Active Surveillance of Powassan Virus in Massachusetts Ixodes scapularis Ticks, Comparing Detection Using a New Triplex Real-Time PCR Assay with a Luminex Vector-Borne Panel
Abstract
:1. Introduction
2. Materials and Methods
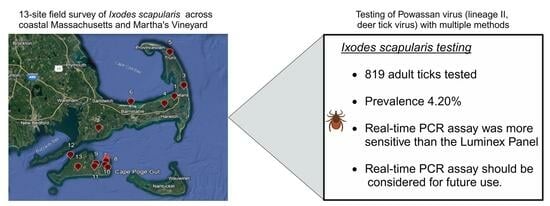
2.1. Study Sites and Tick Collection
2.2. Tick Species Identification and Total Nucleic Acid Extraction
2.3. Taqman Real-Time PCR Assay
2.4. Internal Control
2.5. xMAP MultiFLEX Vector-Borne Panel
2.6. Assay Comparison
3. Results
3.1. Real-Time PCR Powassan Virus Testing of Field-Collected Ticks
3.2. Comparison of Taqman Real-Time PCR Assay with xMAP MultiFLEX Panel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gholam, B.I.; Puksa, S.; Provias, J.P. Powassan Encephalitis: A Case Report with Neuropathology and Literature Review. Can. Med. Assoc. J. 1999, 161, 1419–1422. [Google Scholar]
- Hermance, M.E.; Thangamani, S. Powassan virus: An Emerging Arbovirus of Public Health Concern in North America. Vector Borne Zoonotic Dis. 2017, 17, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Mc, L.D.; Donohue, W.L. Powassan virus: Isolation of Virus from a Fatal Case of Encephalitis. Can. Med. Assoc. J. 1959, 80, 708–711. [Google Scholar]
- Goldfield, M.; Austin, S.M.; Black, H.C.; Taylor, B.F.; Altman, R. A Non-Fatal Human Case of Powassan virus Encephalitis. Am. J. Trop. Med. Hyg. 1973, 22, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Powassan Virus Historic Data (2004–2022). National Arbovirus Surveillance System. Available online: https://www.cdc.gov/powassan/statistics-data/historic-data.html (accessed on 2 January 2024).
- Fatmi, S.S.; Zehra, R.; Carpenter, D.O. Powassan Virus—A New Reemerging Tick-Borne Disease. Front. Public Health 2017, 5, 342. [Google Scholar] [CrossRef] [PubMed]
- Main, A.J.; Carey, A.B.; Downs, W.G. Powassan Virus in Ixodes cookei and Mustelidae in New England. J. Wildl. Dis. 1979, 15, 585–591. [Google Scholar] [CrossRef]
- Ebel, G.D.; Spielman, A.; Telford, S.R., 3rd. Phylogeny of North American Powassan virus. J. Gen. Virol. 2001, 82, 1657–1665. [Google Scholar] [CrossRef]
- Ebel, G.D. Update on Powassan virus: Emergence of a North American Tick-Borne Flavivirus. Annu. Rev. Entomol. 2010, 55, 95–110. [Google Scholar] [CrossRef]
- Thomas, L.A.; Kennedy, R.C.; Eklund, C.M. Isolation of a Virus Closely Related to Powassan virus from Dermacentor andersoni Collected along North Cache La Poudre River, Colo. Proc. Soc. Exp. Biol. Med. 1960, 104, 355–359. [Google Scholar] [CrossRef]
- Telford, S.R., 3rd; Armstrong, P.M.; Katavolos, P.; Foppa, I.; Garcia, A.S.; Wilson, M.L.; Spielman, A. A New Tick-Borne Encephalitis-Like Virus Infecting New England Deer Ticks, Ixodes dammini. Emerg. Infect. Dis. 1997, 3, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ei Khoury, M.Y.; Camargo, J.F.; Wormser, G.P. Changing Epidemiology of Powassan Encephalitis in North America Suggests the Emergence of the Deer Tick Virus Subtype. Expert Rev. Anti Infect. Ther. 2013, 11, 983–985. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.M.; Best, J.M.; Mahalingam, S.; Chernesky, M.A.; Wilson, W.E. Powassan virus: Summer Infection Cycle, 1964. Can. Med. Assoc. J. 1964, 91, 1360–1362. [Google Scholar] [PubMed]
- Ebel, G.D.; Campbell, E.N.; Goethert, H.K.; Spielman, A.; Telford, S.R., 3rd. Enzootic Transmission of Deer Tick Virus in New England and Wisconsin Sites. Am. J. Trop. Med. Hyg. 2000, 63, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Goethert, H.K.; Mather, T.N.; Johnson, R.W.; Telford, S.R., III. Incrimination of shrews as a reservoir for Powassan virus. Commun. Biol. 2021, 4, 1319. [Google Scholar] [CrossRef]
- Brackney, D.E.; Vogels, C.B.F. The Known Unknowns of Powassan virus Ecology. J. Med. Entomol. 2023, 6, 1142–1148. [Google Scholar] [CrossRef]
- Hinten, S.R.; Beckett, G.A.; Gensheimer, K.F.; Pritchard, E.; Courtney, T.M.; Sears, S.D.; Woytowicz, J.M.; Preston, D.G.; Smith, R.P., Jr.; Rand, P.W.; et al. Increased Recognition of Powassan Encephalitis in the United States, 1999–2005. Vector Borne Zoonotic Dis. 2008, 8, 733–740. [Google Scholar] [CrossRef]
- Dupuis, A.P., 2nd; Peters, R.J.; Prusinski, M.A.; Falco, R.C.; Ostfeld, R.S.; Kramer, L.D. Isolation of Deer Tick Virus (Powassan virus, Lineage II) from Ixodes scapularis and Detection of Antibody in Vertebrate Hosts Sampled in the Hudson Valley, New York State. Parasites Vectors 2013, 6, 185. [Google Scholar] [CrossRef]
- Rich, S.M.; Siegel, E.L.; Xu, G. What a Tick Can Tell a Doctor: Using the Human-Biting Tick in the Clinical Management of Tick-Borne Disease. J. Clin. Med. 2023, 12, 6522. [Google Scholar] [CrossRef]
- Ebel, G.D.; Kramer, L.D. Short Report: Duration of Tick Attachment Required for Transmission of Powassan virus by Deer Ticks. Am. J. Trop. Med. Hyg. 2004, 71, 268–271. [Google Scholar] [CrossRef]
- Corrin, T.; Greig, J.; Harding, S.; Young, I.; Mascarenhas, M.; Waddell, L.A. Powassan virus, a Scoping Review of the Global Evidence. Zoonoses Public Health 2018, 65, 595–624. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Powassan Virus Diagnostic Testing. 2023. Available online: https://www.cdc.gov/powassan/diagnostic-testing.html#:~:text=Initial%20serological%20testing%20is%20performed,public%20health%20laboratory%20or%20CDC (accessed on 3 January 2024).
- Brackney, D.E.; Nofchissey, R.A.; Fitzpatrick, K.A.; Brown, I.K.; Ebel, G.D. Stable Prevalence of Powassan virus in Ixodes scapularis in a Northern Wisconsin Focus. Am. J. Trop. Med. Hyg. 2008, 79, 971–973. [Google Scholar] [CrossRef]
- Tokarz, R.; Jain, K.; Bennett, A.; Briese, T.; Lipkin, W.I. Assessment of Polymicrobial Infections in Ticks in New York State. Vector Borne Zoonotic Dis. 2010, 10, 217–221. [Google Scholar] [CrossRef]
- Anderson, J.F.; Armstrong, P.M. Prevalence and Genetic Characterization of Powassan virus Strains Infecting Ixodes scapularis in Connecticut. Am. J. Trop. Med. Hyg. 2012, 87, 754–759. [Google Scholar] [CrossRef]
- Knox, K.K.; Thomm, A.M.; Harrington, Y.A.; Ketter, E.; Patitucci, J.M.; Carrigan, D.R. Powassan/Deer Tick Virus and Borrelia burgdorferi Infection in Wisconsin Tick Populations. Vector Borne Zoonotic Dis. 2017, 17, 463–466. [Google Scholar] [CrossRef]
- Aliota, M.T.; Dupuis, A.P., 2nd; Wilczek, M.P.; Peters, R.J.; Ostfeld, R.S.; Kramer, L.D. The Prevalence of Zoonotic Tick-Borne Pathogens in Ixodes Scapularis Collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis. 2014, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, R.; Tagliafierro, T.; Cucura, D.M.; Rochlin, I.; Sameroff, S.; Lipkin, W.I. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan virus in Ticks by a Multiplex Real-Time Reverse Transcription-PCR Assay. mSphere 2017, 2, e00125-17. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, S.A. Applications of Luminex xMAP Technology for Rapid, High-Throughput Multiplexed Nucleic Acid Detection. Clin. Chim. Acta 2006, 363, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E.; Clifford, C.M. The Genus Ixodes in the United States: A Scanning Electron Microscope Study and Key to the Adults. J. Med. Entomol. Suppl. 1978, 2, 1–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Pearson, P.; Dykstra, E.; Andrews, E.S.; Rich, S.M. Human-Biting Ixodes Ticks and Pathogen Prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis. 2019, 19, 106–114. [Google Scholar] [CrossRef]
- Basic Local Alignment Search Tool. National Library of Medicine. National Center for Biotechnical Information. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 1 January 2024).
- Xu, G.; Fang, Q.Q.; Sun, Y.; Keirans, J.E.; Durden, L.A. Hard Tick Calreticulin (CRT) Gene Coding Regions Have Only One Intron with Conserved Positions and Variable Sizes. J. Parasitol. 2005, 91, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Sanchez, M.; Burraco, P.; Gomez-Mestre, I.; Leonard, J.A. Preservation of RNA and DNA from Mammal Samples under Field Conditions. Mol. Ecol. Resour. 2013, 13, 663–673. [Google Scholar] [CrossRef]
- Schwartz, I.; Varde, S.; Nadelman, R.B.; Wormser, G.P.; Fish, D. Inhibition of Efficient Polymerase Chain Reaction Amplification of Borrelia Burgdorferi DNA in Blood-Fed Ticks. Am. J. Trop. Med. Hyg. 1997, 56, 339–342. [Google Scholar] [CrossRef]
- Schwaiger, M.; Cassinotti, P. Development of a Quantitative Real-Time RT-PCR Assay with Internal Control for the Laboratory Detection of Tick Borne Encephalitis Virus (TBEV) RNA. J. Clin. Virol. 2003, 27, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.; Yang, X.; Chen, M.; Bockenstedt, L.K.; Anderson, J.F.; Flavell, R.A.; Norgard, M.V.; Fikrig, E. OspC Facilitates Borrelia burgdorferi Invasion of Ixodes scapularis Salivary Glands. J. Clin. Investig. 2004, 113, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Mather, T.N.; Hollingsworth, C.S.; Rich, S.M. Passive Surveillance of Ixodes scapularis (Say), Their Biting Activity, and Associated Pathogens in Massachusetts. Vector Borne Zoonotic Dis. 2016, 16, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Robich, R.M.; Cosenza, D.S.; Elias, S.P.; Henderson, E.F.; Lubelczyk, C.B.; Welch, M.; Smith, R.P. Prevalence and Genetic Characterization of Deer Tick Virus (Powassan virus, Lineage II) in Ixodes scapularis Ticks Collected in Maine. Am. J. Trop. Med. Hyg. 2019, 101, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Nofchissey, R.A.; Deardorff, E.R.; Blevins, T.M.; Anishchenko, M.; Bosco-Lauth, A.; Berl, E.; Lubelczyk, C.; Mutebi, J.P.; Brault, A.C.; Ebel, G.D.; et al. Seroprevalence of Powassan virus in New England Deer, 1979–2010. Am. J. Trop. Med. Hyg. 2013, 88, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Arboviral disease—United States, 1994. MMWR Morb. Mortal. Wkly. Rep. 1995, 44, 641–644. [Google Scholar]
- Tavakoli, N.P.; Wang, H.; Dupuis, M.; Hull, R.; Ebel, G.D.; Gilmore, E.J.; Faust, P.L. Fatal Case of Deer Tick Virus Encephalitis. N. Engl. J. Med. 2009, 360, 2099–2107. [Google Scholar] [CrossRef]
- Piantadosi, A.; Rubin, D.B.; McQuillen, D.P.; Hsu, L.; Lederer, P.A.; Ashbaugh, C.D.; Duffalo, C.; Duncan, R.; Thon, J.; Bhattacharyya, S.; et al. Emerging Cases of Powassan Virus Encephalitis in New England: Clinical Presentation, Imaging, and Review of the Literature. Clin. Infect. Dis. 2016, 62, 707–713. [Google Scholar] [CrossRef]

| Target | Gene | Type | Sequences (5′-3′) | Con. (nM) |
|---|---|---|---|---|
| Ixodes tick RNA | calreticulin | Forward | CCAAGGTGTACCTCAAGGAAGAG | 200 |
| Reverse | TTGAAAGTTCCCTGCTCGCTTC | 400 | ||
| Probe | Cy5-ZEN-TCGCCGACGGAG(INTRON)ACGCCTGGAC- Iowa Black RQ | 300 | ||
| Standard Curve | Y = −3.469 × LOG(X) + 15.11, Eff. = 94.2%, RSq = 99.8% | |||
| POWV lineage I and II | ns5 | Forward | TGACAGACACAACAGCGTTTGG | 200 |
| Reverse | TCACTCACHGCTCTCATGATCAC | 300 | ||
| Probe | HEX-ZEN-CTGGTGCCTGGCTGYGGYTCYTGGG- Iowa Black FQ | 100 | ||
| Standard Curve | Y = −3.578 × LOG(X) + 16.29, Eff. = 90.3%, RSq = 99.4% | |||
| POWV lineage II (DTV) | ns5 | Forward | GATCATGAGAGCGGTGAGTGACT | 400 |
| Reverse | GGATCTCACCTTTGCTATGAATTCA | 400 | ||
| Probe | FAM-ZEN-TGAGCACCTTCACAGCCGAGCCAG- Iowa Black FQ | 300 | ||
| Standard Curve | Y = −3.541 × LOG(X ) + 18.46, Eff. = 91.60%, RSq = 99.7% |
| Location | Site | n Ticks Collected | n Ticks Passed RNA Control | POWV All+ | DTV+ |
|---|---|---|---|---|---|
| Cape Cod, Brewster | Nickerson State Park (1) | 130 | 129 | 9 (6.98%) | 9 (6.98%) |
| Cape Cod, East Falmouth | Waquoit (2) | 127 | 101 | 5 (4.95%) | 5 (4.95%) |
| Cape Cod, Eastham | Fort Hill (3) | 118 | 118 | 3 (2.54%) | 3 (2.54%) |
| Cape Cod, Harwich | Punkhorn (4) | 116 | 114 | 0 (0%) | 0 (0%) |
| Cape Cod, Truro | Truro (5) | 116 | 115 | 12 (10.43%) | 12 (10.43%) |
| Cape Cod, West Barnstable | Sandy Neck (6) | 38 | 38 | 0 (0%) | 0 (0%) |
| Martha’s Vineyard, Chappaquiddick | Four Poster (7) | 25 | 24 | 2 (8.33%) | 2 (8.33%) |
| Martha’s Vineyard, Chappaquiddick | Waque (8) | 24 | 24 | 0 (0%) | 0 (0%) |
| Martha’s Vineyard, Edgartown | Brine’s Pond (9) | 25 | 22 | 2 (9.09) | 2 (9.09) |
| Martha’s Vineyard, Edgartown | Cape Pogue (10) | 25 | 25 | 0 (0%) | 0 (0%) |
| Martha’s Vineyard, Edgartown | Correslis State Park (11) | 25 | 25 | 0 (0%) | 0 (0%) |
| Martha’s Vineyard, Vinyard haven | Cedar Tree Neck (12) | 25 | 25 | 0 (0%) | 0 (0%) |
| Martha’s Vineyard, Vinyard haven | Seppiesa Point (13) | 25 | 25 | 0 (0%) | 0 (0%) |
| Total | 819 | 785 | 33 (4.20%) | 33 (4.20%) |
| Triplex qPCR | Wadsworth Center qPCR | xMAP MultiFLEX Vector-Borne Panel | |
|---|---|---|---|
| Total ticks | 819 | 819 | 819 |
| n failed internal control | 34 | N/A 1 | N/A 1 |
| POWV-positive ticks | 33 | 33 | 30 |
| POWV-negative ticks | 752 | 786 | 789 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Siegel, E.; Fernandez, N.; Bechtold, E.; Daly, T.; Dupuis, A.P., II; Ciota, A.; Rich, S.M. Active Surveillance of Powassan Virus in Massachusetts Ixodes scapularis Ticks, Comparing Detection Using a New Triplex Real-Time PCR Assay with a Luminex Vector-Borne Panel. Viruses 2024, 16, 250. https://doi.org/10.3390/v16020250
Xu G, Siegel E, Fernandez N, Bechtold E, Daly T, Dupuis AP II, Ciota A, Rich SM. Active Surveillance of Powassan Virus in Massachusetts Ixodes scapularis Ticks, Comparing Detection Using a New Triplex Real-Time PCR Assay with a Luminex Vector-Borne Panel. Viruses. 2024; 16(2):250. https://doi.org/10.3390/v16020250
Chicago/Turabian StyleXu, Guang, Eric Siegel, Nolan Fernandez, Emily Bechtold, Timothy Daly, Alan P. Dupuis, II, Alexander Ciota, and Stephen M. Rich. 2024. "Active Surveillance of Powassan Virus in Massachusetts Ixodes scapularis Ticks, Comparing Detection Using a New Triplex Real-Time PCR Assay with a Luminex Vector-Borne Panel" Viruses 16, no. 2: 250. https://doi.org/10.3390/v16020250