HHV-6A Infection of Papillary Thyroid Cancer Cells Induces Several Effects Related to Cancer Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures, Infection, and Treatments
2.2. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
2.3. Cell Viability Assay
2.4. FACS Analysis
2.5. RNA Extraction and RT-qPCR
2.6. Western Blot Analysis
2.7. Antibodies
2.8. Chemiluminescent Immunometric Assay (Luminex Assay)
2.9. Densitometric Analysis
2.10. Statistical Analysis
3. Results
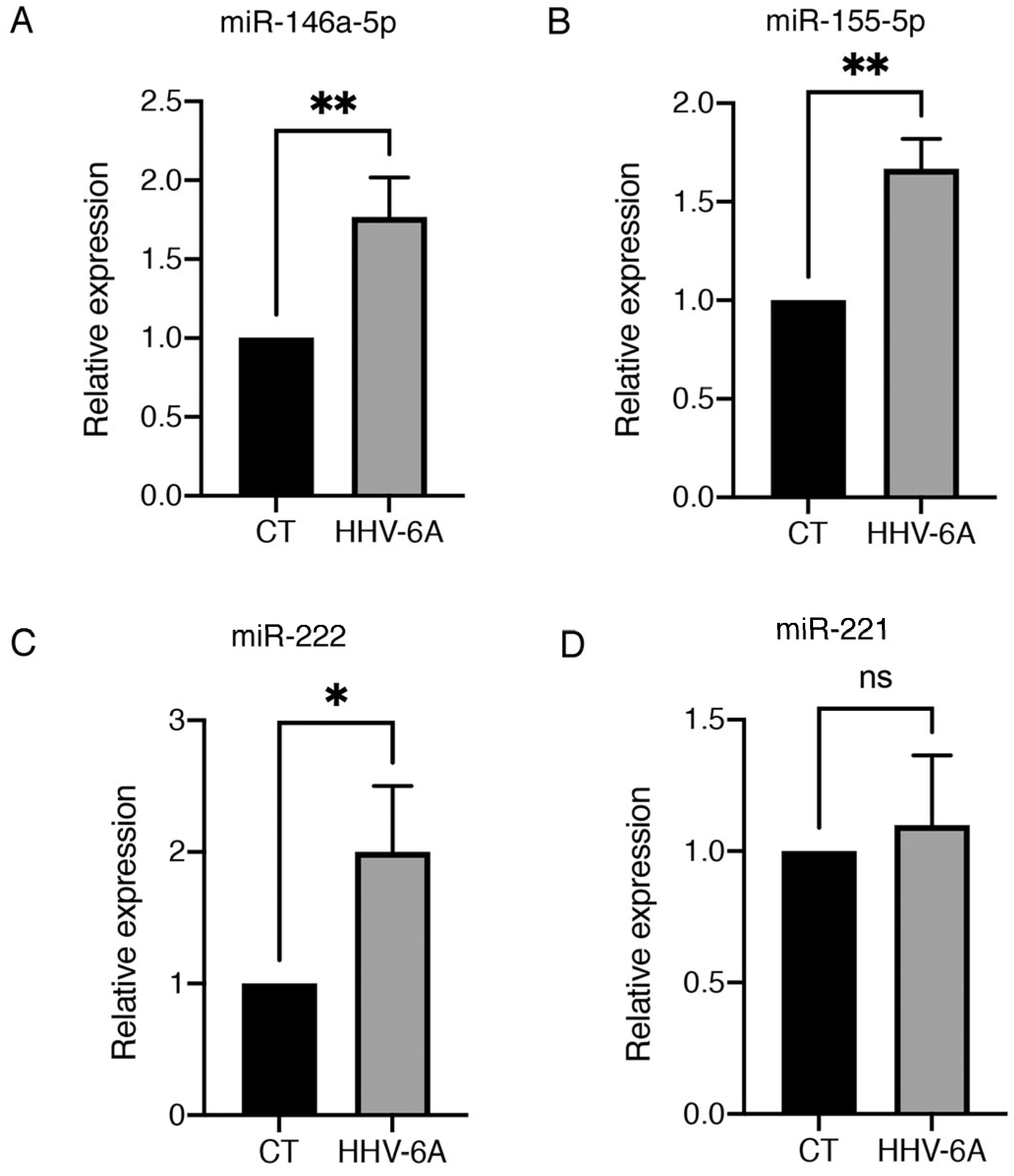
3.1. HHV-6 Infection of BCPAP Cells Does Not Induce Cytopathic Effects and Dysregulates Expression of Several miRNAs
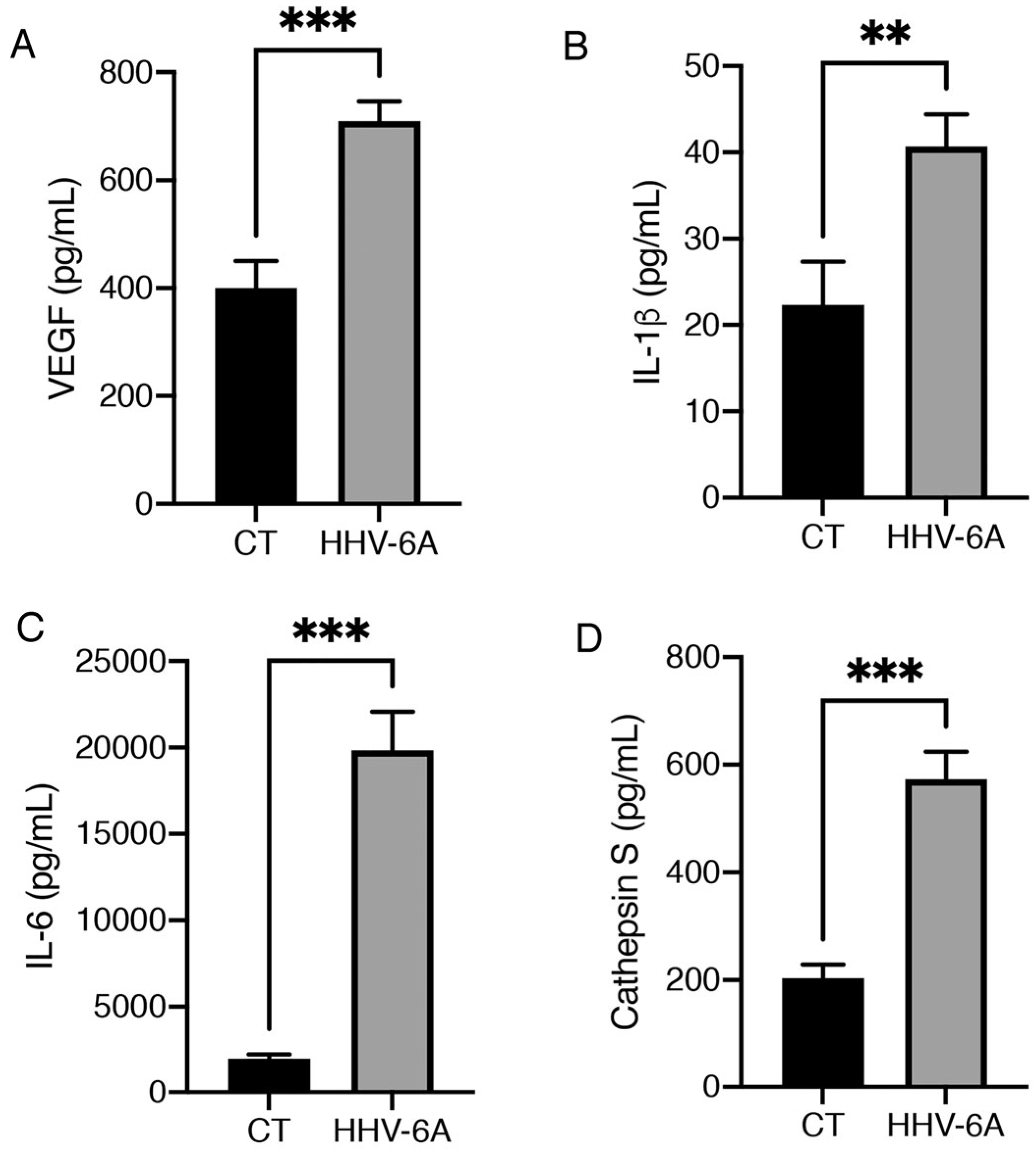
3.2. HHV-6A Promotes the Release of Pro-Inflammatory Cytokines by Infected BCPAP Cells
3.3. HHV-6A Increases Genomic Instability in BCPAP Cells and Up-Regulates PTEN/mutp53/c-Myc/Bcl-xL Axis and miR-9 to Promote EMT
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Odate, T.; Oishi, N.; Kawai, M.; Tahara, I.; Mochizuki, K.; Akaishi, J.; Ito, K.; Katoh, R.; Kondo, T. Progression of Papillary Thyroid Carcinoma to Anaplastic Carcinoma in Metastatic Lymph Nodes: Solid/Insular Growth and Hobnail Cell Change in Lymph Nodes Are Predictors of Subsequent Anaplastic Transformation. Endocr. Pathol. 2021, 32, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Koo, J.S. Cell Component and Function of Tumor Microenvironment in Thyroid Cancer. Int. J. Mol. Sci. 2022, 23, 12578. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, W.; Zhang, H. Roles and new Insights of Macrophages in the Tumor Microenvironment of Thyroid Cancer. Front. Pharmacol. 2022, 13, 875384. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Varricchi, G.; Marone, G. The immune network in thyroid cancer. OncoImmunology 2016, 5, e1168556. [Google Scholar] [CrossRef] [PubMed]
- Visciano, C.; Prevete, N.; Liotti, F.; Marone, G. Tumor-Associated Mast Cells in Thyroid Cancer. Int. J. Endocrinol. 2015, 2015, 705169. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Tang, Y.; Guo, Y.; Wen, S. Immune Microenvironment of Thyroid Cancer. J. Cancer 2020, 11, 4884–4896. [Google Scholar] [CrossRef]
- Lai, X.; Xia, Y.; Zhang, B.; Li, J.; Jiang, Y. A meta-analysis of Hashimoto’s thyroiditis and papillary thyroid carcinoma risk. Oncotarget 2017, 8, 62414–62424. [Google Scholar] [CrossRef]
- Mardente, S.; Zicari, A.; Consorti, F.; Mari, E.; Di Vito, M.; Leopizzi, M.; Della Rocca, C.; Antonaci, A. Cross-talk between NO and HMGB1 in lymphocytic thyroiditis and papillary thyroid cancer. Oncol. Rep. 2010, 24, 1455–1461. [Google Scholar] [CrossRef]
- Mardente, S.; Mari, E.; Massimi, I.; Fico, F.; Faggioni, A.; Pulcinelli, F.; Antonaci, A.; Zicari, A. HMGB1-Induced Cross Talk between PTEN and miRs 221/222 in Thyroid Cancer. BioMed Res. Int. 2015, 2015, 512027. [Google Scholar] [CrossRef]
- Singh, A.; Ham, J.; Po, J.W.; Niles, N.; Roberts, T.; Lee, C.S. The Genomic Landscape of Thyroid Cancer Tumourigenesis and Implications for Immunotherapy. Cells 2021, 10, 1082. [Google Scholar] [CrossRef]
- Kim, K.-H.; Suh, K.-S.; Kang, D.-W.; Kang, D.-Y. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto’s thyroiditis. Pathol. Int. 2005, 55, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.D.; Jackson, L.N.; Riall, T.S.; Uchida, T.; Thomas, R.P.; Qiu, S.; Evers, M.B. Increased Incidence of Well-Differentiated Thyroid Cancer Associated with Hashimoto Thyroiditis and the Role of the PI3k/Akt Pathway. J. Am. Coll. Surg. 2007, 204, 764–773, discussion 773–765. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Carroll, C.; Asa, S.; Ezzat, S. Genetic Events in the Evolution of Thyroid Cancer. J. Otolaryngol. 2002, 31, 202–206. [Google Scholar] [CrossRef]
- Garofalo, M.; Quintavalle, C.; Romano, G.; Croce, C.M.; Condorelli, G. miR221/222 in Cancer: Their Role in Tumor Progression and Response to Therapy. Curr. Mol. Med. 2012, 12, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ouyang, Y.; Che, J.; Li, X.; Zhao, Y.; Yang, K.; Zhao, X.; Chen, Y.; Fan, C.; Yuan, W. Potential Value of miR-221/222 as Diagnostic, Prognostic, and Therapeutic Biomarkers for Diseases. Front. Immunol. 2017, 8, 56. [Google Scholar] [CrossRef]
- Menicali, E.; Guzzetti, M.; Morelli, S.; Moretti, S.; Puxeddu, E. Immune Landscape of Thyroid Cancers: New Insights. Front. Endocrinol. 2021, 11, 637826. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shirvani-Farsani, Z.; Taheri, M. The role of microRNAs in the pathogenesis of thyroid cancer. Non-Coding RNA Res. 2020, 5, 88–98. [Google Scholar] [CrossRef]
- Jensen, K.; Patel, A.; Larin, A.; Hoperia, V.; Saji, M.; Bauer, A.; Yim, K.; Hemming, V.; Vasko, V. Human herpes simplex viruses in benign and malignant thyroid tumours. J. Pathol. 2010, 221, 193–200. [Google Scholar] [CrossRef]
- Caselli, E.; Zatelli, M.C.; Rizzo, R.; Benedetti, S.; Martorelli, D.; Trasforini, G.; Cassai, E.; degli Uberti, E.C.; Di Luca, D.; Dolcetti, R. Virologic and Immunologic Evidence Supporting an Association between HHV-6 and Hashimoto’s Thyroiditis. PLoS Pathog. 2012, 8, e1002951. [Google Scholar] [CrossRef]
- Eliassen, E.; Lum, E.; Pritchett, J.; Ongradi, J.; Krueger, G.; Crawford, J.R.; Phan, T.L.; Ablashi, D.; Hudnall, S.D. Human Herpesvirus 6 and Malignancy: A Review. Front. Oncol. 2018, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, L.; Angeloni, A.; Zompetta, C.; Cirone, M.; Calogero, A.; Frati, L.; Ragona, G.; Faggioni, A.; Ascherio, A.; Munger, K.L.; et al. Human Herpesvirus 6 Variant A, but Not Variant B, Infects EBV-Positive B Lymphoid Cells, Activating the Latent EBV Genome through a BZLF-1-Dependent Mechanism. AIDS Res. Hum. Retroviruses 1995, 11, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.A.; Tayyar, Y.; Idris, A.; McMillan, N.A. A “hit-and-run” affair—A possible link for cancer progression in virally driven cancers. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188476. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, M.R.; Cirone, M.; Pavan, A.; Zompetta, C.; Barile, G.; Frati, L.; Faggioni, A. Localization of Epstein-Barr virus envelope glycoproteins on the inner nuclear membrane of virus-producing cells. J. Virol. 1989, 63, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Montani, M.S.G.; Romeo, M.A.; Benedetti, R.; Gaeta, A.; Cirone, M. DNA damage triggers an interplay between wtp53 and c-Myc affecting lymphoma cell proliferation and Kaposi sarcoma herpesvirus replication. Biochim. Biophys. Acta-Mol. Cell Res. 2021, 1869, 119168. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Kobawala, T.P.; Trivedi, T.I.; Gajjar, K.K.; Patel, D.H.; Patel, G.H.; Ghosh, N.R. Significance of Interleukin-6 in Papillary Thyroid Carcinoma. J. Thyroid. Res. 2016, 2016, 6178921. [Google Scholar] [CrossRef]
- Fuchs, N.; Meta, M.; Schuppan, D.; Nuhn, L.; Schirmeister, T. Novel Opportunities for Cathepsin S Inhibitors in Cancer Immunotherapy by Nanocarrier-Mediated Delivery. Cells 2020, 9, 2021. [Google Scholar] [CrossRef] [PubMed]
- Min, A.K.T.; Mimura, K.; Nakajima, S.; Okayama, H.; Saito, K.; Sakamoto, W.; Fujita, S.; Endo, H.; Saito, M.; Saze, Z.; et al. Therapeutic potential of anti-VEGF receptor 2 therapy targeting for M2-tumor-associated macrophages in colorectal cancer. Cancer Immunol. Immunother. 2021, 70, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Mardente, S.; Aventaggiato, M.; Splendiani, E.; Mari, E.; Zicari, A.; Catanzaro, G.; Po, A.; Coppola, L.; Tafani, M. Extra-Cellular Vesicles Derived from Thyroid Cancer Cells Promote the Epithelial to Mesenchymal Transition (EMT) and the Transfer of Malignant Phenotypes through Immune Mediated Mechanisms. Int. J. Mol. Sci. 2023, 24, 2754. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guessous, F.; Kwon, S.; Kumar, M.; Ibidapo, O.; Fuller, L.; Johnson, E.; Lal, B.; Hussaini, I.; Bao, Y.; et al. PTEN Has Tumor-Promoting Properties in the Setting of Gain-of-Function p53 Mutations. Cancer Res. 2008, 68, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Y.; Tang, Y.; Butler, N.; Kim, J.; Guessous, F.; Schiff, D.; Mandell, J.; Abounader, R. A Novel PTEN/Mutant p53/c-Myc/Bcl-XL Axis Mediates Context-Dependent Oncogenic Effects of PTEN with Implications for Cancer Prognosis and Therapy. Neoplasia 2013, 15, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L. MicroRNA control of epithelial–mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012, 31, 653–662. [Google Scholar] [CrossRef]
- Romeo, M.A.; Montani, M.S.G.; Gaeta, A.; D’Orazi, G.; Faggioni, A.; Cirone, M. HHV-6A infection dysregulates autophagy/UPR interplay increasing beta amyloid production and tau phosphorylation in astrocytoma cells as well as in primary neurons, possible molecular mechanisms linking viral infection to Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165647. [Google Scholar] [CrossRef]
- Broccolo, F.; Fusetti, L.; Ceccherini-Nelli, L. Possible Role of Human Herpesvirus 6 as a Trigger of Autoimmune Disease. Sci. World J. 2013, 2013, 867389. [Google Scholar] [CrossRef]
- Sultanova, A.; Cistjakovs, M.; Gravelsina, S.; Chapenko, S.; Roga, S.; Cunskis, E.; Nora-Krukle, Z.; Groma, V.; Ventina, I.; Murovska, M. Association of active human herpesvirus-6 (HHV-6) infection with autoimmune thyroid gland diseases. Clin. Microbiol. Infect. 2017, 23, 50.e1–50.e5. [Google Scholar] [CrossRef]
- Caselli, E.; D’accolti, M.; Soffritti, I.; Zatelli, M.C.; Rossi, R.; degli Uberti, E.; Di Luca, D. HHV-6A in vitro infection of thyrocytes and T cells alters the expression of miRNA associated to autoimmune thyroiditis. Virol. J. 2017, 14, 3. [Google Scholar] [CrossRef]
- Patel, P.D.; Alghareeb, R.; Hussain, A.; Maheshwari, M.V.; Khalid, N. The Association of Epstein-Barr Virus with Cancer. Cureus 2022, 14, e26314. [Google Scholar] [CrossRef] [PubMed]
- Cirone, M. EBV and KSHV Infection Dysregulates Autophagy to Optimize Viral Replication, Prevent Immune Recognition and Promote Tumorigenesis. Viruses 2018, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Cirone, M. Cancer cells dysregulate PI3K/AKT/mTOR pathway activation to ensure their survival and proliferation: Mimicking them is a smart strategy of gammaherpesviruses. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Mittra, I. Origin of Genome Instability and Determinants of Mutational Landscape in Cancer Cells. Genes 2020, 11, 1101. [Google Scholar] [CrossRef]
- Zhang, J.; Veeramachaneni, N. Targeting interleukin-1β and inflammation in lung cancer. Biomark. Res. 2022, 10, 5. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, G.; Li, X.; Wei, X.; Liu, C.; Derwahl, M. Effect of IL-6 on proliferation of human thyroid anaplastic cancer stem cells. Int. J. Clin. Exp. Pathol. 2019, 12, 3992–4001. [Google Scholar]
- Cardoso, A.P.F.; Banerjee, M.; Nail, A.N.; Lykoudi, A.; States, J.C. miRNA dysregulation is an emerging modulator of genomic instability. Semin. Cancer Biol. 2021, 76, 120–131. [Google Scholar] [CrossRef]
- Schulz-Heddergott, R.; Stark, N.; Edmunds, S.J.; Li, J.; Conradi, L.-C.; Bohnenberger, H.; Ceteci, F.; Greten, F.R.; Dobbelstein, M.; Moll, U.M. Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion. Cancer Cell 2018, 34, 298–314.e7. [Google Scholar] [CrossRef]
- Romeo, M.A.; Gilardini Montani, M.S.; Benedetti, R.; Santarelli, R.; D’Orazi, G.; Cirone, M. STAT3 and mutp53 Engage a Positive Feedback Loop Involving HSP90 and the Mevalonate Pathway. Front. Oncol. 2020, 10, 1102. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Doerr, H.W.; Cinatl, J. The Story of Human Cytomegalovirus and Cancer: Increasing Evidence and Open Questions. Neoplasia 2009, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardente, S.; Romeo, M.A.; Asquino, A.; Po, A.; Gilardini Montani, M.S.; Cirone, M. HHV-6A Infection of Papillary Thyroid Cancer Cells Induces Several Effects Related to Cancer Progression. Viruses 2023, 15, 2122. https://doi.org/10.3390/v15102122
Mardente S, Romeo MA, Asquino A, Po A, Gilardini Montani MS, Cirone M. HHV-6A Infection of Papillary Thyroid Cancer Cells Induces Several Effects Related to Cancer Progression. Viruses. 2023; 15(10):2122. https://doi.org/10.3390/v15102122
Chicago/Turabian StyleMardente, Stefania, Maria Anele Romeo, Angela Asquino, Agnese Po, Maria Saveria Gilardini Montani, and Mara Cirone. 2023. "HHV-6A Infection of Papillary Thyroid Cancer Cells Induces Several Effects Related to Cancer Progression" Viruses 15, no. 10: 2122. https://doi.org/10.3390/v15102122




