A COVID-19 Infection Model Considering the Factors of Environmental Vectors and Re-Positives and Its Application to Data Fitting in Japan and Italy
Abstract
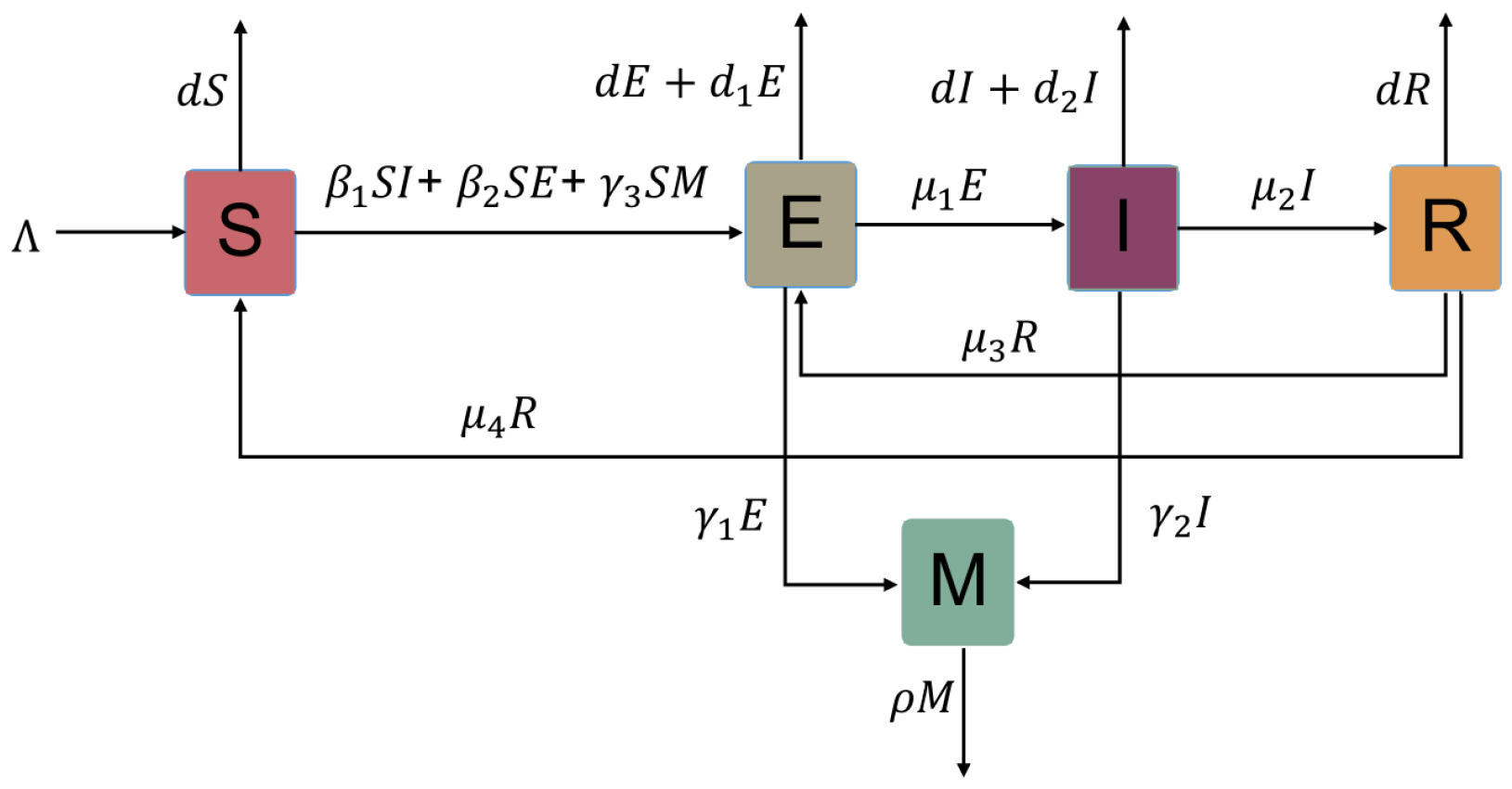
:1. Introduction
2. Stability and Uniform Persistence
2.1. Basic Reproduction Number and Classification of Equilibria
2.2. Global Stability of the Disease-Free Equilibrium
2.3. Uniform Persistence and Global Stability of the Endemic Equilibrium
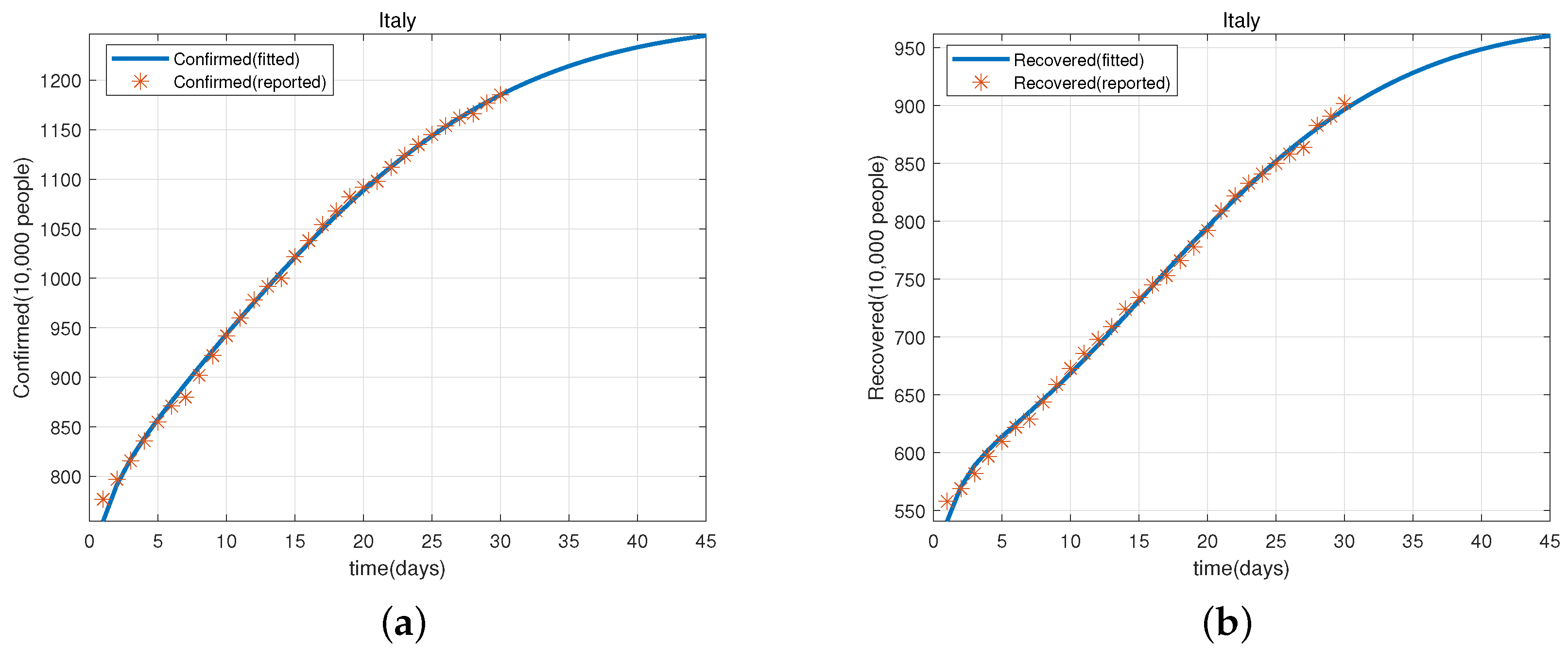
3. Applications of the Model in Japan and Italy
3.1. Predicted Cases for Cumulative Confirmed and Recovered Cases Based on Data in Japan and Italy
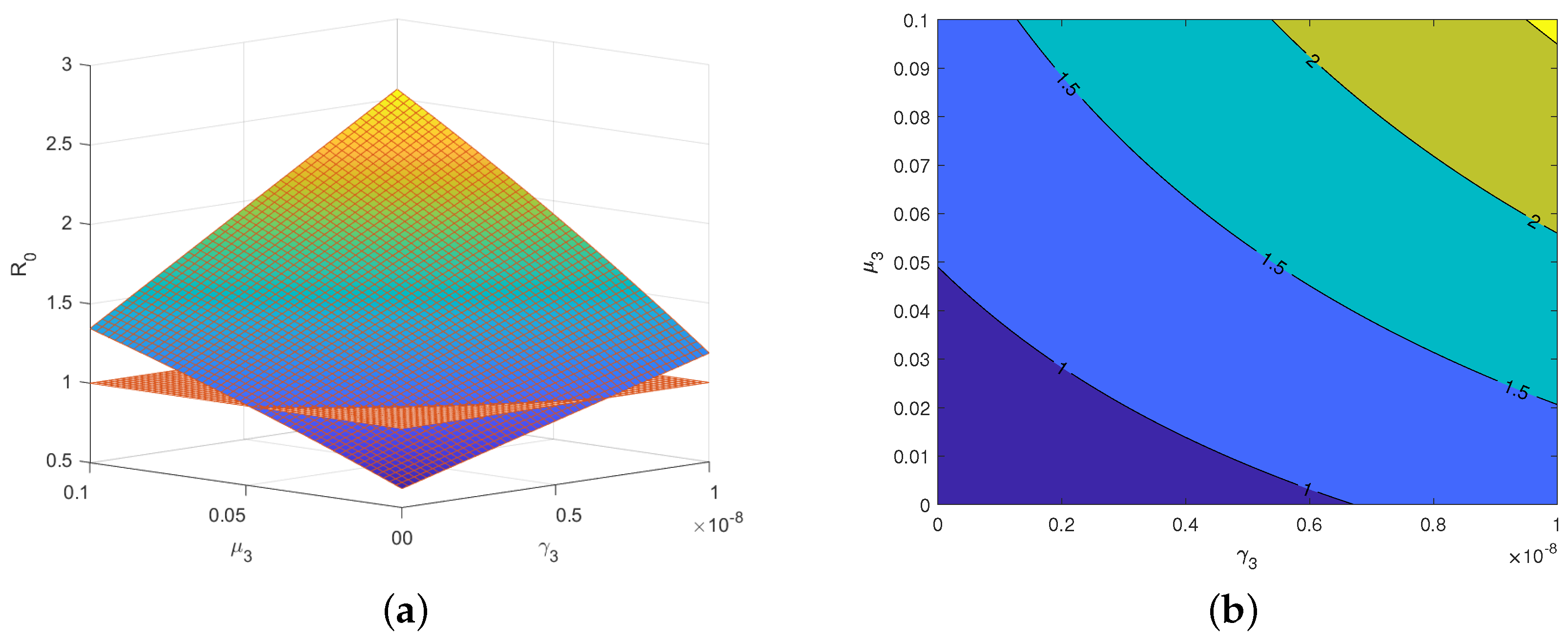
3.2. Sensitivity Analysis Based on the Basic Reproduction Number and Data from Italy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.H.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus-Public Health Emergency. Available online: https://time.com/5774747/coronavirus-whopublic-health-emergency/ (accessed on 11 July 2020).
- COVID-19 Biggest Global Crisis for Children in Our 75-Year History. Available online: https://www.unicef.cn/press-releases/covid-19-biggest-global-crisis-children-our-75-year-history-unicef (accessed on 18 July 2021).
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Huang, L.X.; Wang, Y.M.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of covid- 19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 22 August 2022).
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 18 July 2022).
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xia, F.; Wu, J.H. Lessons drawn from China and South Korea for managing COVID-19 epidemic: Insights from a comparative modeling study. ISA Trans. 2020, 124, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, X.; Li, Q.; Bragazzi, N.L.; Tang, S.; Xiao, Y.; Wu, J. Estimation of the Transmission Risk of the 2019-nCoV and Its Implication for Public Health Interventions. J. Clin. Med. 2020, 9, 462. [Google Scholar] [CrossRef]
- Yang, Z.F.; Zeng, Z.Q.; Wang, K.; Wong, S.S.; Liang, W.; Zanin, M.; Liu, P.; Cao, X.; Gao, Z.; Mai, Z.; et al. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J. Thorac. Dis. 2020, 12, 165–174. [Google Scholar] [CrossRef]
- Kucharski, A.J.; Russell, T.W.; Diamond, C.; Liu, Y.; Edmunds, J.; Funk, S.; Eggo, R.M.; Sun, F.; Jit, M.; Munday, J.D.; et al. Early dynamics of transmission and control of COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 553–558. [Google Scholar] [CrossRef]
- Sarkar, K.; Khajanchi, S.; Nieto, J.J. Modeling and forecasting the COVID-19 pandemic in India. Chaos Solitons Fractals 2020, 139, 110049. [Google Scholar] [CrossRef] [PubMed]
- Nadim, S.S.; Chattopadhyay, J. Occurrence of backward bifurcation and prediction of disease transmission with imperfect lockdown: A case study on COVID-19. Chaos Solitons Fractals 2020, 140, 110163. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Wang, J. A mathematical model for the novel coronavirus epidemic in Wuhan, China. Math. Biosci. Eng. 2020, 17, 2708–2724. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.M.; Yang, L.; Chu, H.D.; Fan, M. Effect of delay in diagnosis on transmission of COVID-19. Math. Biosci. Eng. 2020, 17, 2725–2740. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Russo, L.; Tsakris, A.; Siettos, C. Data-based analysis, modelling and forecasting of the COVID-19 outbreak. PLoS ONE. 2020, 15, e0230405. [Google Scholar] [CrossRef]
- Zhang, T.L.; Li, Z.M. Analysis of COVID-19 epidemic transmission trend based on a time-delayed dynamic model. Commun. Pure Appl. Anal. 2023, 22, 1–18. [Google Scholar] [CrossRef]
- Li, T.J.; Xiao, Y.N. Linking the disease transmission to information dissemination dynamics: An insight from a multi-scale model study. J. Theor. Biol. 2021, 526, 110796. [Google Scholar] [CrossRef]
- Zu, J.; Shen, M.W.; Li, Y. Investigating the relationship between reopening the economy and implementing control measures during the COVID-19 pandemic. Public Health 2021, 200, 15e21. [Google Scholar] [CrossRef]
- Duan, M.; Jin, Z. The heterogeneous mixing model of COVID-19 with interventions. J. Theor. Biol. 2022, 553, 111258. [Google Scholar] [CrossRef]
- Tu, H.; Wang, X.; Tang, S. Exploring COVID-19 transmission patterns and key factors during epidemics caused by three major strains in Asia. J. Theor. Biol. 2023, 557, 111336. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, B.; Tang, S. The resurgence risk of COVID-19 in China in the presence of immunity waning and ADE: A mathematical modelling study. Vaccine 2022, 40, 7141–7150. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Liu, R.; Tang, S. A quantitative method to project the probability of the end of an epidemic: Application to the COVID-19 outbreak in Wuhan. J. Theor. Biol. 2022, 545, 111149. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.F.; Xiao, Y.N.; Zhao, X.Q. A cross-infection model with diffusive environmental bacteria. J. Math. Anal. Appl. 2022, 505, 125637. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Doremalen, N.V.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, S.; Fazil, A.; Li, M.; Mangat, C.S.; Peterson, S.W.; Daigle, J.; Langner, S.; Shurgold, J.; D’Aoust, P.; Delatolla, R.; et al. A wastewater-based epidemic model for SARS-CoV-2 with application to three Canadian cities. Epidemics 2022, 39, 100560. [Google Scholar] [CrossRef]
- Vallejo, J.A.; Trigo-Tasende, N.; Rumbo-Feal, S.; Conde-Pérez, K.; López-Oriona, Á.; Barbeito, I.; Vaamonde, M.; Tarrío-Saavedra, J.; Reif, R.; Ladra, S.; et al. Modeling the number of people infected with SARS-COV-2 from wastewater viral load in Northwest Spain. Sci. Total Environ. 2021, 811, 152334. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J. A two-patch model for the COVID-19 transmission dynamics in China. J. Appl. Anal. Comput. 2021, 11, 1982–2016. [Google Scholar] [CrossRef]
- Naik, P.A.; Zu, J.; Ghori, M.B.; Naik, M. Modeling the effects of the contaminated environments on COVID-19 transmission in India. Results Phys. 2021, 29, 104774. [Google Scholar] [CrossRef]
- Rai, R.K.; Khajanchi, S.; Tiwari, P.K.; Venturino, E.; Misra, A.K. Impact of social media advertisements on the transmission dynamics of COVID-19 pandemic in India. J. Appl. Math. Comput. 2022, 68, 19–44. [Google Scholar] [CrossRef]
- Chen, T.M.; Rui, J.; Wang, Q.X.; Zhao, Z.Y.; Cui, J.A.; Yin, L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect. Dis. Poverty. 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.N.; Tang, S.Y.; Wu, J.H. Media impact switching surface during an infectious disease outbreak. Sci. Rep. 2015, 5, 7838. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.F.; Yang, P.; Xiang, H. Stability and bifurcation for an SEIS epidemic model with the impact of media. Phys. A 2018, 490, 702–720. [Google Scholar] [CrossRef]
- Cui, J.A.; Sun, Y.H.; Zhu, H.P. The impact of media on the control of infectious diseases. J. Dyn. Differ. Equ. 2008, 20, 31–53. [Google Scholar] [CrossRef]
- Yan, Q.L.; Tang, S.Y.; Gabriele, S.; Wu, J.H. Media coverage and hospital notifications: Correlation analysis and optimal media impact duration to manage a pandemic. J. Theor. Biol. 2016, 390, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Song, P.F.; Xiao, Y.N. Analysis of an Epidemic System with Two Response Delays in Media Impact Function. Bull. Math. Biol. 2019, 81, 1582–1612. [Google Scholar] [CrossRef]
- Musa, S.S.; Yusuf, A.; Zhao, S.; Abdullahi, Z.U.; Abu-Odah, H.; Saad, F.T.; Adamu, L.; He, D. Transmission dynamics of COVID-19 pandemic with combined effects of relapse, reinfection and environmental contribution:A modeling analysis. Results Phys. 2022, 38, 105653. [Google Scholar] [CrossRef]
- Garba, S.M.; Lubuma, J.M.; Tsanou, B. Modeling the transmission dynamics of the COVID-19 pandemic in South Africa. Math Biosci. 2020, 328, 108441. [Google Scholar] [CrossRef]
- Lan, L.; Xu, D.; Ye, G.; Xia, C.; Wang, S.; Li, Y.; Xu, H. Positive RT-PCR test results in patients recovered from COVID-19. JAMA 2020, 323, 1502–1503. [Google Scholar] [CrossRef]
- Mei, Q.; Li, J.; Du, R.; Yuan, X.; Li, M.; Li, J. Assessment of patients who tested positive for COVID-19 after recovery. Lancet Infect. Dis. 2020, 20, 1004–1005. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, S.; He, D.; Yang, L.; Wang, M.H.; Li, Y.; Mei, S.; Zou, X. Positive RT-PCR tests among discharged COVID-19 patients in Shenzhen, China. Infect Control. Hosp Epidemiol. 2020, 41, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liao, X.; Xiao, T.; Qian, S.; Yuan, J.; Ye, H.; Qi, F.; Shen, C.; Wang, L.; Liu, Y.; et al. Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Ann. Transl. Med. 2020, 8, 1084. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Hung, I.F.; Chu, A.W.; Chan, W.M.; Chan, W.M.; Tam, A.R.; Fong, C.H.; Yuan, S.; Tsoi, H.W.; Ng, A.C.; et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020, ciaa1275. [Google Scholar] [CrossRef]
- Murillo-Zamora, E.; Mendoza-Cano, O.; Delgado-Enciso, I.; Hernandez-Suarez, C.M. Predictors of severe symptomatic laboratory-confirmed SARS-COV-2 reinfection. Public Health 2021, 193, 113–115. [Google Scholar] [CrossRef]
- Buskermolen, M.; Paske, K.; Beek, J.; Kortbeek, T.; Götz, H.; Fanoy, E.; Feenstra, S.; Richardus, J.H.; Vollaard, A. Relapse in the first 8 weeks after onset of COVID-19 disease in outpatients: Viral reactivation or inflammatory rebound? J. Infect. 2021, 83, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Reinfection with SARS-CoV: Considerations for Public Health Response: ECDC. Available online: https://www.ecdc.europa.eu/ (accessed on 18 August 2021).
- Diekmann, O.; Heesterbeek, J.A.P.; Metz, J.A.J. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Driessche, P.V.D.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Wang, W.D. Global behavior of an SEIRS epidemic model with time delays. Appl. Math. Lett. 2002, 15, 423–428. [Google Scholar] [CrossRef]
- Guo, S.B.; Ma, W.B. Global behavior of delay differential equations model of HIV infection with apoptosis. Discret. Contin. Dyn. Syst.-Ser. B 2015, 21, 103–119. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, W.H.; Wang, H.B. Global analysis in delayed ratio-dependent gause-type predator-prey models. J. Appl. Anal. Comput. 2017, 7, 1095–1111. [Google Scholar] [CrossRef]
- Guo, K.; Ma, W.B. Permanence and extinction for a nonautonomous Kawasaki disease model with time delays. Appl. Math. Lett. 2021, 122, 107511. [Google Scholar] [CrossRef]
- Martin, R.H. Logarithmic norms and projections applied to linear differential systems. J. Math. Anal. Appl. 1974, 45, 432–454. [Google Scholar] [CrossRef]
- Li, M.Y.; Muldowney, J.S. A Geometric Approach to Global-Stability Problems. SIAM J. Math. Anal. 1996, 27, 1070–1083. [Google Scholar] [CrossRef]
- Feng, X.M.; Ruan, S.G.; Teng, Z.D.; Wang, K. Stability and backward bifurcation in a malaria transmission model with applications to the control of malaria in China. Math. Biosci. 2015, 266, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.H.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Covid-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/countries (accessed on 14 August 2022).
- Stephen, A.D. Forecasting: Principles and Applications; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Lewis, C.D. Industrial and Business Forecasting Methods: A Practical Guide to Exponential Smoothing and Curve Fitting; Butterworth-Heinemann: Oxford, UK, 1982. [Google Scholar]
- Chitnis, N.; Hyman, J.M.; Cushing, J.M. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 2008, 70, 1272–1296. [Google Scholar] [CrossRef]



| Parameter | Description | Unit |
|---|---|---|
| Infection rate of infected individuals | /10,000 persons × day | |
| Infection rate of exposed individuals | /10,000 persons × day | |
| Rate of virus release into the environment by exposed individuals | 10,000 viruses/10,000 persons × day | |
| Rate of virus release into the environment by infected individuals | 10,000 viruses/10,000 persons × day | |
| Infection rate due to environmental vectors | /10,000 viruses × day | |
| Rate of decay or clearance of free virus particles in environmental vectors | /day | |
| Population input rate | 10,000 persons/day | |
| Rate at which exposed individuals become infected | /day | |
| Recovery rate of infected people | /day | |
| Rate of re-positives | /day | |
| Rate at which recovered individuals without immune protection return to the susceptible class | /day | |
| d | Natural death rate | /day |
| Death rate of exposed individuals caused by virus | /day | |
| Death rate of infected individuals caused by virus | /day |
| Para-Meter | Definitions | Value (Japan) | Value (Italy) | Unit | Source |
|---|---|---|---|---|---|
| See Table 1 | /10,000 persons × day | LSM | |||
| See Table 1 | /10,000 persons × day | LSM | |||
| See Table 1 | 0.6513 | 0.3966 | 10,000 viruses/10,000 persons × day | LSM | |
| See Table 1 | 0.2791 | 0.6091 | 10,000 viruses/10,000 persons × day | LSM | |
| See Table 1 | /10,000 viruses × day | LSM | |||
| See Table 1 | 0.8901 | 0.5957 | /day | LSM | |
| See Table 1 | 0.3916 | 0.3358 | 10,000 persons/day | LSM | |
| Incubation period | 5.2 | 5.2 | day | [3] | |
| Infectious period | 20 | 20 | day | [60] | |
| See Table 1 | 0.03051 | 0.09186 | /day | LSM | |
| Time of immune protection | 180 | 180 | day | [61] | |
| Mean lifetime | day | WHO | |||
| See Table 1 | /day | LSM | |||
| See Table 1 | /day | [62] |
| Data | Cumulative Confirmed Cases (Japan) | |
|---|---|---|
| Reported | Predicted | |
| Data | Cumulative Recovered Cases (Japan) | |
|---|---|---|
| Reported | Predicted | |
| Data | Cumulative Confirmed Cases (Italy) | |
|---|---|---|
| Reported | Predicted | |
| Data | Cumulative Recovered Cases (Italy) | |
|---|---|---|
| Reported | Predicted | |
| MAPE/RMSPE | Predictive Ability |
|---|---|
| <10% | Precision prediction |
| 10–20% | Good prediction |
| 20–50% | Reasonable prediction |
| >50% | Unreasonable prediction |
| Data Type | MAPE | Predictive Ability | RMSPE | Predictive Ability |
|---|---|---|---|---|
| Confirmed cases in Japan | Precision prediction | Precision prediction | ||
| Reovered cases in Japan | Precision prediction | Precision prediction | ||
| Confirmed cases in Italy | Precision prediction | Precision prediction | ||
| Recovered cases in Italy | Precision prediction | Precision prediction |
| Parameter | ||||||
| Sensitivity Index | 0.5827 | 0.2993 | −0.2501 | −0.1816 | 0.2618 | −0.0833 |
| Parameter | ||||||
| Sensitivity Index | 0.0200 | 0.0979 | 0.1180 | −0.1180 | −0.0041 | −0.0713 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Lv, J.; Ma, W.; Pradeep, B.G.S.A. A COVID-19 Infection Model Considering the Factors of Environmental Vectors and Re-Positives and Its Application to Data Fitting in Japan and Italy. Viruses 2023, 15, 1201. https://doi.org/10.3390/v15051201
Dong S, Lv J, Ma W, Pradeep BGSA. A COVID-19 Infection Model Considering the Factors of Environmental Vectors and Re-Positives and Its Application to Data Fitting in Japan and Italy. Viruses. 2023; 15(5):1201. https://doi.org/10.3390/v15051201
Chicago/Turabian StyleDong, Shimeng, Jinlong Lv, Wanbiao Ma, and Boralahala Gamage Sampath Aruna Pradeep. 2023. "A COVID-19 Infection Model Considering the Factors of Environmental Vectors and Re-Positives and Its Application to Data Fitting in Japan and Italy" Viruses 15, no. 5: 1201. https://doi.org/10.3390/v15051201





