Disease Virus, China. Emerg. Infect. Dis. 2020, 26, 3081–3083. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Murota, K.; Hayama, Y. Endemic and Emerging Arboviruses in Domestic Ruminants in East Asia. Front. Veter- Sci. 2020, 7, 168. [Google Scholar] [CrossRef]
- Cottingham, S.; White, Z.; Wisely, S.; Campos-Krauer, J. A Mortality-Based Description of EHDV and BTV Prevalence in Farmed White-Tailed Deer (Odocoileus virginianus) in Florida, USA. Viruses 2021, 13, 1443. [Google Scholar] [CrossRef]
- Temizel, E.M.; Yesilbag, K.; Batten, C.; Senturk, S.; Maan, N.S.; Mertens, P.; Batmaz, H. Epizootic Hemorrhagic Disease in Cattle, Western Turkey. Emerg. Infect. Dis. 2009, 15, 317–319. [Google Scholar] [CrossRef]
- Borkent, A.; Dominiak, P. Catalog of the Biting Midges of the World (Diptera: Ceratopogonidae). Zootaxa 2020, 4787, 1–377. [Google Scholar] [CrossRef]
- ICTV ICTV Taxonomy History: Peribunyaviridae. Available online: https://talk.ictvonline.org/taxonomy/p/taxonomy-history?taxnode_id=202000081 (accessed on 21 February 2022).
- Xing, S.; Guo, X.; Zhang, X.; Zhao, Q.; Li, L.; Zuo, S.; An, X.; Pei, G.; Sun, Q.; Cheng, S.; et al. A novel mosquito-borne reassortant Orbivirus isolated from Xishuangbanna, China. Virol. Sin. 2016, 32, 159–162. [Google Scholar] [CrossRef]
- Duan, Y.L.; Yang, Z.X.; Bellis, G.; Li, L. Additional file 2 of Isolation of Tibet Orbivirus from Culicoides jacobsoni (Diptera, Ceratopogonidae) in China. Parasites Vectors 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Z.; Zhang, F.; Zhu, J. Studies on bluetongue disease in the People’s Republic of China. Veter. Ital. 2010, 40, 51–56. [Google Scholar]
- Duan, Y.L.; Miao, H.S.; Liao, D.F.; Kou, M.L.; Li, Z.H.; Wang, Z.; Li, H.C.; Li, L. The serologic investigation and viral isolation of bluetongue virus in Shangri-La in Southwest China. Transbound. Emerg. Dis. 2019, 66, 2353–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Wang, F.; Chang, J.; Zhang, Y.; Zhu, J.; Li, H.; Yu, L. Identification and complete-genome phylogenetic analysis of an epizootic hemorrhagic disease virus serotype 7 strain isolated in China. Arch. Virol. 2019, 164, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Lun, Z.R.; James, A.A.; Chen, X.G. Review: Dengue Fever in mainland China. Am. J. Trop Med. Hyg. 2010, 83, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.-C.; Zhao, H.; Li, L.-H.; Jiang, T.; Hong, W.-X.; Wang, J.; Zhao, L.-Z.; Yang, H.-Q.; Ma, D.-H.; Bai, C.-H.; et al. Severe dengue outbreak in Yunnan, China, 2013. Int. J. Infect. Dis. 2014, 27, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, Y.; Lu, S.; Dong, J.; Xu, H.; Zhang, Q.; Weng, R.; Yin, Y.; He, R.; Fang, P.; et al. Epidemiological survey and screening strategy for dengue virus in blood donors from Yunnan Province. BMC Infect. Dis. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Zhang, N.Z.; Zhang, K.L.; Li, Z.H.; Chan-yu, N.C.; Hu, Y.L.; Li, G.; Zhao, K.; Zou, F.Z.; Xu, W.Z.; Li, S.X.; et al. A Report of the Investigation and Research of Bluetongue on Sheep. Yunnan J. Anim. Sci. Vet. Med. 1989, 18, 3–13. [Google Scholar]
- Kirkland, P.D.; Zhang, N.; Hawkes, R.A.; Li, Z.; Zhang, F.; Davis, R.J.; Sanders, D.A.; Li, H.; Zhang, K.; Jiang, B.F.; et al. Studies on the epidemiology of bluetongue virus in China. Epidemiol. Infect. 2002, 128, 257–263. [Google Scholar] [CrossRef]
- Attoui, H.; Jaafar, F.M.; Belhouchet, M.; Biagini, P.; Cantaloube, J.-F.; de Micco, P.; de Lamballerie, X. Expansion of family Reoviridae to include nine-segmented dsRNA viruses: Isolation and characterization of a new virus designated Aedes pseudoscutellaris reovirus assigned to a proposed genus (Dinovernavirus). Virology 2005, 343, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Brownlie, J.; Tesh, R.; Attoui, H.; Mertens PP, C. Genetic Characterization of the Tick-Borne Orbiviruses. Viruses 2015, 7, 2185–2209. [Google Scholar] [CrossRef]
- Maan, S.; Rao, S.; Maan, N.S.; Anthony, S.J.; Attoui, H.; Samuel, A.R.; Mertens PP, C. Rapid cDNA synthesis and sequencing techniques for the genetic study of bluetongue and other dsRNA viruses. J. Virol. Methods 2007, 143, 132–139. [Google Scholar] [CrossRef]
- Kato, T.; Shirafuji, H.; Tanaka, S.; Sato, M.; Yamakawa, M.; Tsuda, T.; Yanase, T. Bovine Arboviruses in Culicoides Biting Midges and Sentinel Cattle in Southern Japan from 2003 to 2013. Transbound. Emerg. Dis. 2015, 63, e160–e172. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.L.; Bellis, G.; Li, L.; Li, H.C.; Miao, H.S.; Kou, M.L.; Liao, D.F.; Wang, Z.; Gao, L.; Li, J.Z. Potential vectors of bluetongue virus in high altitude areas of Yunnan Province, China. Parasites Vectors 2019, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.L.; Li, L.; Bellis, G.; Yang, Z.X.; Li, H.C. Detection of bluetongue virus in Culicoides spp. in southern Yunnan Province, China. Parasites Vectors 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.J.C. On Culicoides as a Vector of Onchocerca gibsoni (Cleland & Johnston, 1910). J. Helminthol. 1938, 16, 121–158. [Google Scholar] [CrossRef]
- Attoui, H.; Stirling, J.M.; Munderloh, U.G.; Billoir, F.; Brookes, S.M.; Burroughs, J.N.; De Micco, P.; Mertens, P.; De Lamballerie, X. Complete sequence characterization of the genome of the St Croix River virus, a new Orbivirus isolated from cells of Ixodes scapularis. J. Gen. Virol. 2001, 82, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Attoui, H.; Jaafar, F.M.; Belhouchet, M.; Aldrovandi, N.; Tao, S.; Chen, B.; Liang, G.; Tesh, R.B.; De Micco, P.; De Lamballerie, X. Yunnan Orbivirus, a new Orbivirus species isolated from Culex tritaeniorhynchus mosquitoes in China. J. Gen. Virol. 2005, 86, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.M.; Burroughs, J.N.; Gouet, P.; Diprose, J.M.; Malby, R.L.; Ziéntara, S.; Mertens, P.; Stuart, D. The atomic structure of the bluetongue virus core. Nature 1998, 395, 470–478. [Google Scholar] [CrossRef]
- Grimes, J.M.; Jakana, J.; Ghosh, M.; Basak, A.K.; Roy, P.; Chiu, W.I.; Stuart, D.; Prasad, B.V. An atomic model of the outer layer of the bluetongue virus core derived from X-ray crystallography and electron cryomicroscopy. Structure 1997, 5, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Burroughs, J.; Grimes, J.; Mertens, P.; Stuart, D. Crystallization and Preliminary X-ray Analysis of the Core Particle of Bluetongue Virus. Virology 1995, 210, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Strand, M.R. The insect cellular immune response. Insect. Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Mukherjee, D.; Das, S.; Begum, F.; Mal, S.; Ray, U. The Mosquito Immune System and the Life of Dengue Virus: What We Know and Do Not Know. Pathogens 2019, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, G.; Boros, Á.; Delwart, E.; Pankovics, P. Novel Seadornavirus (family Reoviridae) related to Banna virus in Europe. Arch. Virol. 2013, 158, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Mah, M.G.; Deerain, J.M.; Warrilow, D.; Colmant AM, G.; O’Brien, C.A.; Harrison, J.J.; McLean, B.J.; Hewlett, E.K.; Piyasena TB, H.; et al. New genotypes of Liao ning virus (LNV) in Australia exhibit an insect-specific phenotype. J. Gen. Virol. 2018, 99, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, F.M.; Attoui, H.; Mertens PP, C.; De Micco, P.; De Lamballerie, X. Structural organization of an encephalitic human isolate of Banna virus (genus Seadornavirus, family Reoviridae). J. Gen. Virol. 2005, 86, 1147–1157. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Nomikou, K.; Veronesi, E.; Bachanek-Bankowska, K.; Belaganahalli, M.N.; Attoui, H.; Mertens PP, C. Complete Genome Characterisation of a Novel 26th Bluetongue Virus Serotype from Kuwait. PLoS ONE 2011, 6, e26147. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wu, J.; Zhu, J.; Wang, J.; Lyu, M.; Xiao, L.; Yang, Z.; Liao, D.; Li, H.; Yang, H. Isolation and genetic characterization of epizootic hemorrhagic disease virus serotype 1 strains prevalent in China from 2013 to 2019. J. South. Agric. 2021, 52, 2043–2062. [Google Scholar]
- Zhang, Y.X.; Lin, J.; Cao, Y.Y.; Zhu, J.B.; Du, Y.C.; Yang, Z.X.; Yao, J.; Li, H.C.; Wu, J.M. Investigation of the serotypes of epizootic hemorrhagic disease virus and analysis of their distribution in Guangxi. Shanghai J. Anim. Husb. Vet. Med. 2016, 61, 19–21. [Google Scholar]
- Kou, M.; Yang, Z.; Li, L.; Zhu, J.; Gao, L.; Miao, H. Serological investigation and serotype identification of epidemic hemorrhagic virus in Yunnan border area. China Anim. Husb. Vet. Med. 2019, 46, 3065–3074. [Google Scholar]
- Xue, D.M.; Zhang, G.X.; Liu, A.D.; Zhang, Q.Y.; Li, D.C.; Guo, Y.Z.; Huang, D.J.; Yang, B.S. Suspicious Ibaraki disease in cattle. J. Anim. Sci. Vet. Med. 1987, 6, 43–44. [Google Scholar]
- Omori, T.; Inaba, Y.; Morimoto, T.; Tanaka, Y.; Ishitani, R. Ibaraki virus, an agent of epizootic disease of cattle resembling bluetongue. I. Epidemiologic, clinical and pathologic observations and experimental transmission to calves. Jpn. J. Microbiol. 1969, 13, 139–157. [Google Scholar] [CrossRef] [Green Version]

| Isolate | Cell | Target Virus Species | |||||
|---|---|---|---|---|---|---|---|
| AKAV | BAV | BTV | EHDV | PALV | TIBOV | ||
| YNV/01-1 | C6/36 | NA | 16.2 | NA | NA | NA | NA |
| YNV/KM3 | BHK-21 | NA | NA | NA | 26.3 | NA | NA |
| YNV/03-2 | BHK-21 | NA | NA | NA | 27.4 | NA | NA |
| Seg | YNV/01-1 (BAV) | YNV/KM3 (EHDV-1) | YNV/03-2 (EHDV-7) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Length (bp) | Gene | GenBank No. | Length (bp) | Gene | GenBank No. | Length (bp) | Gene | GenBank No. | |
| 1 | 3762 | VP1 | OM953801 | 3942 | VP1 | OM953791 | 3942 | VP1 | OM953813 |
| 2 | 3050 | VP2 (T2) | OM953802 | 2968 | VP2 | OM953792 | 3002 | VP2 | OM953814 |
| 3 | 2399 | VP3 | OM953803 | 2768 | VP3 (T2) | OM953793 | 2768 | VP3 (T2) | OM953815 |
| 4 | 2032 | VP4 | OM953804 | 1984 | VP4 | OM953794 | 1984 | VP4 | OM953816 |
| 5 | 1686 | VP5 | OM953805 | 1769 | NS1 | OM953795 | 1769 | NS1 | OM953817 |
| 6 | 1671 | VP6 | OM953806 | 1640 | VP5 | OM953796 | 1641 | VP5 | OM953818 |
| 7 | 1137 | VP7 | OM953807 | 1162 | VP7 (T13) | OM953797 | 1162 | VP7 (T13) | OM953819 |
| 8 | 1119 | VP8 (T13) | OM953808 | 1192 | NS2 | OM953798 | 1192 | NS2 | OM953820 |
| 9 | 1100 | VP9 | OM953809 | 1071 | VP6 | OM953799 | 1074 | VP6 | OM953821 |
| 10 | 977 | VP10 | OM953810 | 810 | NS3 | OM953800 | 810 | NS3 | OM953822 |
| 11 | 867 | VP11 | OM953811 | ||||||
| 12 | 861 | VP12 | OM953812 | ||||||
| Isolates | Identification | Hosts | Collection Date | Cells for Isolation | |
|---|---|---|---|---|---|
| C6/36 | BHK-21 | ||||
| YNV/KM3 | EHDV-1 | Culicoides spp. | 20 August 2019 | + | + |
| YNV/01-1 | BAV | C. tainanus | 20 May2020 | + | - |
| YNV/03-2 | EHDV-7 | C. orientalis | 9 June 2020 | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Yang, Z.; Bellis, G.; Xie, J.; Li, L. Full Genome Sequencing of Three Sedoreoviridae Viruses Isolated from Culicoides spp. (Diptera, Ceratopogonidae) in China. Viruses 2022, 14, 971. https://doi.org/10.3390/v14050971
Duan Y, Yang Z, Bellis G, Xie J, Li L. Full Genome Sequencing of Three Sedoreoviridae Viruses Isolated from Culicoides spp. (Diptera, Ceratopogonidae) in China. Viruses. 2022; 14(5):971. https://doi.org/10.3390/v14050971
Chicago/Turabian StyleDuan, Yingliang, Zhenxing Yang, Glenn Bellis, Jiarui Xie, and Le Li. 2022. "Full Genome Sequencing of Three Sedoreoviridae Viruses Isolated from Culicoides spp. (Diptera, Ceratopogonidae) in China" Viruses 14, no. 5: 971. https://doi.org/10.3390/v14050971





