Spatio-Temporal Dynamics of Viruses are Differentially Affected by Parasitoids Depending on the Mode of Transmission
Abstract
:1. Introduction
2. Results and Discussion
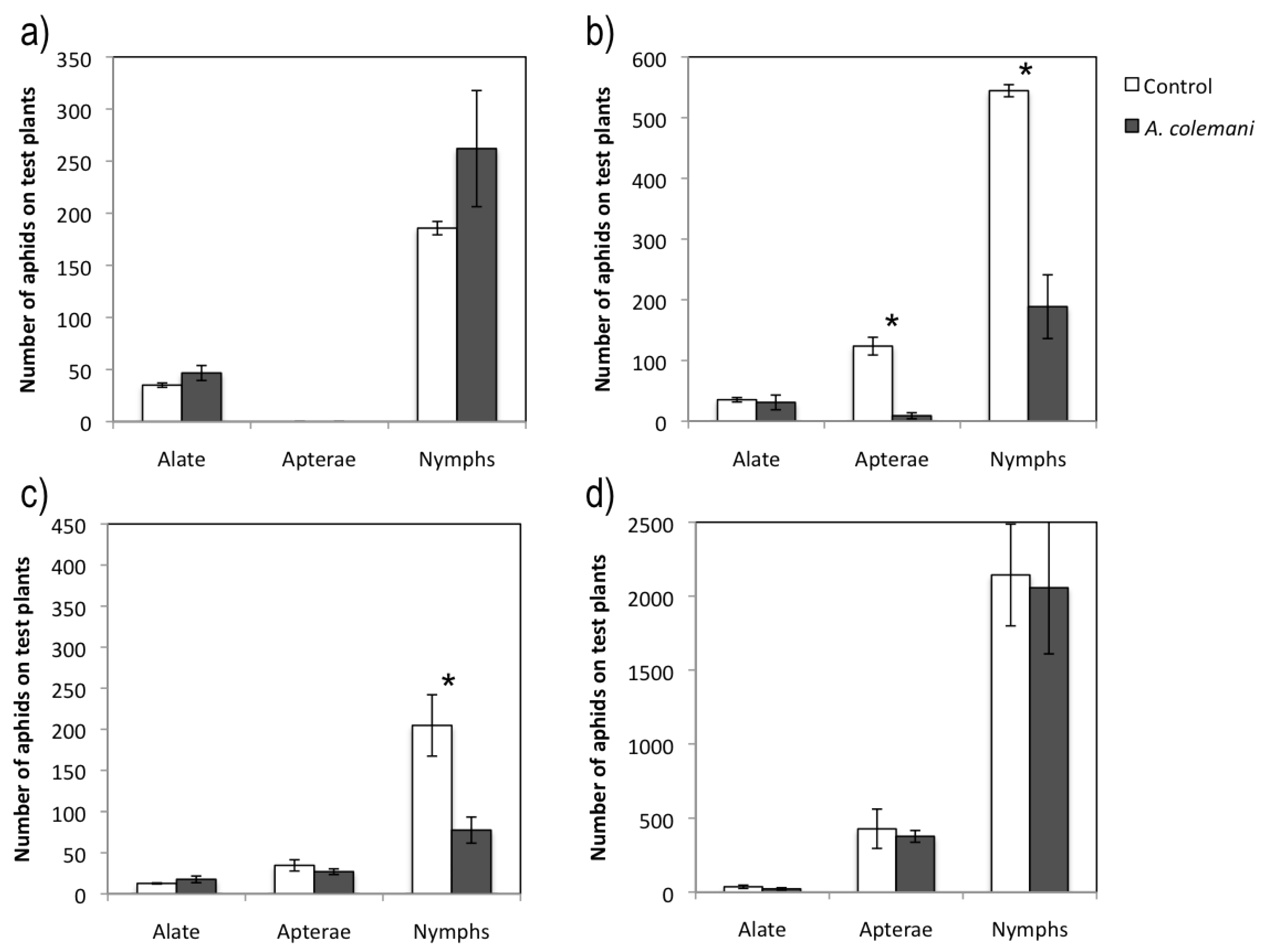
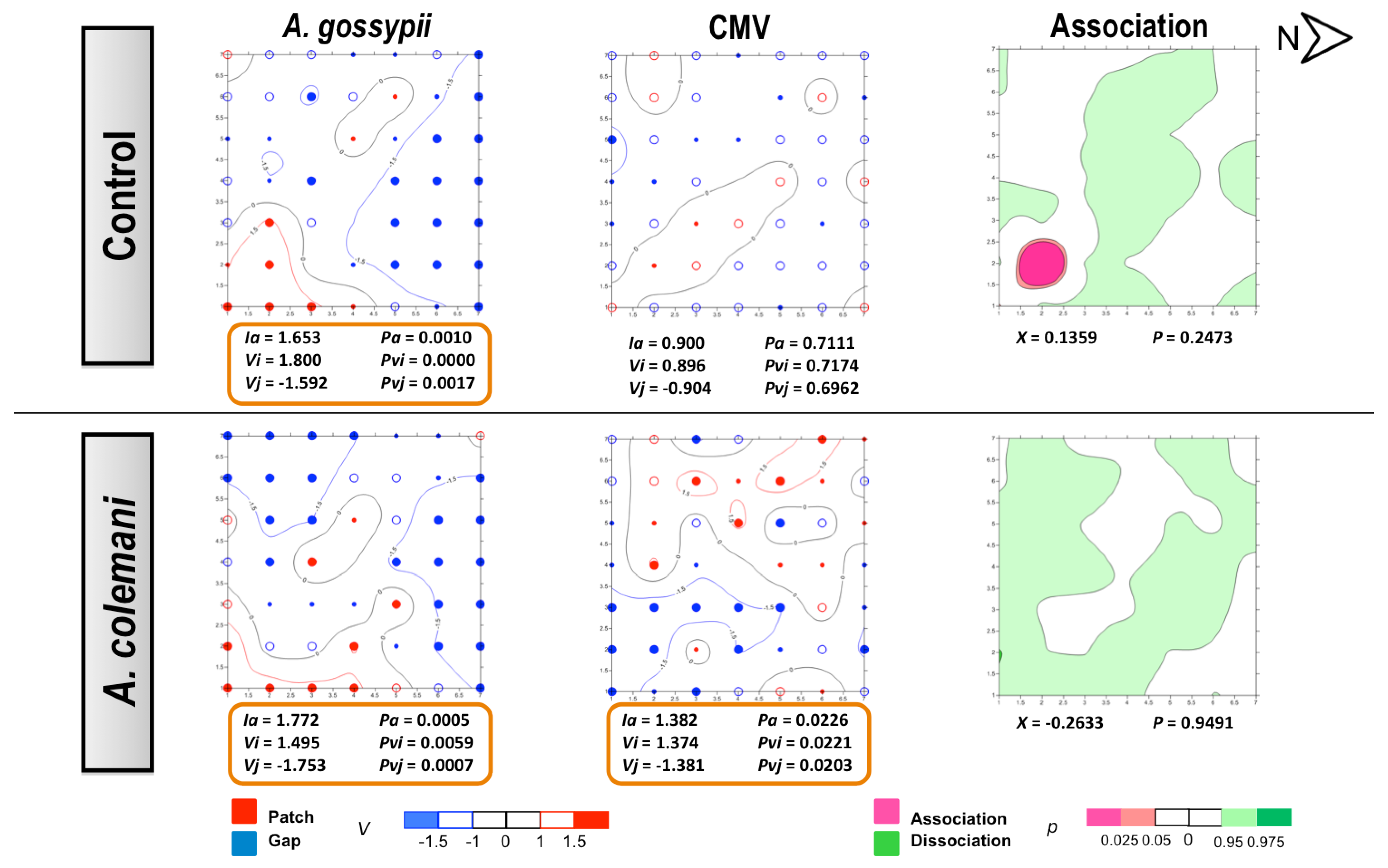
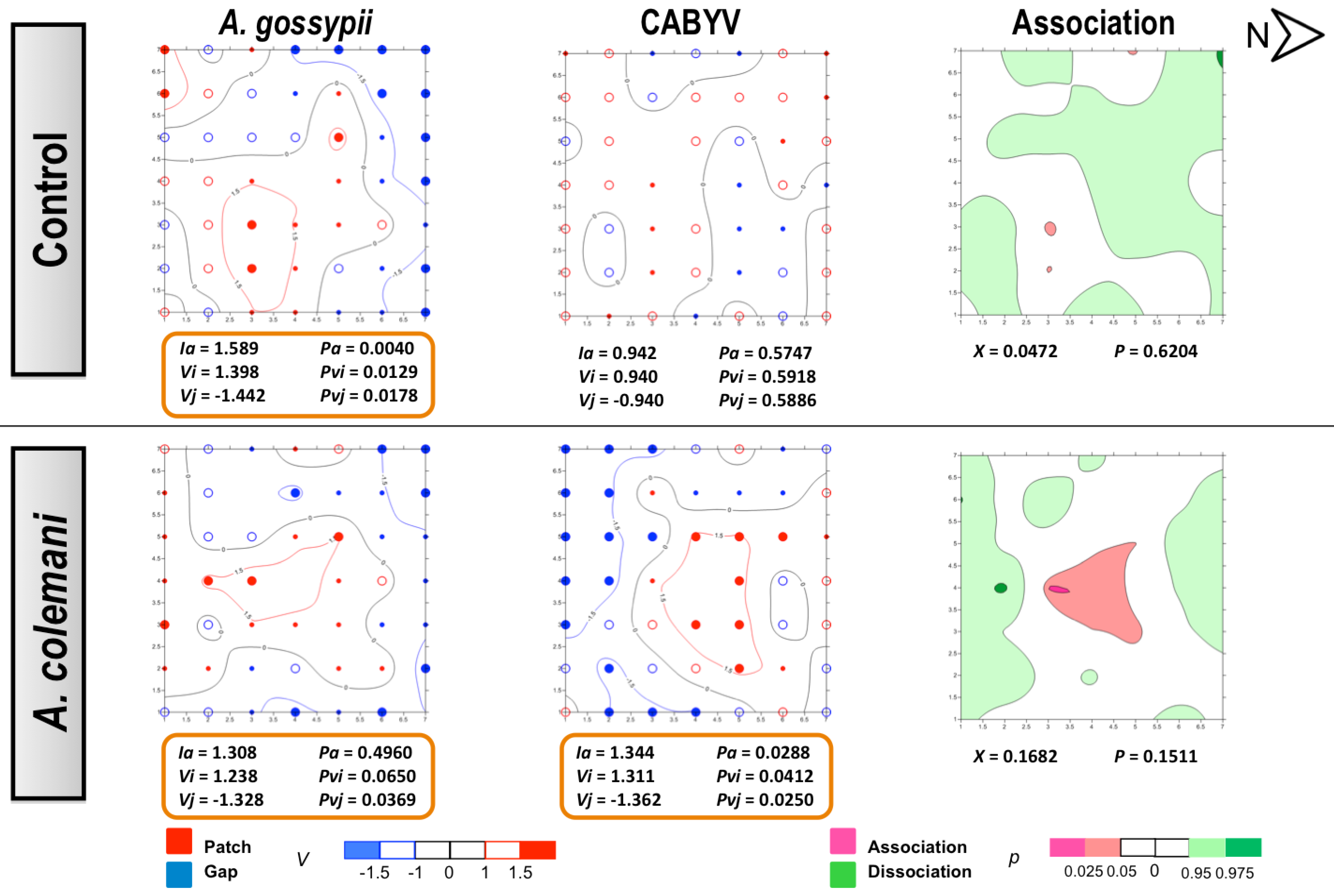
2.1. Effect of Aphidius Colemani on Aphid Dispersal and the Spread of Cucumber Mosaic Virus
| 2 days | 7 days | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | A. colemani | t | p | Control | A. colemani | t | p | |
| Alate | 24.7 ± 5.5 | 18.0 ± 5.3 | 0.873 | 0.947 | 11.0 ± 3.2 | 9.0 ± 2.5 | 0.524 | 0.628 |
| Apterae | - | - | - | - | 10.7 ± 4.7 | 6.3 ± 3.0 | 0.882 | 0.428 |
| Nymphs | 176.3 ± 55.1 | 128.7 ± 26.6 | 0.745 | 0.498 | 170.0 ± 50.0 | 99.7 ± 33.3 | 1.227 | 0.287 |
| 2 days | 7 days | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | A. colemani | X 2 | p | Control | A. colemani | X 2 | p | |
| Alate | 38.9 ± 2.5 | 54.2 ± 7.9 | 9.930 | 0.002 | 42.4 ± 3.5 | 33.3 ± 8.7 | 2.495 | 0.117 |
| Apterae | - | - | - | - | 38.2 ± 2.8 | 6.3 ± 3.2 | 42.509 | <0.001 |
| Nymphs | 38.2 ± 4.2 | 50.7 ± 8.2 | 4.556 | 0.044 | 50.7 ± 2.5 | 38.2 ± 7.8 | 4.556 | 0.034 |
| Aphids | Virus | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | A. colemani | t | p | Control | A. colemani | t | p | |
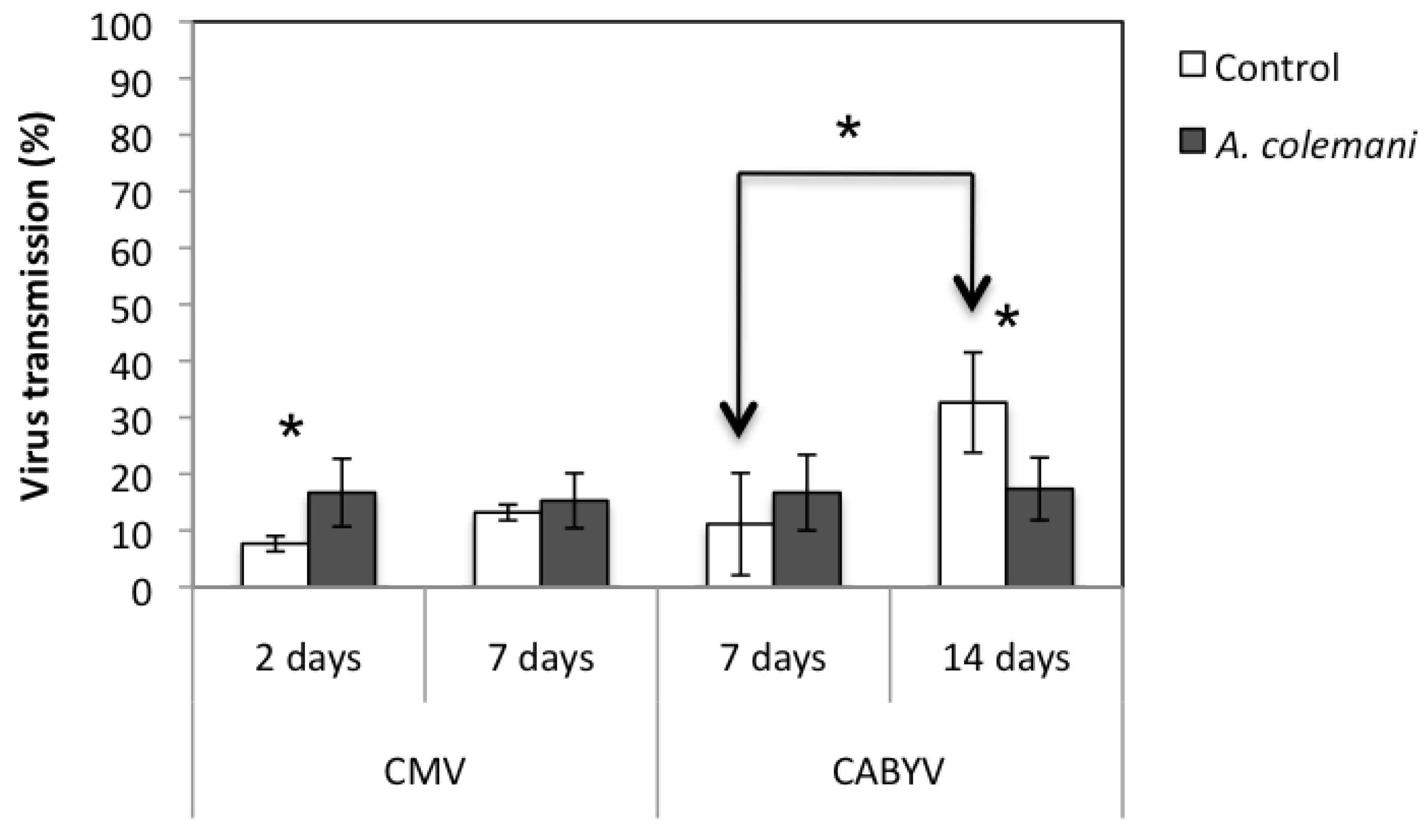
| 2 days | 1.9 ± 0.2 | 1.6 ± 0.2 | 0.758 | 0.491 | 0.9 ± 0.1 | 1.4 ± 0.6 | –0.690 | 0.528 |
| 7 days | 1.3 ± 0.1 | 1.5 ± 0.4 | –0.539 | 0.619 | 1.2 ± 0.4 | 1.0 ± 0.4 | 0.215 | 0.840 |
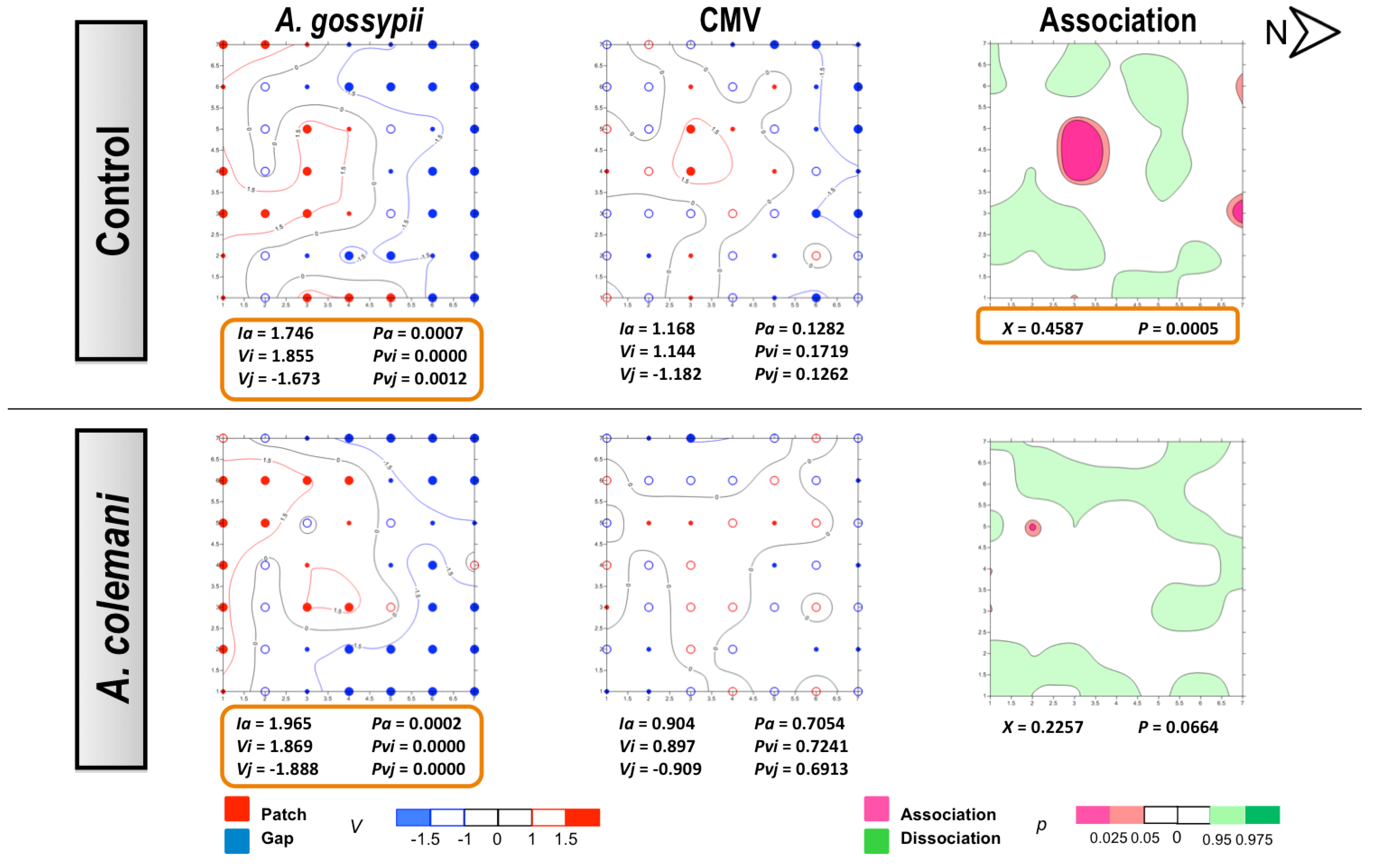
2.2. Effect of Aphidius Colemani on Aphid Dispersal and the Spread of Cucumber Aphid-Borne Yellows Virus
| 7 days | 14 days | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | A. colemani | t | p | Control | A. colemani | t | p | |
| Alate | 36.0 ± 1.7 | 24.3 ± 7.8 | 1.443 | 0.222 | 20.0 ± 6.7 | 10.0 ± 1.2 | 1.359 | 0.246 |
| Apterae | 60.3 ± 9.9 | 17.0 ± 1.5 | 6.524 | 0.003 | 179.7 ± 54.3 | 185.0 ± 8.2 | 0.168 | 0.874 |
| Nymphs | 388.0 ± 39.1 | 160.0 ± 10.4 | 7.026 | 0.020 | 1624.7 ± 297.8 | 1212.0 ± 55.6 | 1.465 | 0.217 |
| 7 days | 14 days | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | A. colemani | X 2 | p | Control | A. colemani | X 2 | p | |
| Alate | 18.8 ± 1.2 | 24.3 ± 4.2 | 1.315 | 0.256 | 37.5 ± 9.8 | 30.6 ± 9.1 | 1.547 | 0.263 |
| Apterae | 20.9 ± 5.2 | 16.0 ± 3.0 | 1.133 | 0.293 | 63.9 ± 15.2 | 60.4 ± 1.2 | 0.369 | 0.627 |
| Nymphs | 27.8 ± 2.8 | 29.8 ± 4.6 | 0.152 | 0.670 | 75.0 ± 11.0 | 72.2 ± 0.7 | 0.286 | 0.596 |
| Aphids | Virus | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | A. colemani | t | p | Control | A. colemani | t | p | |
| 7 days | 0.8 ± 0.1 | 1.2 ± 0.2 | –1.496 | 0.209 | 2.2 ± 1.0 | 1.0 ± 0.3 | 1.154 | 0.313 |
| 14 days | 0.8 ± 0.3 | 0.8 ± 0.1 | –0.112 | 0.916 | 1.4 ± 0.4 | 1.0 ± 0.0 | 1.117 | 0.326 |

3. Experimental Section
3.1. Insect Cultures
3.2. Virus Sources and Test Plants
3.3. Effect of Aphidius Colemani on Aphid Dispersal and Virus Spread

3.4. Statistical Methods and Spatial Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ng, J.C.K.; Perry, K.L. Transmission of plant viruses by aphid vectors. Mol. Plant. Pathol. 2004, 5, 505–511. [Google Scholar] [CrossRef]
- Nault, L.R. Arthropod transmission of plant viruses: A new synthesis. Ann. Entomol. Soc. Am. 1997, 90, 521–541. [Google Scholar]
- Hull, R. Matthews’ Plant Virology, 4th ed; Academic Press: New York, USA, 2002. [Google Scholar]
- Fereres, A.; Moreno, A. Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 2009, 141, 158–168. [Google Scholar] [CrossRef]
- Döring, T.F.; Chittka, L. Visual ecology of aphids: A critical review on the role of colours in host finding. Arthropod.-Plant Interact. 2007, 1, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vector to inferior hosts. P. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]
- Hodge, S.; Powell, G. Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance? Environ. Entomol. 2008, 38, 1573–1581. [Google Scholar] [CrossRef]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteoviruses pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef]
- Calvo, D.; Fereres, A. The performance of an aphid parasitoid is negatively affected by the presence of a circulative plant virus. BioControl 2011, 56, 747–757. [Google Scholar] [CrossRef]
- Dicke, M.; van Loon, J.J.A. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar]
- Roitberg, B.D.; Myers, J.H. Effect of adult coccinellidae on the spread of a plant virus by an aphid. J. Appl. Ecol. 1978, 15, 775–779. [Google Scholar] [CrossRef]
- Bailey, S.M.; Irwin, M.E.; Kampmeier, G.E.; Eastman, C.E.; Hewings, A.D. Physical and biological perturbations: Their effect on the movement of apterous Rhopalosiphum padi (Homoptera: Aphididae) and localized spread of Barley yellow dwarf virus. Environ. Entomol. 1995, 24, 24–33. [Google Scholar]
- Weber, C.A.; Godfrey, L.D.; Mauk, P.A. Effects of parasitism by Lysiphlebus testaceipes (Hymenoptera: Aphidiidae) on transmission of Beet yellows closterovirus by bean aphid (Homoptera: Aphididae). J. Econ. Entomol. 1996, 89, 1431–1437. [Google Scholar]
- Hodge, S.; Hardie, J.; Powell, G. Parasitoids aid dispersal of a nonpersistently transmitted plant virus by disturbing the aphid vector. Agr. Forest Entomol. 2011, 13, 83–88. [Google Scholar] [CrossRef]
- Hodge, S.; Powell, G. Complex interactions between a plant pathogen and insect parasitoid via the shared vector-host: Consequences for host plant infection. Oecologia 2008, 157, 387–397. [Google Scholar] [CrossRef]
- Smyrnioudis, I.N.; Harrington, R.; Clark, S.J.; Katis, N. The effect of natural enemies on the spread of Barley yellow dwarf virus (BYDV) by Rhopalosiphum padi (Hemiptera: Aphididae). B. Entomol. Res. 2001, 91, 301–306. [Google Scholar] [CrossRef]
- Belliure, B.; Amorós-Jiménez, R.; Fereres, A.; Marcos-García, M.A. Antipredator behaviour of Myzus persicae affects transmission efficiency of Broad bean wilt virus 1. Virus Res. 2011, 159, 206–214. [Google Scholar] [CrossRef]
- Losey, J.E.; Denno, R.F. The escape response of pea aphids to foliar-foraging predators: Factors affecting dropping behaviour. Ecol. Entomol. 1998, 23, 53–61. [Google Scholar] [CrossRef]
- Day, K.R.; Docherty, M.; Leather, S.R.; Kidd, N.A.C. The role of generalist insect predators and pathogens in suppressing green spruce aphid populations through direct mortality and mediation of aphid dropping behaviour. Biol. Control 2006, 38, 223–246. [Google Scholar]
- Jeger, M.J.; Chen, Z.; Powell, G.; Hodge, S.; van den Bosch, F. Interactions in a host plant-virus-vector-parasitoid system: Modelling the consequences for virus transmission and disease dynamics. Virus Res. 2011, 159, 183–193. [Google Scholar] [CrossRef]
- Jeger, M.J.; Chen, Z.; Cunningham, E.; Martin, G.; Powell, G. Population biology and epidemiology of plant virus epidemics: from tripartite to tritrophics interactions. Eur. J. Plant Pathol. 2012, 133, 3–23. [Google Scholar] [CrossRef]
- Díaz, B.M.; Legarrea, S.; Marcos-García, M.A.; Fereres, A. The spatio-temporal relationships among aphids, the entomophthoran fungus, Pandora neoaphidis, and aphidophagous hoverflies in outdoor lettuce. Biol. Control 2010, 53, 304–311. [Google Scholar] [CrossRef]
- Díaz, B.M.; Barrios, L.; Fereres, A. Interplant movement and spatial distribution of alate and apterous morphs of Nasonovia ribisnigri (Homoptera: Aphididae) on lettuce. B. Entomol. Res. 2012, 31, 1–9. [Google Scholar]
- Perry, J.N. Spatial analysis by distance indices. J. Anim. Ecol. 1995, 64, 303–314. [Google Scholar] [CrossRef]
- Perry, J.N. Measures of spatial pattern for counts. Ecology 1998, 79, 1008–1017. [Google Scholar] [CrossRef]
- Perry, J.N.; Nixon, P. A new method to measure spatial association for ecological data. Ecoscience 2002, 9, 133–141. [Google Scholar]
- Perry, J.N.; Winder, L.; Holland, J.M.; Alston, R.D. Red-blue cages for detecting clusters in count data. Ecol. Lett. 1999, 2, 106–113. [Google Scholar]
- Moreno, A.; Nebreda, M.; Díaz, B.M.; García, M.; Salas, F.; Fereres, A. Temporal and spatial spread of Lettuce mosaic virus in lettuce crops in central Spain: Factors involved in Lettuce mosaic virus epidemics. Ann. Appl. Biol. 2007, 150, 351–360. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Coutts, B.A.; Latham, L.J.; McKirdy, S.J. Cucumber mosaic virus infection of chickpea stands: Temporal and spatial patterns of spread and yield-limiting potential. Plant Pathol. 2008, 57, 842–853. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World Crops: An Identification and Information Guide, 2nd ed; Wiley: New York, USA, 2000. [Google Scholar]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid parasitoids in biological control. Can. J. Plant Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Lecoq, H.; Bourdin, D.; Wipf-Scheibel, C.; Bon, M.; Lot, H.; Lemaire, O.; Herrbach, E. A new yellowing disease of cucurbits caused by a Luteovirus, Cucurbit aphid-borne yellows virus. Plant Pathol. 1992, 41, 749–761. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; Hemenway, C.; Foster, G.D. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Falk, B.W. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Ann. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Du, Y.J.; Poppy, G.M.; Powell, W. Relative importance of semiochemicals from first and second trophic levels in host foraging behavior of Aphidius ervi. J. Chem. Ecol. 1996, 22, 1591–1605. [Google Scholar] [CrossRef]
- Guerrieri, E.; Pennacchio, F.; Tremblay, E. Effect of adult experience on in-flight orientation to plant and plant-host complex volatiles in Aphidius ervi Haliday (Hymenoptera, Braconidae). Biol. Control 1997, 10, 159–165. [Google Scholar] [CrossRef]
- Michaud, J.P.; Mackauer, M. The use of visual cues in host evaluation by aphidiid wasps. Entomol. Exp. Appl. 1995, 74, 267–275. [Google Scholar] [CrossRef]
- Yang, S.; Xu, R.; Yang, S.-Y.; Kuang, R.P. Olfactory responses of Aphidius gifuensis to odors of host plants and aphid-plant complexes. Insect Sci. 2009, 16, 503–510. [Google Scholar] [CrossRef]
- Christiansen-Weniger, P.; Powell, G.; Hardie, J. Plant virus and parasitoid interactions in a shared insect vector/host. Entomol. Exp. Appl. 1998, 86, 205–213. [Google Scholar]
- Zamani, A.A.; Talebi, A.; Fathipour, Y.; Baniameri, V. Effect of temperature on life history of Aphidius colemani and Aphidius matricariae (Hymenoptera: Braconidae), two parasitoids of Aphis gossypii and Myzus persicae (Homoptera: Aphididae). Environ. Entomol. 2007, 36, 263–271. [Google Scholar] [CrossRef]
- Irwin, M.E.; Thresh, J.M. Epidemiology of Barley yellow dwarf: A study in ecological complexity. Annu. Rev. Phytopathol. 1990, 28, 393–424. [Google Scholar] [CrossRef]
- Díaz, J.A.; Mallor, C.; Soria, C.; Camero, R.; Garzo, E.; Fereres, A.; Álvarez, J.M.; Gómez-Guillamón, A.L.; Luis-Arteaga, M.; Moriones, E. Potential sources of resistance for melon to nonpersistently aphid-borne viruses. Plant Dis. 2003, 87, 960–964. [Google Scholar] [CrossRef]
- Moreno, A.; Garzo, E.; Fernández-Mata, G.; Kassem, M.; Aranda, M.A.; Fereres, A. Aphids secrete watery saliva into plant tissues from the onset of stylet penetration. Entomol. Exp. Appl. 2011, 139, 145–153. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked inmunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- SPSS Inc. IBM Statistics SPSS 19.0 Package for Windows, Chicago, USA, 2010.
- Golden Software Inc. Golden Surfer Software 9.0 version, Colorado, USA, 2009.
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host-vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dáder, B.; Moreno, A.; Viñuela, E.; Fereres, A. Spatio-Temporal Dynamics of Viruses are Differentially Affected by Parasitoids Depending on the Mode of Transmission. Viruses 2012, 4, 3069-3089. https://doi.org/10.3390/v4113069
Dáder B, Moreno A, Viñuela E, Fereres A. Spatio-Temporal Dynamics of Viruses are Differentially Affected by Parasitoids Depending on the Mode of Transmission. Viruses. 2012; 4(11):3069-3089. https://doi.org/10.3390/v4113069
Chicago/Turabian StyleDáder, Beatriz, Aránzazu Moreno, Elisa Viñuela, and Alberto Fereres. 2012. "Spatio-Temporal Dynamics of Viruses are Differentially Affected by Parasitoids Depending on the Mode of Transmission" Viruses 4, no. 11: 3069-3089. https://doi.org/10.3390/v4113069
APA StyleDáder, B., Moreno, A., Viñuela, E., & Fereres, A. (2012). Spatio-Temporal Dynamics of Viruses are Differentially Affected by Parasitoids Depending on the Mode of Transmission. Viruses, 4(11), 3069-3089. https://doi.org/10.3390/v4113069






