Integrase Defective Lentiviral Vector Promoter Impacts Transgene Expression in Target Cells and Magnitude of Vector-Induced Immune Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid Construction and Lentiviral Vector Production
2.2. LV Transduction of 293T Lenti-X Cells
2.3. LV Transduction of Mouse-Bone-Marrow-Derived Dendritic Cells
2.4. LV Transduction of Monkey-Monocyte-Derived Dendritic Cells
2.5. LV Transduction of Human-Monocyte-Derived Dendritic Cells
2.6. LV Transduction of Monkey Skeletal Muscle Cells
2.7. Mouse Immunization Study
2.8. IFN-γ ELISpot Assay
3. Results
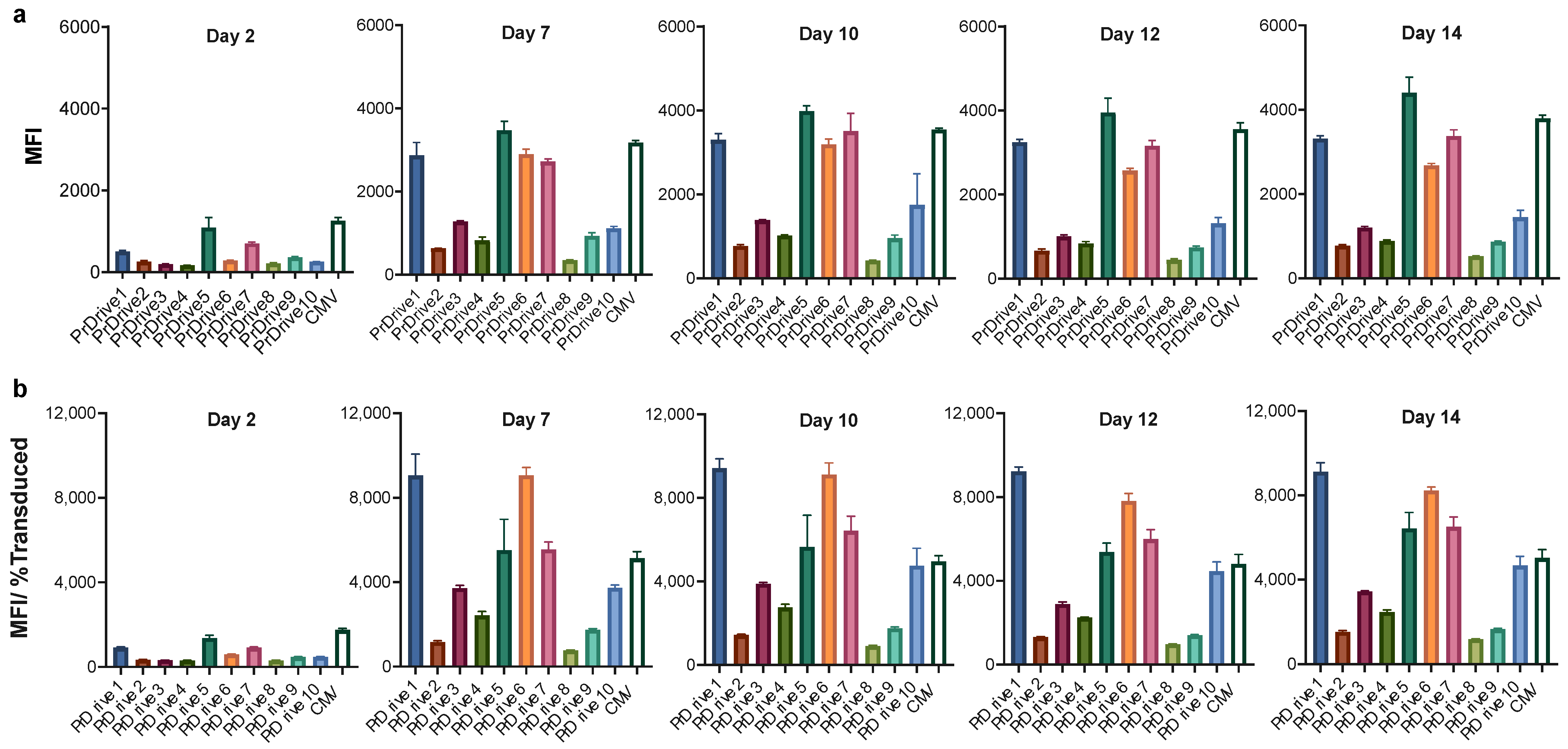
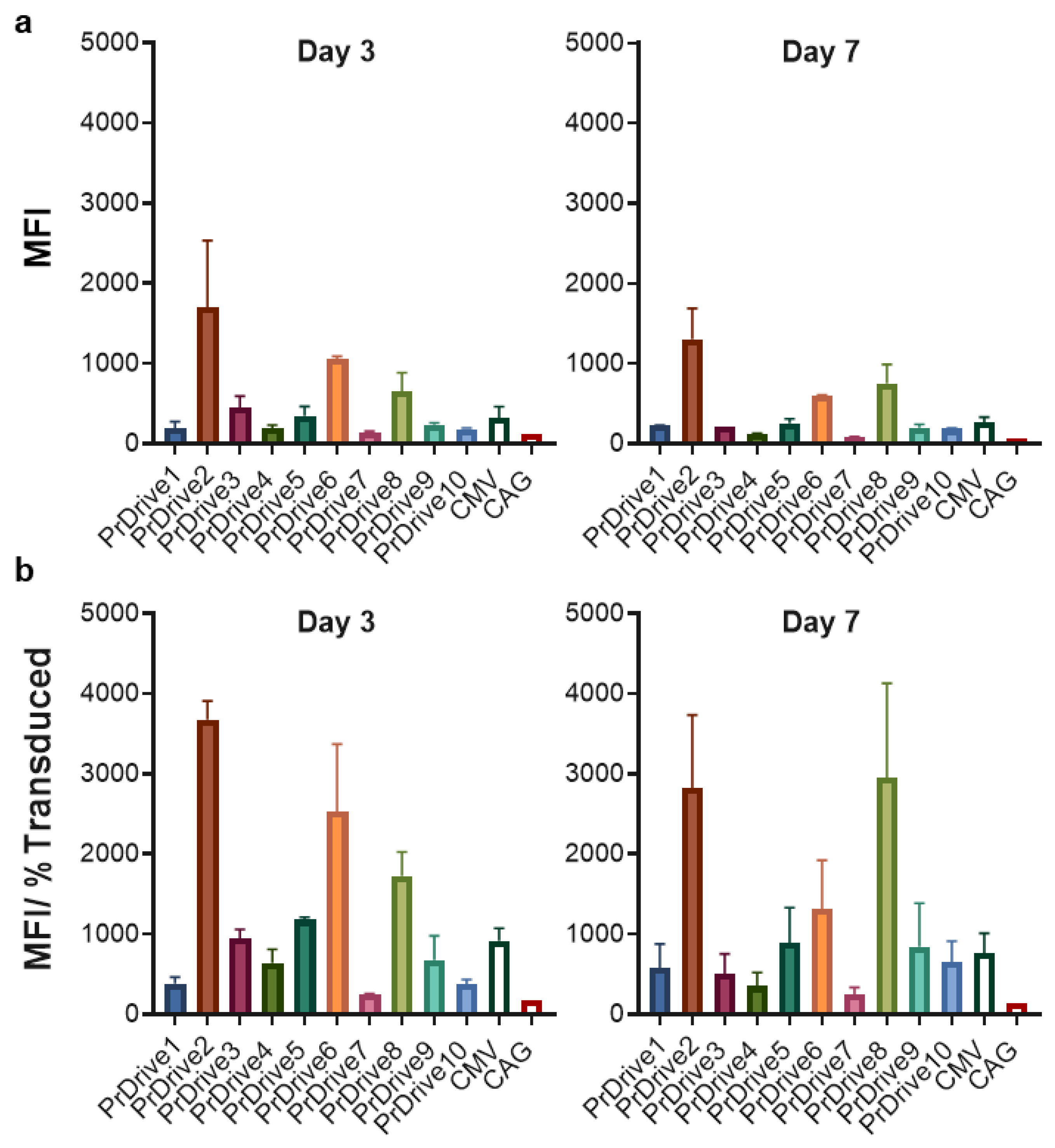
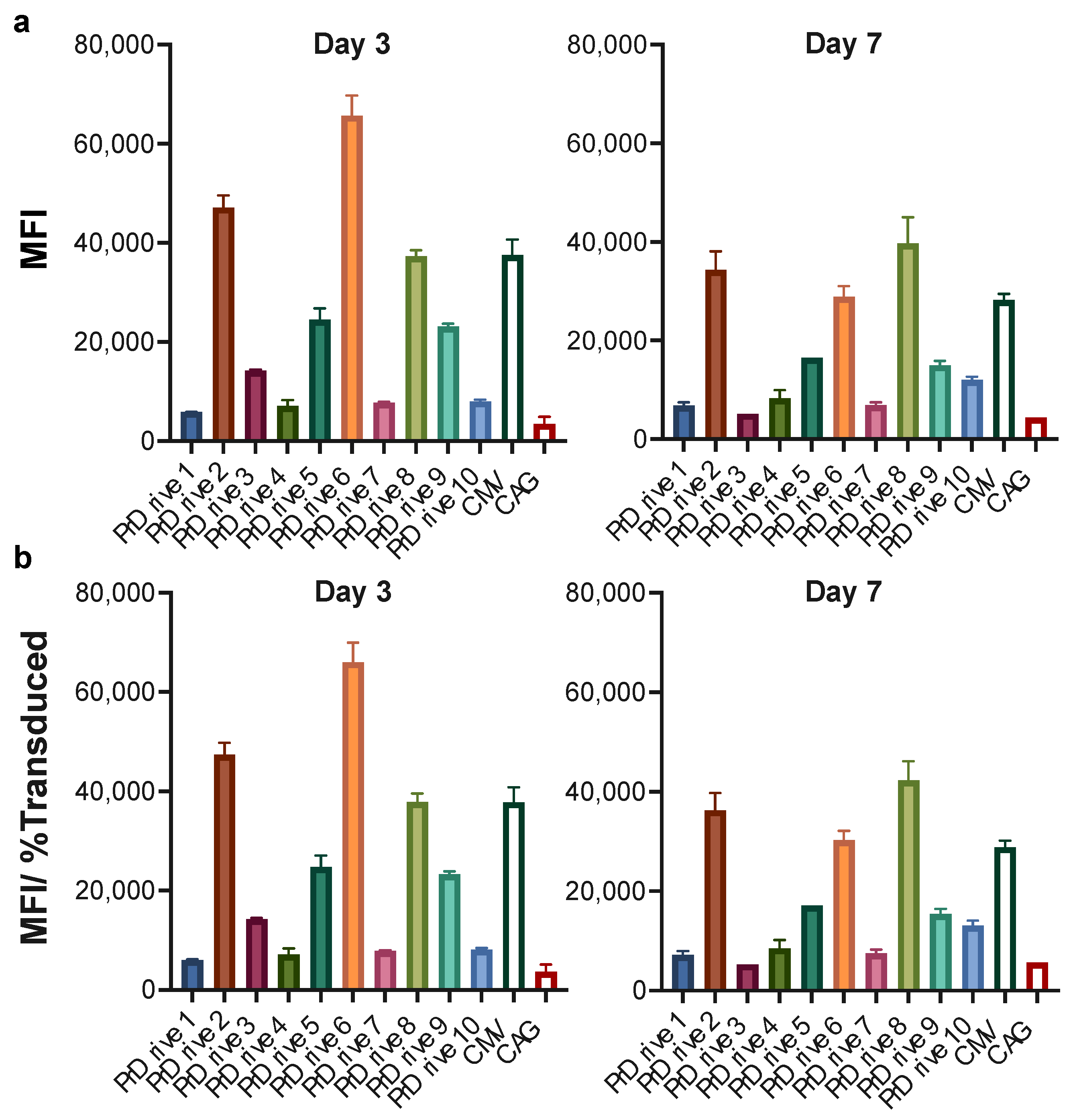
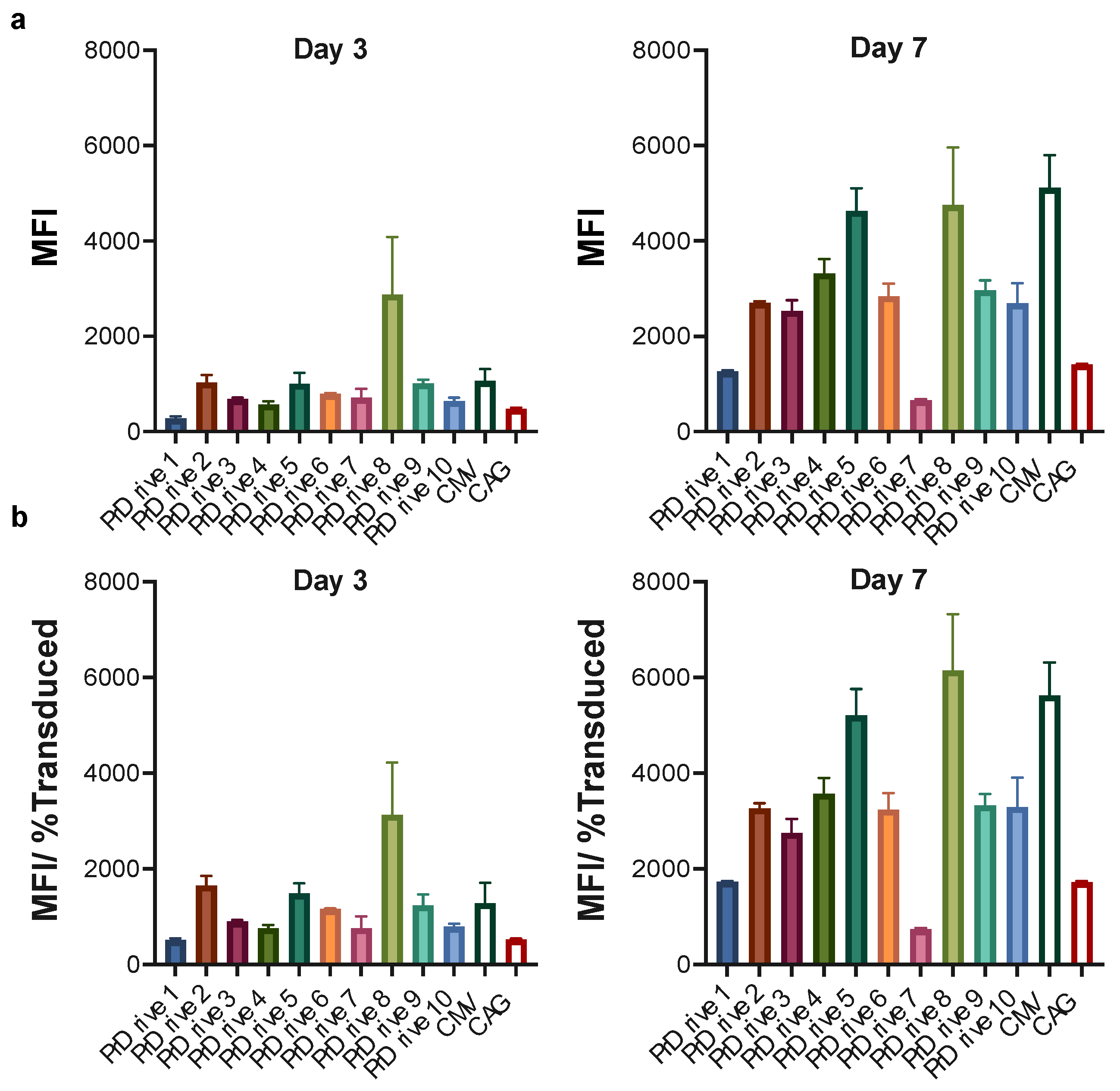
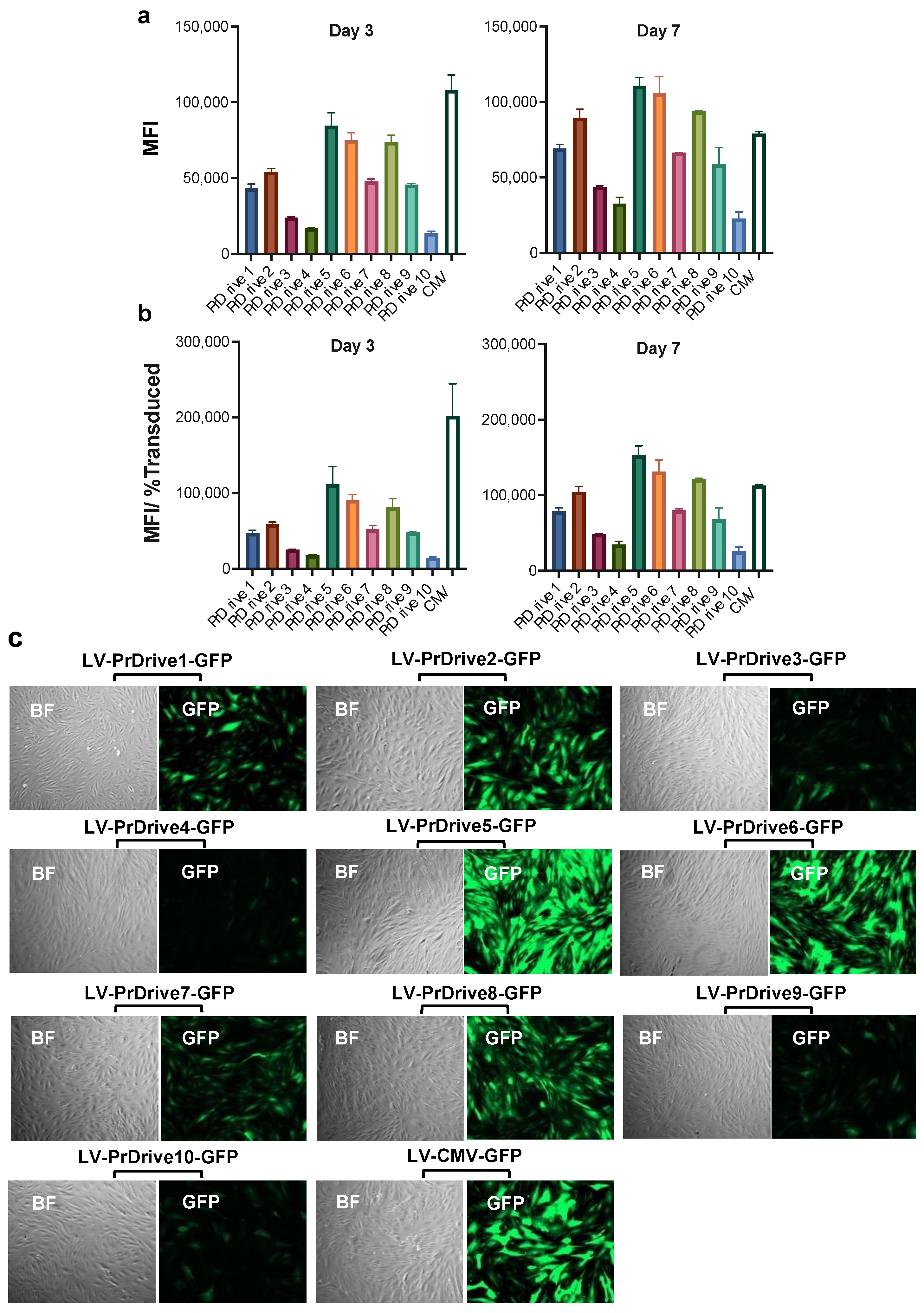
3.1. Vector Promoters Impact Transgene Expression Levels in Different Cell Types
3.2. Impact of Vector Promoter on the Magnitude of IDLV-Induced T Cell Responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Tai, A.; Wang, P. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol. Rev. 2011, 239, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.W.; Charneau, P.; Majlessi, L. Use of lentiviral vectors in vaccination. Expert Rev. Vaccines 2021, 20, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Poletti, V.; Mavilio, F. Designing Lentiviral Vectors for Gene Therapy of Genetic Diseases. Viruses 2021, 13, 1526. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.; Blasi, M. The use of viral vectors in vaccine development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.H.; Mikkelsen, J.G. Delivering genes with human immunodeficiency virus-derived vehicles: Still state-of-the-art after 25 years. J. Biomed. Sci. 2022, 29, 79. [Google Scholar] [CrossRef]
- Fontana, J.M.; Christos, P.J.; Michelini, Z.; Negri, D.; Cara, A.; Salvatore, M. Mucosal immunization with integrase-defective lentiviral vectors protects against influenza virus challenge in mice. PLoS ONE 2014, 9, e97270. [Google Scholar] [CrossRef]
- Grasso, F.; Negri, D.R.; Mochi, S.; Rossi, A.; Cesolini, A.; Giovannelli, A.; Chiantore, M.V.; Leone, P.; Giorgi, C.; Cara, A. Successful therapeutic vaccination with integrase defective lentiviral vector expressing nononcogenic human papillomavirus E7 protein. Int. J. Cancer 2013, 132, 335–344. [Google Scholar] [CrossRef]
- Michelini, Z.; Negri, D.R.; Baroncelli, S.; Spada, M.; Leone, P.; Bona, R.; Klotman, M.E.; Cara, A. Development and use of SIV-based Integrase defective lentiviral vector for immunization. Vaccine 2009, 27, 4622–4629. [Google Scholar] [CrossRef]
- Negri, D.; Blasi, M.; LaBranche, C.; Parks, R.; Balachandran, H.; Lifton, M.; Shen, X.; Denny, T.; Ferrari, G.; Vescio, M.F.; et al. Immunization with an SIV-based IDLV Expressing HIV-1 Env 1086 Clade C Elicits Durable Humoral and Cellular Responses in Rhesus Macaques. Mol. Ther. 2016, 24, 2021–2032. [Google Scholar] [CrossRef]
- Negri, D.R.; Michelini, Z.; Baroncelli, S.; Spada, M.; Vendetti, S.; Bona, R.; Leone, P.; Klotman, M.E.; Cara, A. Nonintegrating Lentiviral Vector-Based Vaccine Efficiently Induces Functional and Persistent CD8+ T Cell Responses in Mice. J. Biomed. Biotechnol. 2010, 2010, 534501. [Google Scholar] [CrossRef]
- Negri, D.R.; Michelini, Z.; Baroncelli, S.; Spada, M.; Vendetti, S.; Buffa, V.; Bona, R.; Leone, P.; Klotman, M.E.; Cara, A. Successful immunization with a single injection of non-integrating lentiviral vector. Mol. Ther. 2007, 15, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.; Negri, D.; LaBranche, C.; Alam, S.M.; Baker, E.J.; Brunner, E.C.; Gladden, M.A.; Michelini, Z.; Vandergrift, N.A.; Wiehe, K.J.; et al. IDLV-HIV-1 Env vaccination in non-human primates induces affinity maturation of antigen-specific memory B cells. Commun. Biol. 2018, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.; Negri, D.; Saunders, K.O.; Baker, E.J.; Stadtler, H.; LaBranche, C.; Mildenberg, B.; Morton, G.; Ciarla, A.; Shen, X.; et al. Immunogenicity, safety, and efficacy of sequential immunizations with an SIV-based IDLV expressing CH505 Envs. NPJ Vaccines 2020, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Dai, B.; Wang, P. Vaccines delivered by integration-deficient lentiviral vectors targeting dendritic cells induces strong antigen-specific immunity. Vaccine 2010, 28, 6675–6683. [Google Scholar] [CrossRef]
- Karwacz, K.; Mukherjee, S.; Apolonia, L.; Blundell, M.P.; Bouma, G.; Escors, D.; Collins, M.K.; Thrasher, A.J. Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy. J. Virol. 2009, 83, 3094–3103. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Belle, I.; Blasi, M.; Huang, M.N.; Buckley, A.F.; Rountree, W.; Klotman, M.E.; Cara, A.; Negri, D. Skeletal Muscle Is an Antigen Reservoir in Integrase-Defective Lentiviral Vector-Induced Long-Term Immunity. Mol. Ther. Methods Clin. Dev. 2020, 17, 532–544. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Blasi, M.; Wescott, E.C.; Baker, E.J.; Mildenberg, B.; LaBranche, C.; Rountree, W.; Haynes, B.F.; Saunders, K.O.; Moody, M.A.; Negri, D.; et al. Therapeutic vaccination with IDLV-SIV-Gag results in durable viremia control in chronically SHIV-infected macaques. NPJ Vaccines 2020, 5, 36. [Google Scholar] [CrossRef]
- Borghi, M.; Gallinaro, A.; Pirillo, M.F.; Canitano, A.; Michelini, Z.; De Angelis, M.L.; Cecchetti, S.; Tinari, A.; Falce, C.; Mariotti, S.; et al. Different configurations of SARS-CoV-2 spike protein delivered by integrase-defective lentiviral vectors induce persistent functional immune responses, characterized by distinct immunogenicity profiles. Front. Immunol. 2023, 14, 1147953. [Google Scholar] [CrossRef]
- Gallinaro, A.; Borghi, M.; Bona, R.; Grasso, F.; Calzoletti, L.; Palladino, L.; Cecchetti, S.; Vescio, M.F.; Macchia, D.; Morante, V.; et al. Integrase Defective Lentiviral Vector as a Vaccine Platform for Delivering Influenza Antigens. Front. Immunol. 2018, 9, 171. [Google Scholar] [CrossRef]
- Gallinaro, A.; Pirillo, M.F.; Aldon, Y.; Cecchetti, S.; Michelini, Z.; Tinari, A.; Borghi, M.; Canitano, A.; McKay, P.F.; Bona, R.; et al. Persistent immunogenicity of integrase defective lentiviral vectors delivering membrane-tethered native-like HIV-1 envelope trimers. NPJ Vaccines 2022, 7, 44. [Google Scholar] [CrossRef]
- Pollack, S.M.; Lu, H.; Gnjatic, S.; Somaiah, N.; O’Malley, R.B.; Jones, R.L.; Hsu, F.J.; Ter Meulen, J. First-in-Human Treatment with a Dendritic Cell-targeting Lentiviral Vector-expressing NY-ESO-1, LV305, Induces Deep, Durable Response in Refractory Metastatic Synovial Sarcoma Patient. J. Immunother. 2017, 40, 302–306. [Google Scholar] [CrossRef]
- Somaiah, N.; Block, M.S.; Kim, J.W.; Shapiro, G.I.; Do, K.T.; Hwu, P.; Eder, J.P.; Jones, R.L.; Lu, H.; Ter Meulen, J.H.; et al. First-in-Class, First-in-Human Study Evaluating LV305, a Dendritic-Cell Tropic Lentiviral Vector, in Sarcoma and Other Solid Tumors Expressing NY-ESO-1. Clin. Cancer Res. 2019, 25, 5808–5817. [Google Scholar] [CrossRef]
- Ku, M.W.; Authie, P.; Nevo, F.; Souque, P.; Bourgine, M.; Romano, M.; Charneau, P.; Majlessi, L. Lentiviral vector induces high-quality memory T cells via dendritic cells transduction. Commun. Biol. 2021, 4, 713. [Google Scholar] [CrossRef]
- Nemirov, K.; Bourgine, M.; Anna, F.; Wei, Y.; Charneau, P.; Majlessi, L. Lentiviral Vectors as a Vaccine Platform against Infectious Diseases. Pharmaceutics 2023, 15, 846. [Google Scholar] [CrossRef]
- Lu, W.; Arraes, L.C.; Ferreira, W.T.; Andrieu, J.M. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 2004, 10, 1359–1365. [Google Scholar] [CrossRef]
- Coelho, A.V.; de Moura, R.R.; Kamada, A.J.; da Silva, R.C.; Guimaraes, R.L.; Brandao, L.A.; de Alencar, L.C.; Crovella, S. Dendritic Cell-Based Immunotherapies to Fight HIV: How Far from a Success Story? A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2016, 17, 1985. [Google Scholar] [CrossRef]
- Tada, T.; Norton, T.D.; Leibowitz, R.; Landau, N.R. Directly injected lentiviral vector-based T cell vaccine protects mice against acute and chronic viral infection. JCI Insight 2022, 7, e161598. [Google Scholar] [CrossRef]
- Negri, D.R.; Rossi, A.; Blasi, M.; Michelini, Z.; Leone, P.; Chiantore, M.V.; Baroncelli, S.; Perretta, G.; Cimarelli, A.; Klotman, M.E.; et al. Simian immunodeficiency virus-Vpx for improving integrase defective lentiviral vector-based vaccines. Retrovirology 2012, 9, 69. [Google Scholar] [CrossRef]


| Promoter Name | Size | Enhancer | Core Promoter | 5′ UTR |
|---|---|---|---|---|
| PrDrive1 | 929 bp | hCMV | hEF1 | HTLV |
| PrDrive2 | 947 bp | mCMV | hEF1 | HTLV |
| PrDrive3 | 759 bp | SV40 | hEF1 | HTLV |
| PrDrive4 | 723 bp | mTyr | hEF1 | HTLV |
| PrDrive5 | 827 bp | hCMV | hCMV | HTLV |
| PrDrive6 | 657 bp | SV40 | hCMV | HTLV |
| PrDrive7 | 1656 bp | hCMV | hFerL | chEF1 |
| PrDrive8 | 1674 bp | mCMV | hFerL | chEF1 |
| PrDrive9 | 1486 bp | SV40 | hFerL | chEF1 |
| PrDrive10 | 1530 bp | hAldA | hFerL | chEF1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahesh, S.; Li, J.; Travieso, T.; Psaradelli, D.; Negri, D.; Klotman, M.; Cara, A.; Blasi, M. Integrase Defective Lentiviral Vector Promoter Impacts Transgene Expression in Target Cells and Magnitude of Vector-Induced Immune Responses. Viruses 2023, 15, 2255. https://doi.org/10.3390/v15112255
Mahesh S, Li J, Travieso T, Psaradelli D, Negri D, Klotman M, Cara A, Blasi M. Integrase Defective Lentiviral Vector Promoter Impacts Transgene Expression in Target Cells and Magnitude of Vector-Induced Immune Responses. Viruses. 2023; 15(11):2255. https://doi.org/10.3390/v15112255
Chicago/Turabian StyleMahesh, Sneha, Jenny Li, Tatianna Travieso, Danai Psaradelli, Donatella Negri, Mary Klotman, Andrea Cara, and Maria Blasi. 2023. "Integrase Defective Lentiviral Vector Promoter Impacts Transgene Expression in Target Cells and Magnitude of Vector-Induced Immune Responses" Viruses 15, no. 11: 2255. https://doi.org/10.3390/v15112255





