Random Network Models to Predict the Long-Term Impact of HPV Vaccination on Genital Warts
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of the Data
2.2. Network Model
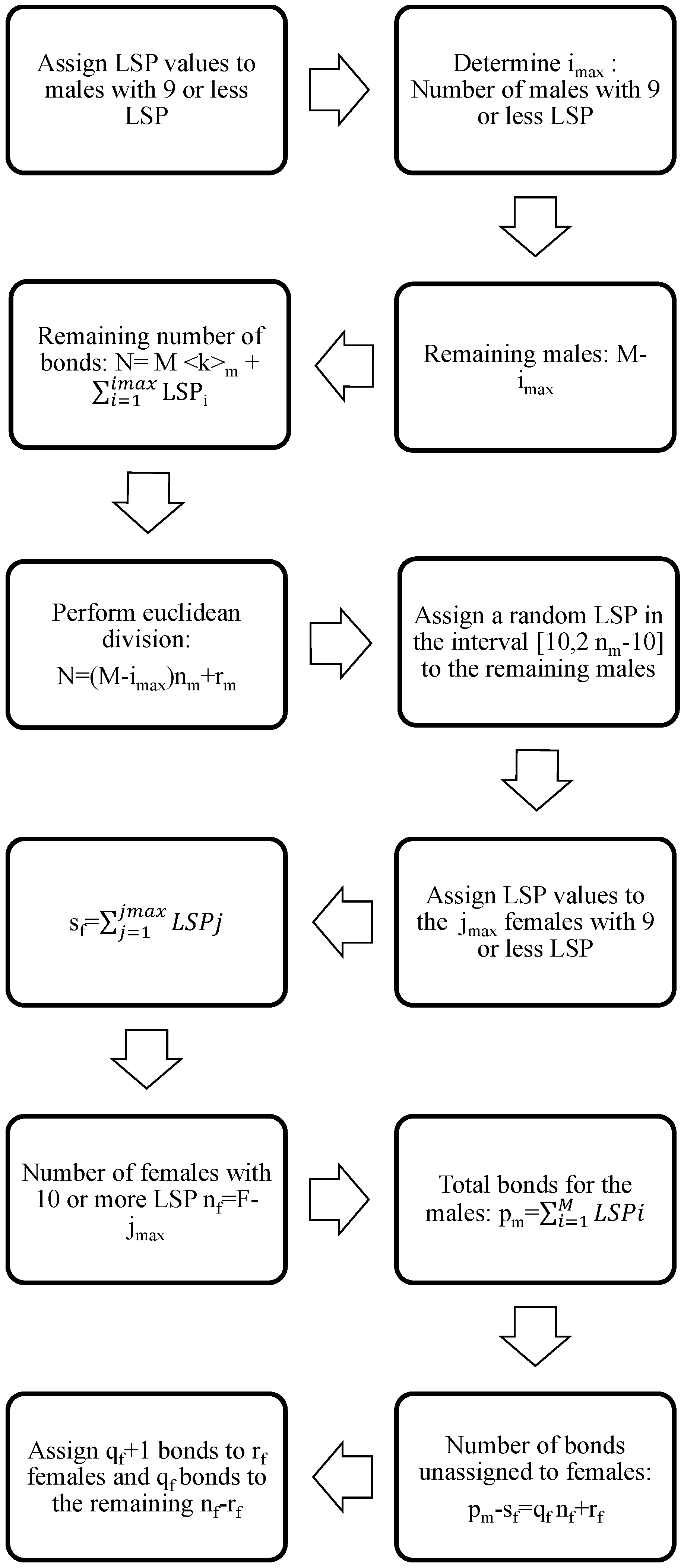
2.3. Semi-Random Construction
- say that the corresponding male does not have an LSP,
- say that the corresponding male has one LSP,
- say that the corresponding male has two LSPs,
- say that the corresponding male has three or four LSPs uniformly distributed,
- say that the corresponding male has five to nine LSPs uniformly distributed,
- say that the corresponding male has 10 or more LSPs.
- is the age of the i-th male, ,
- is the age of the i-th female, ,
- is the number of LSP for the i-th male, ,
- is the number of LSP for the i-th female, .
2.4. The Dynamics of HPV Transmission in the Sexual Network
- We need some probabilities to determine if a sexual partner is going to produce a contagion of another partner in a given time stage. These parameters are different for each age group: 14–17, 18–29, 30–39 and 40–65. Notice that this means that the probability of contagion depends upon the age group of the members of the relationship. Moreover, the probability of connection of these members in the network is also age-dependent as proposed in Equation (2). The values of these probabilities are determined in the process of the model fitting.
- Average time an individual infected by a HR HPV clears the infection and recovers.
- A similar parameter for clearing the LR HPV infection.
- If a partner produces the contagion of his/her partner, we need another four parameters to determine if the high or low risk HPV infection is transmitted from man to woman and vice versa.
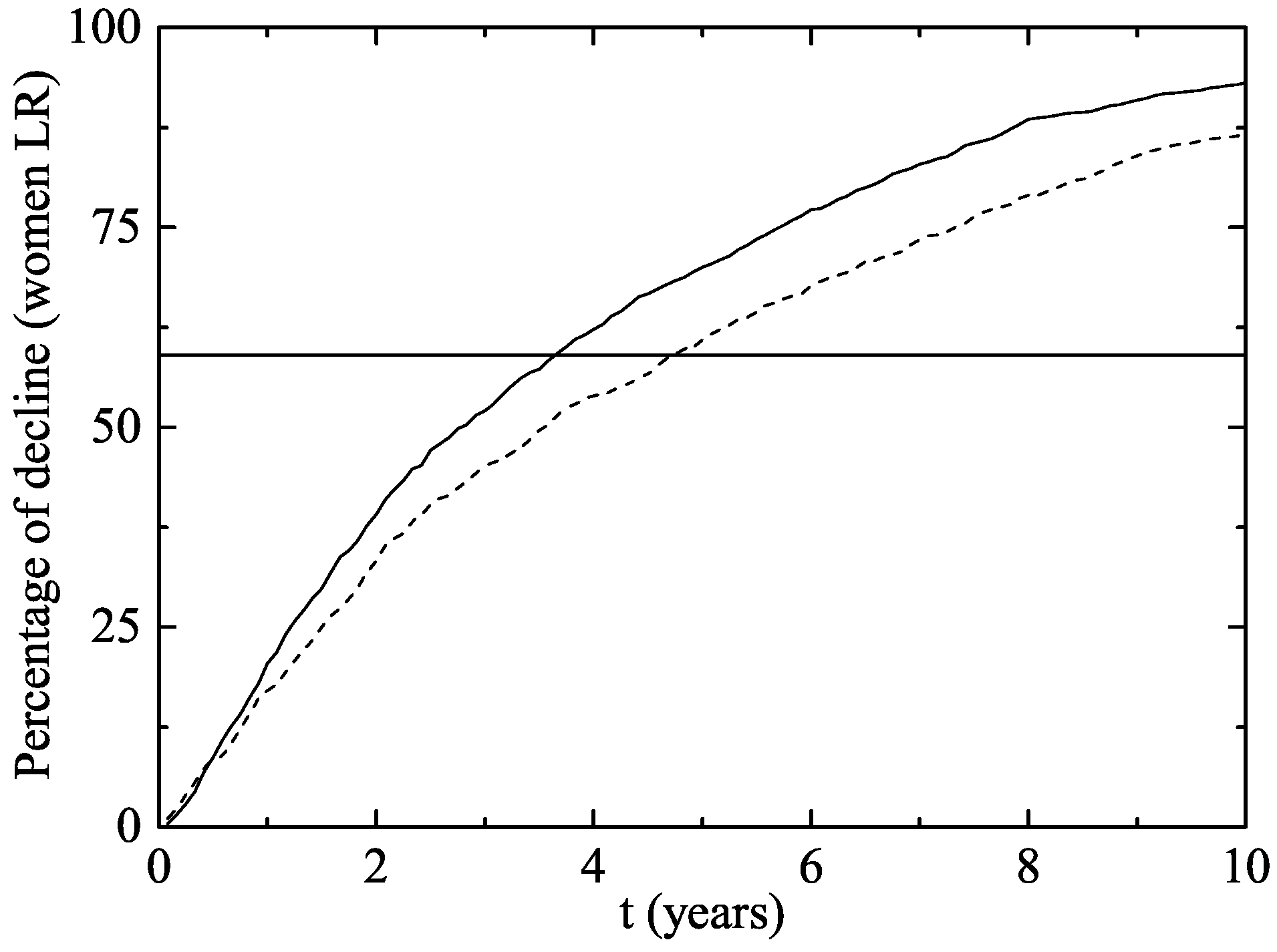
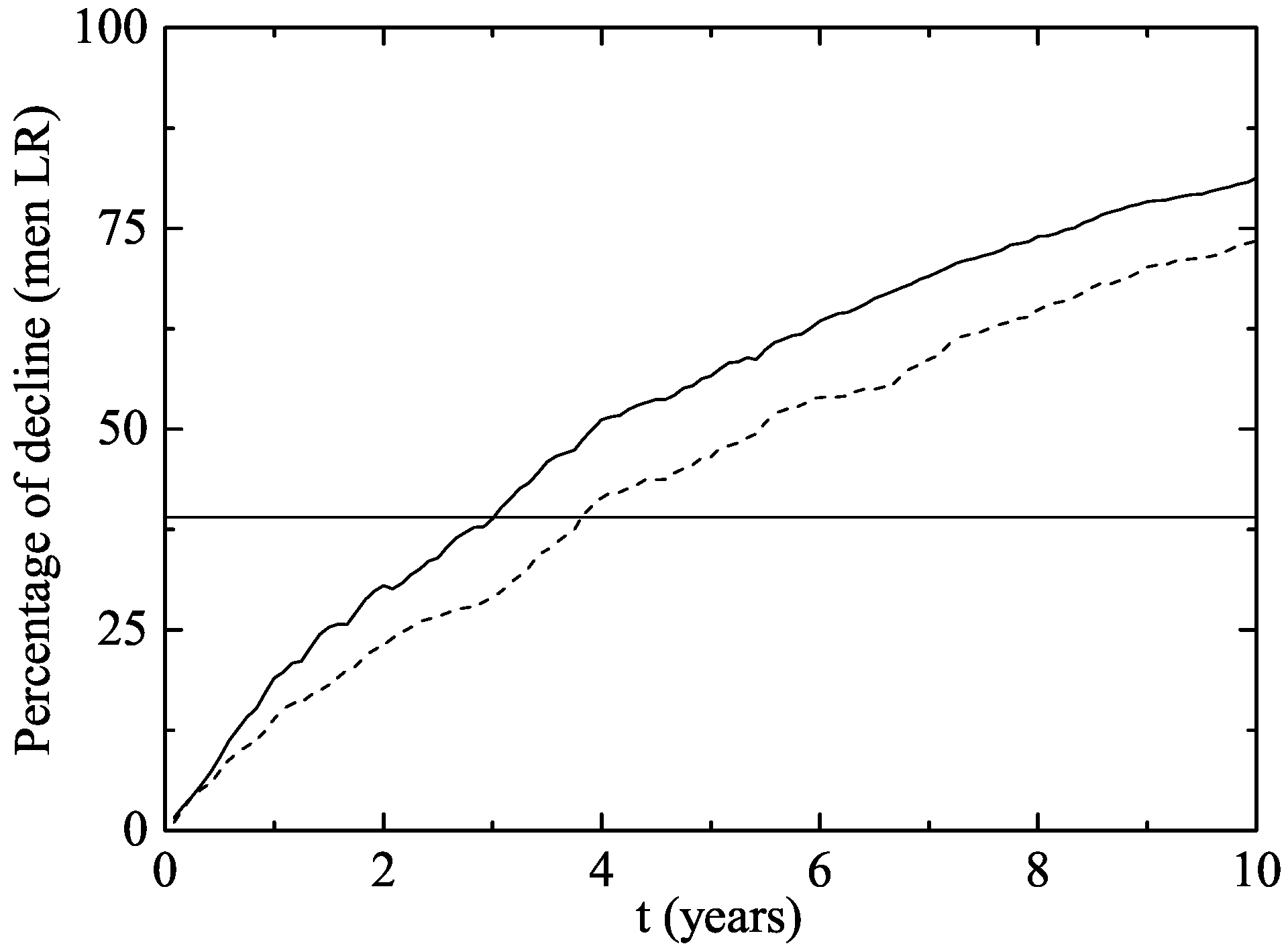
- Two years after the vaccine was introduced, the proportion of GW diagnosed declined by a in vaccine eligible young women aged 12–26 years in 2007, and by in heterosexual men of the same age.
- No significant decline was observed in women or men older than 26 years old, non-resident young women, or men who have sex with men.
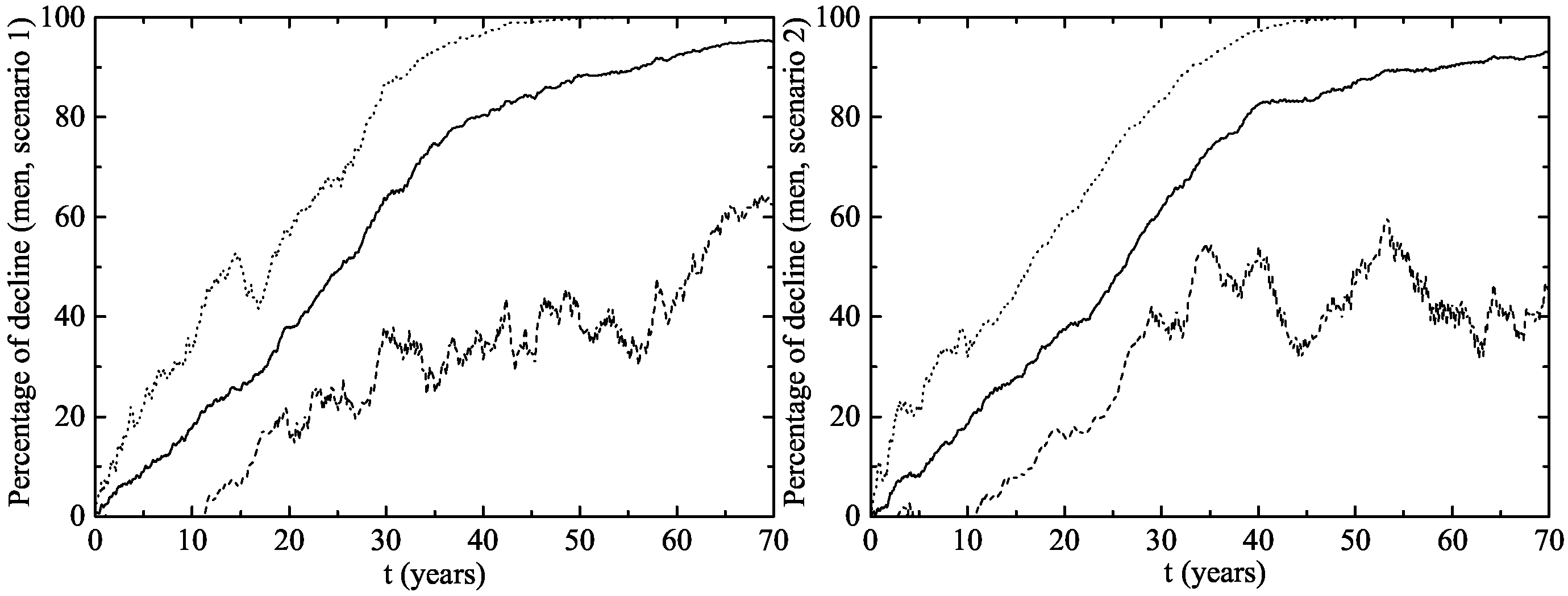
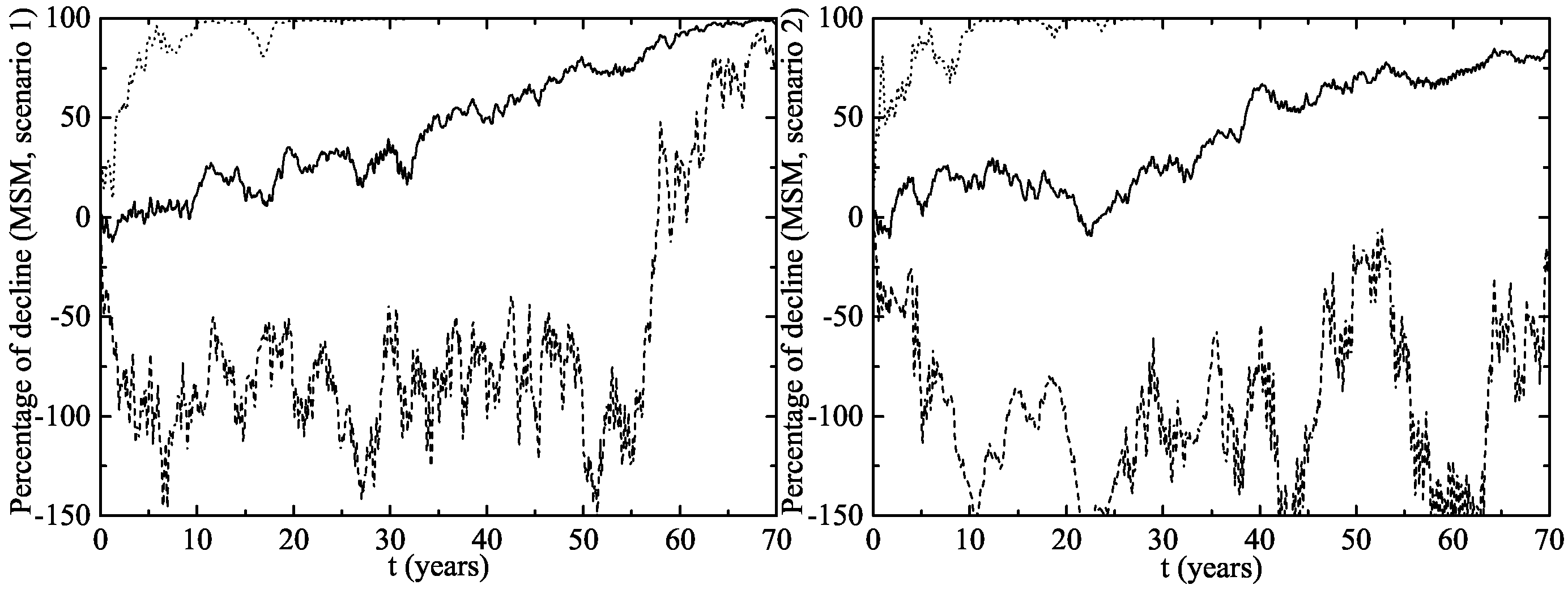
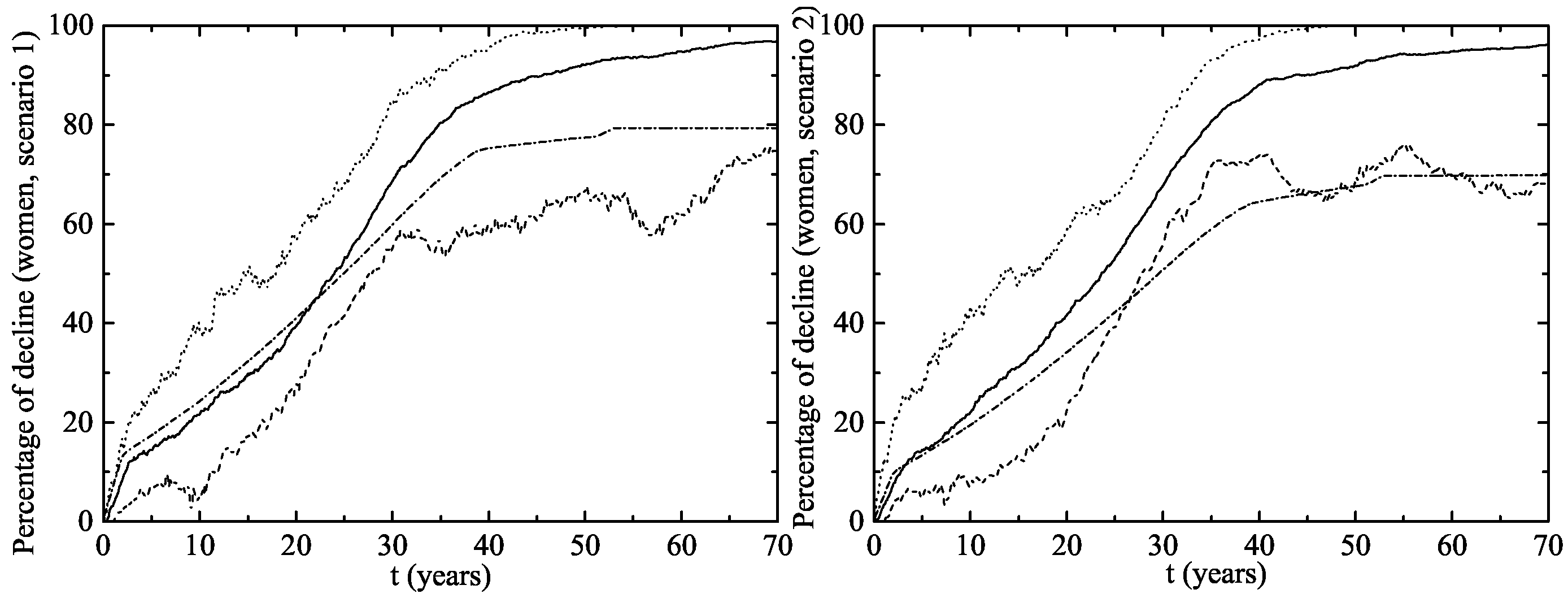
- Scenario 1: vaccination of of the 14 year-old girls (or younger girls) plus a catch-up with coverage for 14–26 year-old women.
- Scenario 2: vaccination of of 14 year-old girls (or younger girls) plus a catch-up with a vaccination coverage of for 14–26 year-old women.
2.5. Calculation of the Number of Infections
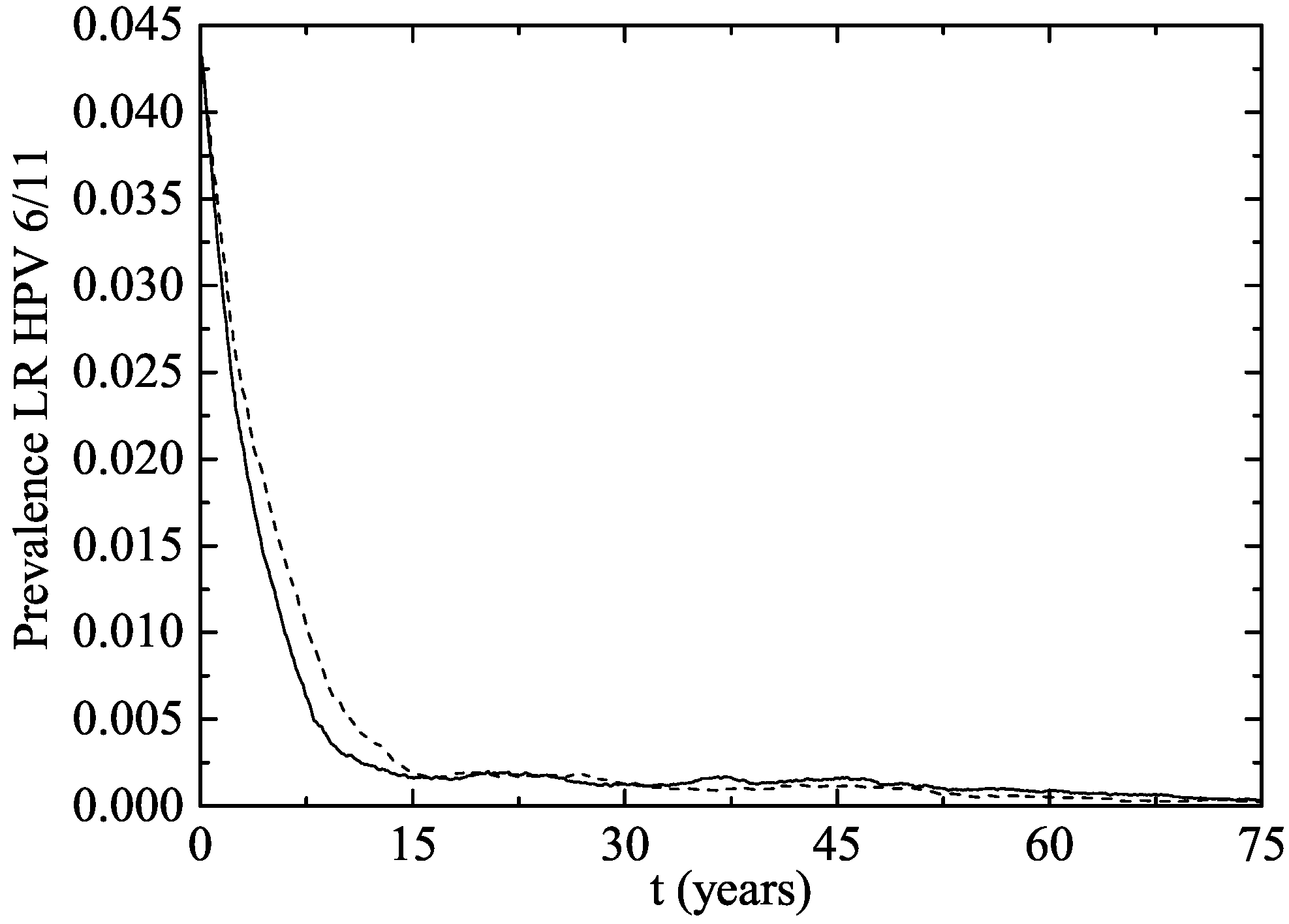
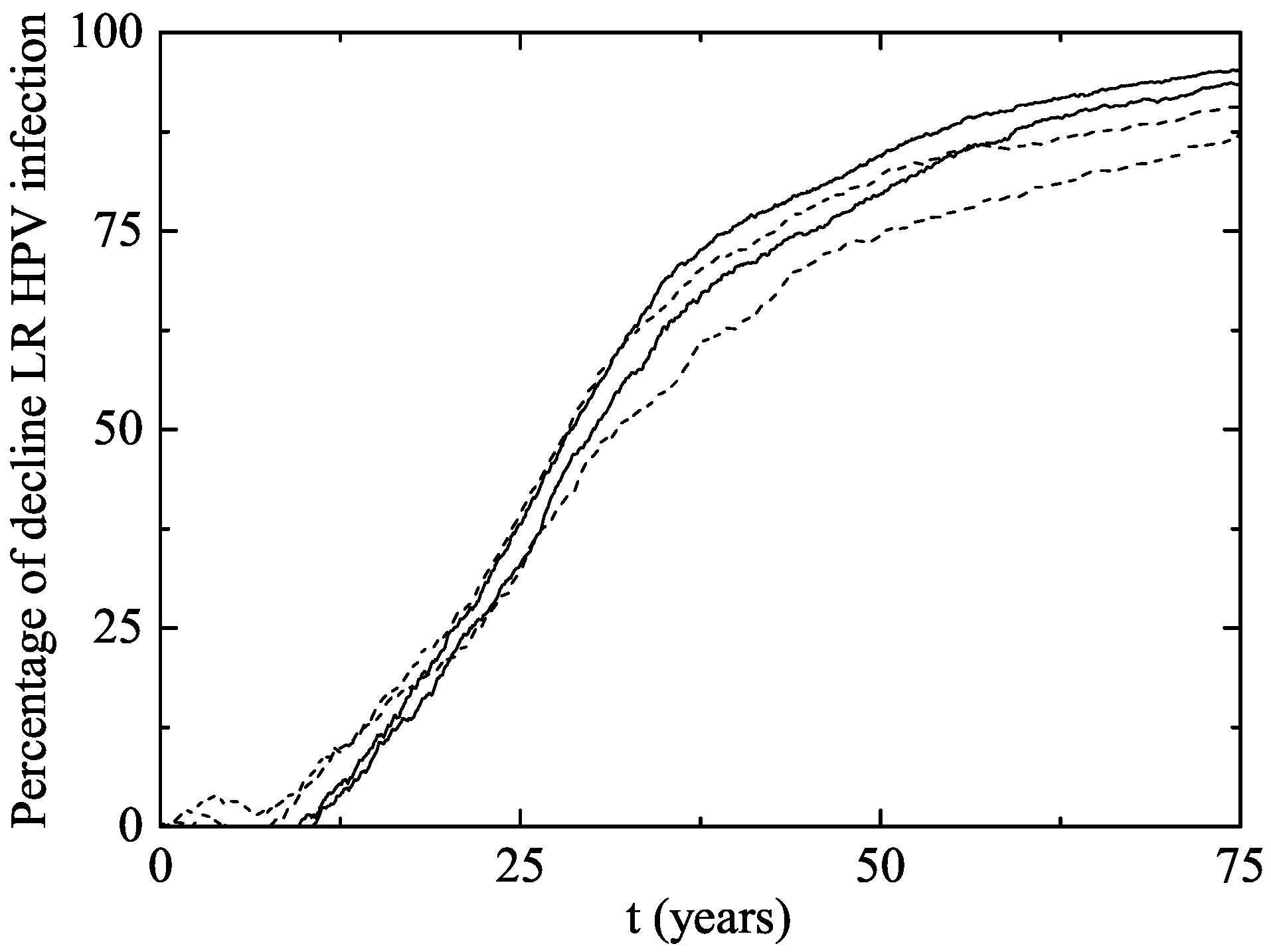
3. Results
The Australian Scenario
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VLP | Virus-like particles |
| STI | Sexually transmitted infection |
| HPV | Human papillomavirus |
| LSP | Lifetime sexual partner |
| HIV | Human immunodeficiency virus |
| PSO | Particle Swarm Optimization |
| MSM | Men having sex with men |
| GW | Genital warts |
References
- Sirjänen, K.; Sirjänen, S. Papillomavirus Infections in Human Pathology; Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Roberts, J.S.C. Vaccine for Genital Warts. In Vaccines for Human Papillomavirus Infections and Anogenital Disease; Tindle, R.W., Ed.; Medical Intellegence Unit 14, R. G. Landes Company: Austin, TX, USA, 1999. [Google Scholar]
- Castellsagué, X.; Iftner, T.; Roura, E.; Antonio Vidart, J.; Kjaer, S.K.; Bosch, F.X.; Muñoz, N.; Palacios, S.; San Martin Rodriguez, M.; Serradell, L.; et al. Prevalence and genotype distribution of human papillomavirus infection of the cervix in Spain: the CLEOPATRE study. J. Med. Virol. 2012, 84, 947–956. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C. Who invented the VLP Cervical Cancer Vaccines? J. Natl. Cancer Inst. 2006, 98, 433. [Google Scholar] [CrossRef] [PubMed]
- Elbasha, E.H.; Dasbach, E.J.; Insinga, R.P. Model for assessing human papillomavirus vaccination strategies. Emerg. Infect. Dis. 2007, 13, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Elbasha, E.H.; Galvani, A.P. Vaccination against multiple HPV types. Math. Biosci. 2005, 197, 88–117. [Google Scholar] [CrossRef] [PubMed]
- Fairley, G.; Hocking, J.; Chen, M.; Donovan, B.C. Rapid decline in warts after national quadrivalent HPV vaccine program. In Proceedings of the 25th International Papillomavirus Conference, Malmo, Sweden, 8–14 May 2009. [Google Scholar]
- Ali, H.; Donovan, B.; Wand, H.; Read, T.R.H.; Regan, D.G.; Grulich, A.E.; Fairley, C.K.; Guy, R.J. Genital warts in young australians five years into national human papillomavirus vaccination programme: National surveillance data. BMJ 2013, 346, f2032. [Google Scholar] [CrossRef] [PubMed]
- Bogaards, J.A.; Wallinga, J.; Brakenhoff, R.H.; Meijer Chris, J.L.M.; Berkhof, J. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: Bayesian evidence synthesis. BMJ 2015, 350, h2016. [Google Scholar] [CrossRef] [PubMed]
- Acedo, L.; Moraño, J.A.; Villanueva, R.J.; Villanueva-Oller, J.; Díez-Domingo, J. Using random networks to study the dynamics of respiratory syncytial virus (RSV) in the Spanish region of Valencia. Math. Comp. Mod. 2011, 54, 1650–1654. [Google Scholar] [CrossRef]
- Christakis, N.A.; Fowler, J.H. The Spread of Obesity in a Large Social Network over 32 Years. N. Engl. J. Med. 2007, 357, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Burchell, A.; Richardson, H.; Mahmud, S.M.; Trottier, H.; Tellier, P.P.; Hanley, J.; Coutlée, F.; Franco, E.L. Modeling the Sexual Transmissibility of Human Papillomavirus Infection using Stochastic Computer Simulation and Empirical Data from a Cohort Study of Young Women in Montreal, Canada. Am. J. Epidemol. 2006, 169, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Liljeros, F.; Edling, C.R.; Nunes, L.A.; Stanley, H.E.; Åberg, Y. The web of human sexual contacts. Nature 2001, 411, 907–908. [Google Scholar] [CrossRef] [PubMed]
- Bearman, P.S.; Moody, J.; Stovel, K. Chains of Affection: The Structure of Adolescent Romantic and Sexual Networks. Am. J. Sociol. 2004, 110, 44–91. [Google Scholar] [CrossRef]
- Helleringer, S.; Kohler, H.P. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS 2007, 21, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.V.; Kretzschmar, M. Determinants of sexual network structure and their impact on cumulative network measures. PLoS Comput. Biol. 2012, 8, e1002470. [Google Scholar] [CrossRef] [PubMed]
- Portal Estadistico de la Generalitat Valenciana (Statistical Portal of the Government of the Community of Valencia) (2013). Available online: http://www.ive.es (accessed on 6 March 2017).
- Encuesta de Salud y Hábitos Sexuales 2003. (Health and Sexual Habits Survey 2003). July 2004. Instituto Nacional de Estadística. Available online: http://www.ine.es (accessed on 6 March 2017).
- Acedo, L.; Moraño, J.-A. Brain oscillations in a random neural network. Math. Comput. Model. 2013, 57, 1768–1772. [Google Scholar] [CrossRef]
- Dorogovtsev, S.N.; Mendes, J.F.F. Evolution of Networks: From Biological Networks to the Internet and WWW; Oxford University Press: Oxford, UK, 14 January 2014. [Google Scholar]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of small-world networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics from the National Survey of Family Growth. Available online: http://www.cdc.gov/nchs/nsfg/key_statistics/n.htm (accessed on 14 October 2017).
- Chandra, A.; Mosher, W.D.; Copen, C. Sexual Behavior, Sexual Attraction, and Sexual Identity in the United States: Data From the 2006–2008. National Survey of Family Growth, National Health Statistic Reports, No. 36. 3 March 2011. Available online: http://www.cdc.gov/nchs/data/nhsr/nhsr036.pdf (accessed on 15 October 2017).
- Gentner, D.; Markman, A.B. Structure mapping in analogy and similarity. Am. Psychol. 1997, 52, 45–56. [Google Scholar] [CrossRef]
- Miret, P. La Similitud Entre Los Componentes De Las Parejas Jóvenes en España en la Primera Década Del Siglo XXI. Cada Vez Más Iguales? (The similarity among the Components of Young Couples in Spain in the First Decade of the XXIst Century. Increasingly Equal?). Revista de Estudios de Juventud 2010, 90, 225–255. Available online: http://www.injuve.es/sites/default/files/RJ90-16.pdf (accessed on 15 October 2017).
- Acedo, L.; Burgos, C.; Hidalgo, J.I.; Sánchez-Alonso, V.; Villanueva, R.J.; Villanueva-Oller, J. Calibrating a large network model describing the transmission dynamics of the human papillomavirus using a particle swarm optimization algorithm in a distributed computing environment. Int. J. High Perform. Comput. Appl. 2017. [Google Scholar] [CrossRef]
- Feo, T.A.; Resende, M.G.C. Greedy Randomized Adaptive Search Procedures. J. Glob. Optim. 1995, 6, 109–133. [Google Scholar] [CrossRef]
- Cormen, T.H.; Leiserson, C.E.; Rivest, R.L.; Stein, C. Introduction to Algorithms; MIT Press: Cambridge, MA, USA; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Acedo, L.; Martí, R.; Palmi, F.; Sánchez-Alonso, V.; Santonja, F.J.; Villanueva, R.J.; Villanueva-Oller, J. Building Lifetime Heterosexual Partner Networks. In Modeling Human Behavior. Individuals and Organizations; Jódar, L., de la Poza, E., Acedo, L., Eds.; Nova Publishers: Hauppauge, NY, USA, 2017; Chapter 19; pp. 235–251. [Google Scholar]
- Campos, N.G.; Burger, E.A.; Sy, S. An Updated Natural History Model of Cervical Cancer: Derivation of Model Parameters. Am. J. Epidemiol. 2014, 180, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Estudio de Conducta Sexual Entre Homosexuales; Study of Sexual Behavior among MSM; Technical Report; Durex: Madrid, Spain, 2002. Available online: http://www.sidastudi.org/es/registro/2c9391e41fb402cc011fb442355a4176 (accessed on 26 June 2002).
- Nyitray, A.G.; Chang, M.; Villa, L.L.; Carvalho da Silva, R.J.; Baggio, M.L.; Abrahamsen, M.; Papenfuss, M.; Quiterio, M.; Salmerón, J.; Lazcano-Ponce, E.; et al. The Natural History of Genital Human Papillomavirus Among HIV-Negative Men Having Sex With Men and Men Having Sex With Women. J. Infect. Dis. 2015, 212, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Eubank, S.; Anil Kumar, V.S.; Marathe, M.V.; Srinivasan, A.; Wang, N. Structure of social contact networks and their impact on epidemics. DIMACS Ser. Discrete Math. Theoret. Comput. Sci. 2006, 70, 181–214. [Google Scholar]
- Estadística de movimientos Turísticos en Fronteras (Statistics of turism in Spain). Press Release from the Instituto Nacional de Estadística, Spain. Available online: http://www.ine.es/daco/daco42/frontur/frontur1216.pdf (accessed on 14 October 2017).
| MALES | ||||||
| Age | 0 LSP | 1 LSP | 2 LSP | 3–4 LSP | 5–9 LSP | 10 or More LSP |
| 14–29 | 0.107 | 0.207 | 0.131 | 0.225 | 0.168 | 0.162 |
| 30–39 | 0.027 | 0.225 | 0.128 | 0.21 | 0.17 | 0.24 |
| 40–65 | 0.019 | 0.268 | 0.14 | 0.193 | 0.163 | 0.217 |
| FEMALES | ||||||
| Age | 0 LSP | 1 LSP | 2 LSP | 3–4 LSP | 5–9 LSP | 10 or More LSP |
| 14–29 | 0.138 | 0.43 | 0.186 | 0.158 | 0.056 | 0.032 |
| 30–39 | 0.029 | 0.501 | 0.168 | 0.177 | 0.077 | 0.048 |
| 40–65 | 0.017 | 0.652 | 0.138 | 0.118 | 0.039 | 0.036 |
| Women | HR-Infected | LR-Infected |
|---|---|---|
| 18–29 y.o. | ||
| 30–39 y.o. | ||
| 40–64 y.o. | ||
| 18–64 y.o. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Domingo, J.; Sánchez-Alonso, V.; Villanueva, R.-J.; Acedo, L.; Moraño, J.-A.; Villanueva-Oller, J. Random Network Models to Predict the Long-Term Impact of HPV Vaccination on Genital Warts. Viruses 2017, 9, 300. https://doi.org/10.3390/v9100300
Díez-Domingo J, Sánchez-Alonso V, Villanueva R-J, Acedo L, Moraño J-A, Villanueva-Oller J. Random Network Models to Predict the Long-Term Impact of HPV Vaccination on Genital Warts. Viruses. 2017; 9(10):300. https://doi.org/10.3390/v9100300
Chicago/Turabian StyleDíez-Domingo, Javier, Víctor Sánchez-Alonso, Rafael-J. Villanueva, Luis Acedo, José-Antonio Moraño, and Javier Villanueva-Oller. 2017. "Random Network Models to Predict the Long-Term Impact of HPV Vaccination on Genital Warts" Viruses 9, no. 10: 300. https://doi.org/10.3390/v9100300
APA StyleDíez-Domingo, J., Sánchez-Alonso, V., Villanueva, R.-J., Acedo, L., Moraño, J.-A., & Villanueva-Oller, J. (2017). Random Network Models to Predict the Long-Term Impact of HPV Vaccination on Genital Warts. Viruses, 9(10), 300. https://doi.org/10.3390/v9100300








