The Characteristics of Herpes Simplex Virus Type 1 Infection in Rhesus Macaques and the Associated Pathological Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus and Cells
2.2. Animal Feeding and Care
2.3. Experimental Design and Sample Collection
2.4. Virus Titration
2.5. Quantitative Detection of Viral Genomic DNA and qRT-PCR Analysis of Viral Transcript mRNA
2.6. Histopathological and Immunohistopathological Detection
2.7. Co-Culture of Nervous Tissues and Vero Cells
2.8. Hybridization of LAT mRNA in Nervous Tissues
2.9. ELISpot
2.10. Statistical Analysis
3. Results
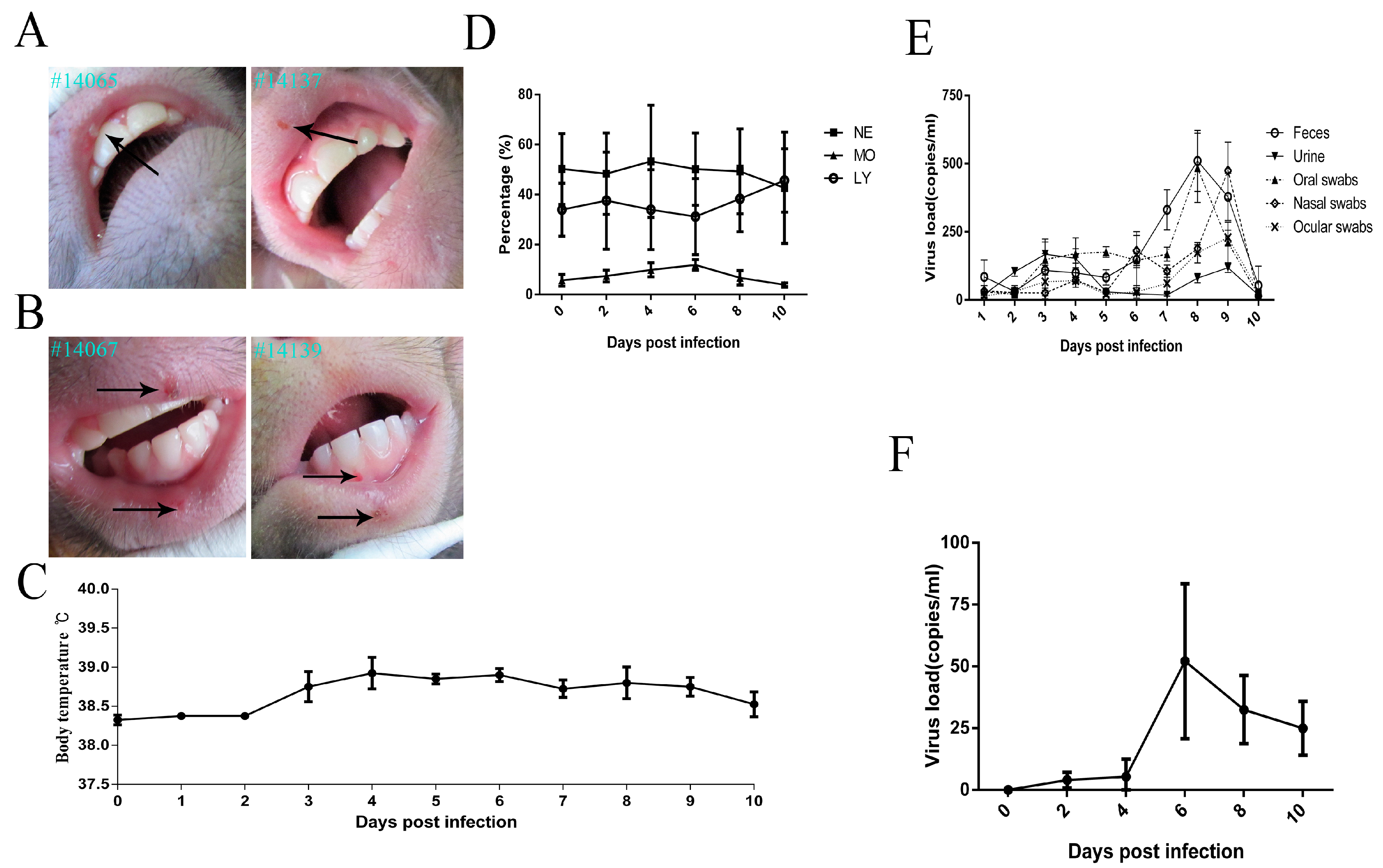
3.1. Clinical ManifestationsInduced in Rhesus Macaques Infected by HSV1
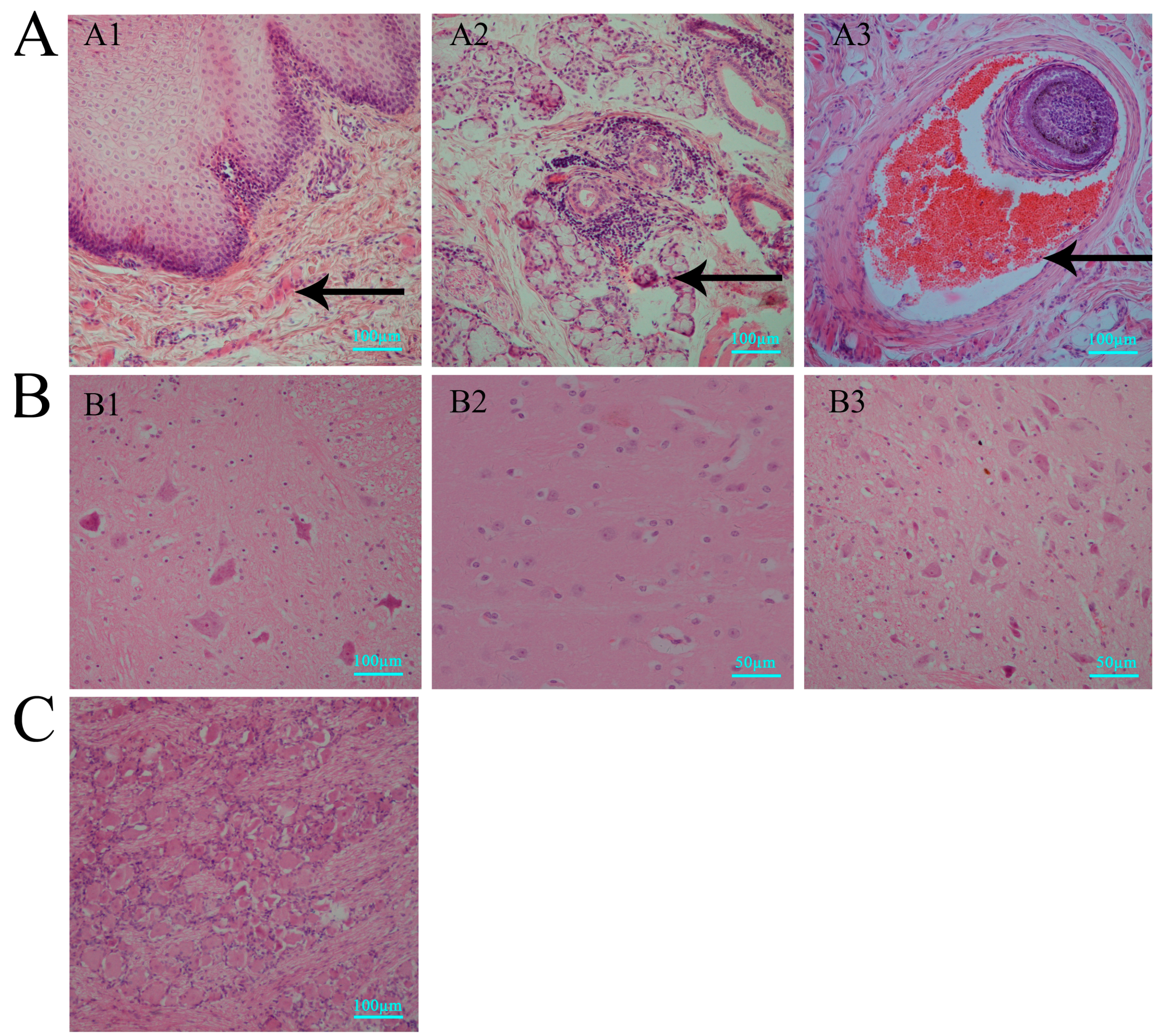
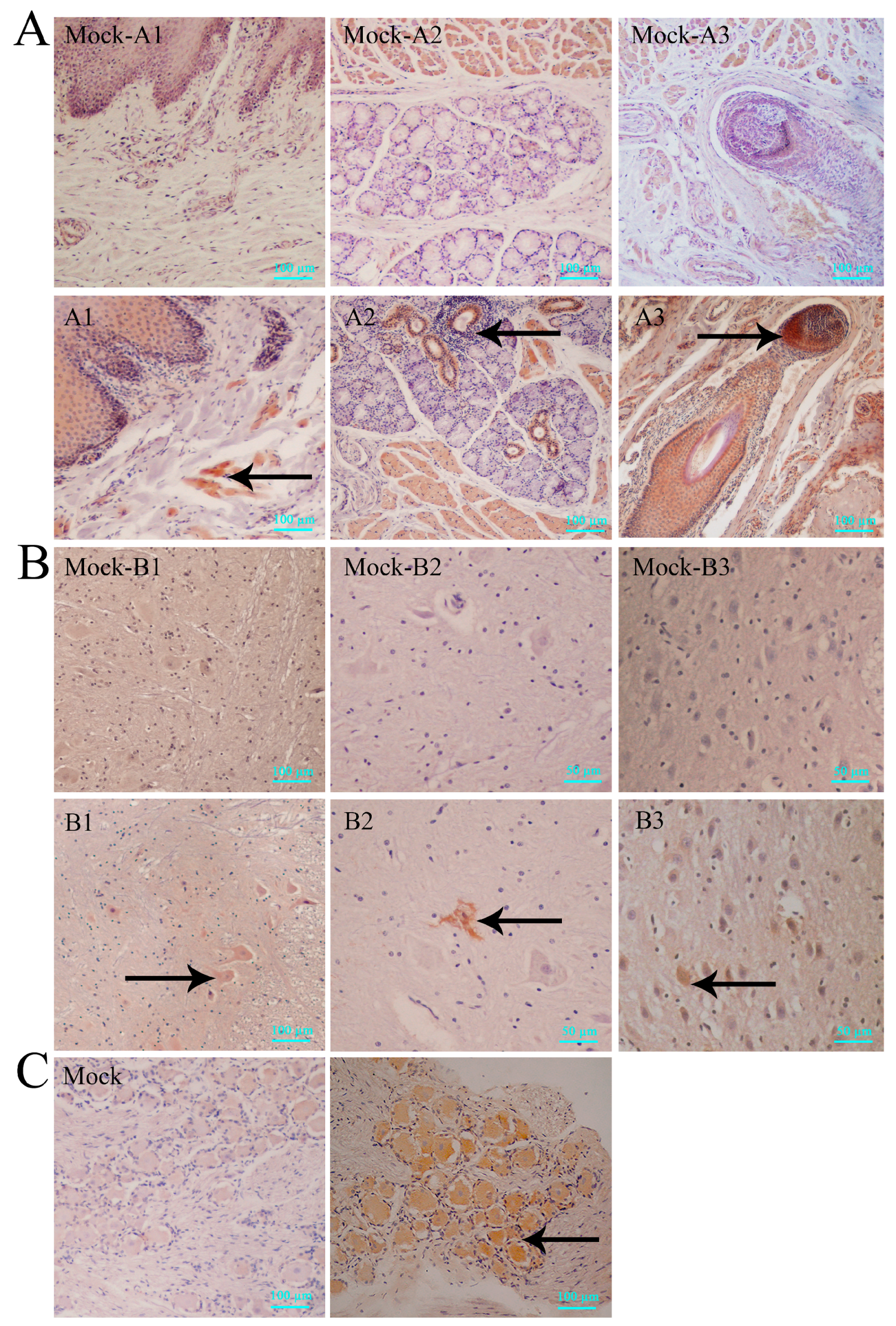
3.2. Histopathologic Features of Macaques in Acute HSV1 Infection
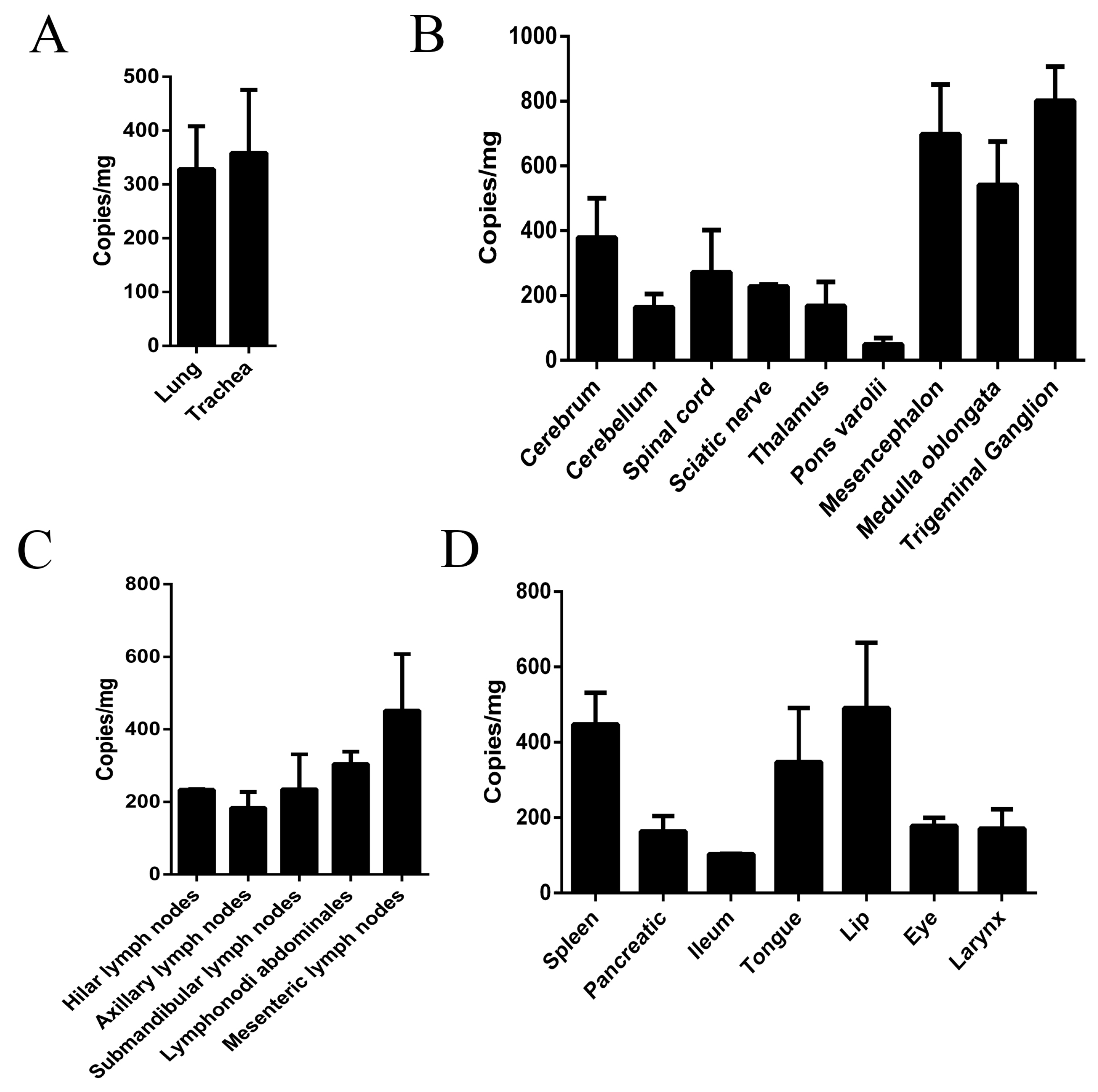
3.3. Virus Distribution in Various Macaque Tissues on Day 10 after HSV1 Infection
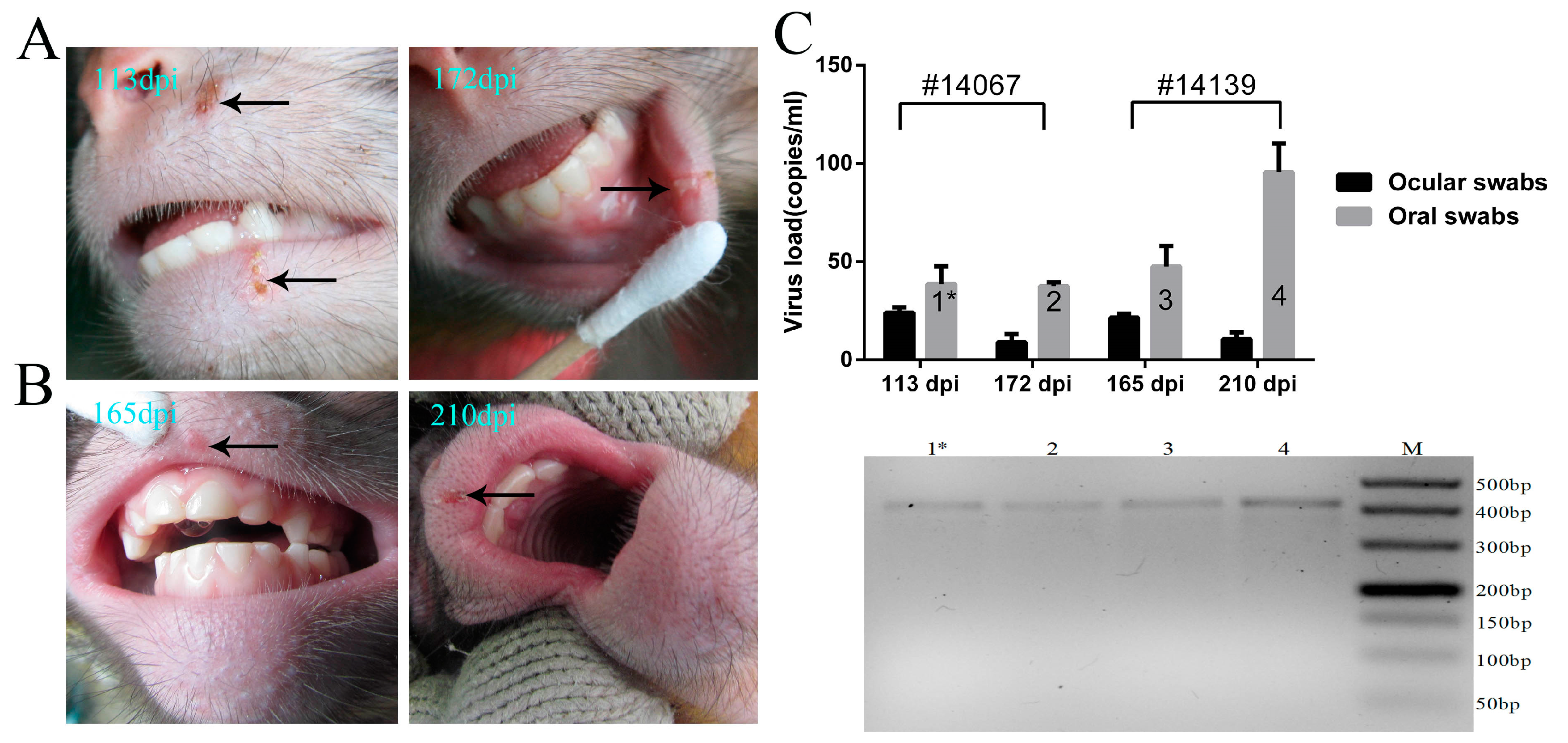
3.4. Recurrence of Oral Blebs and Associated Virus Shedding in Macaques within Four to Five Months Post-Infection
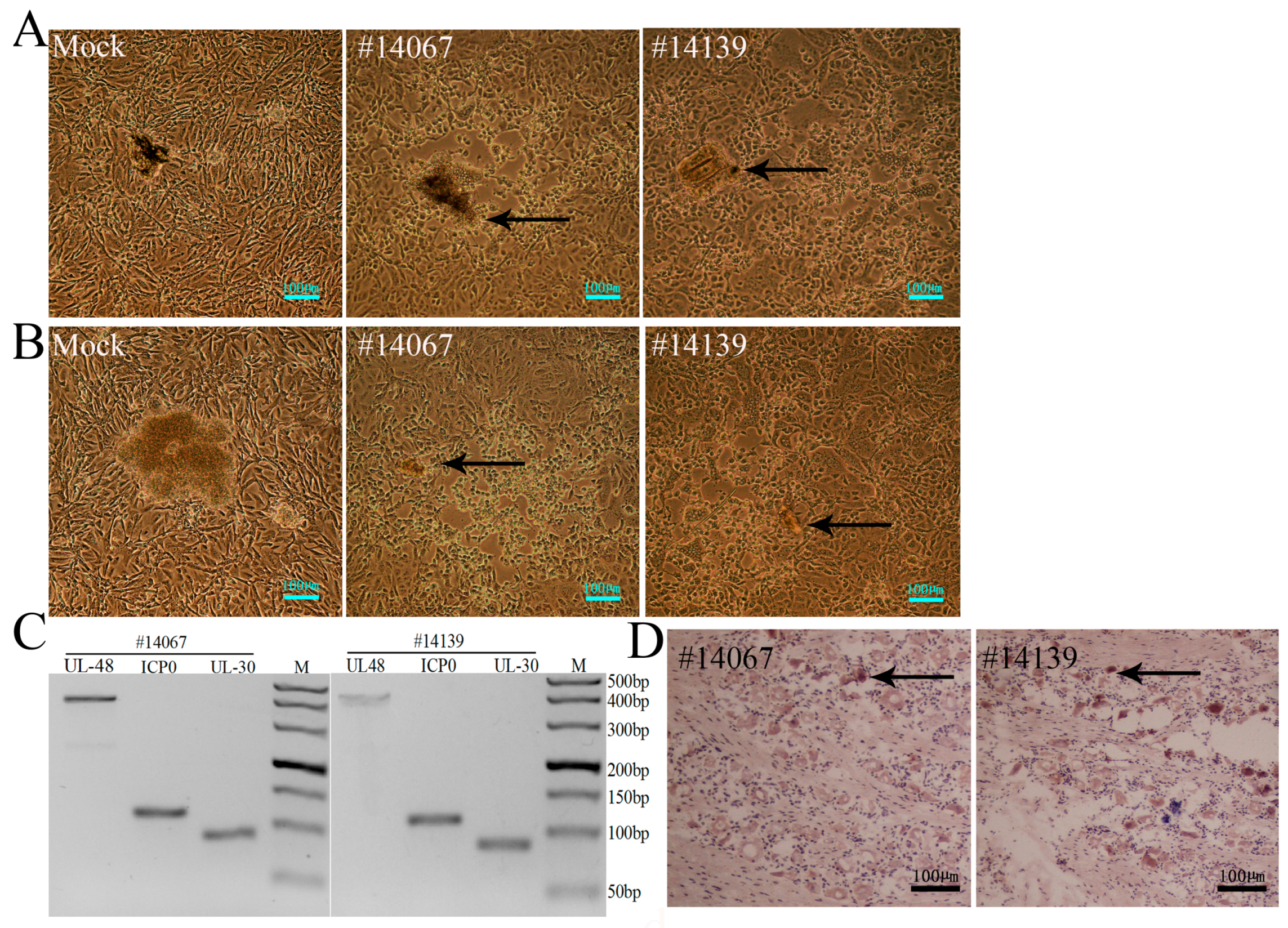
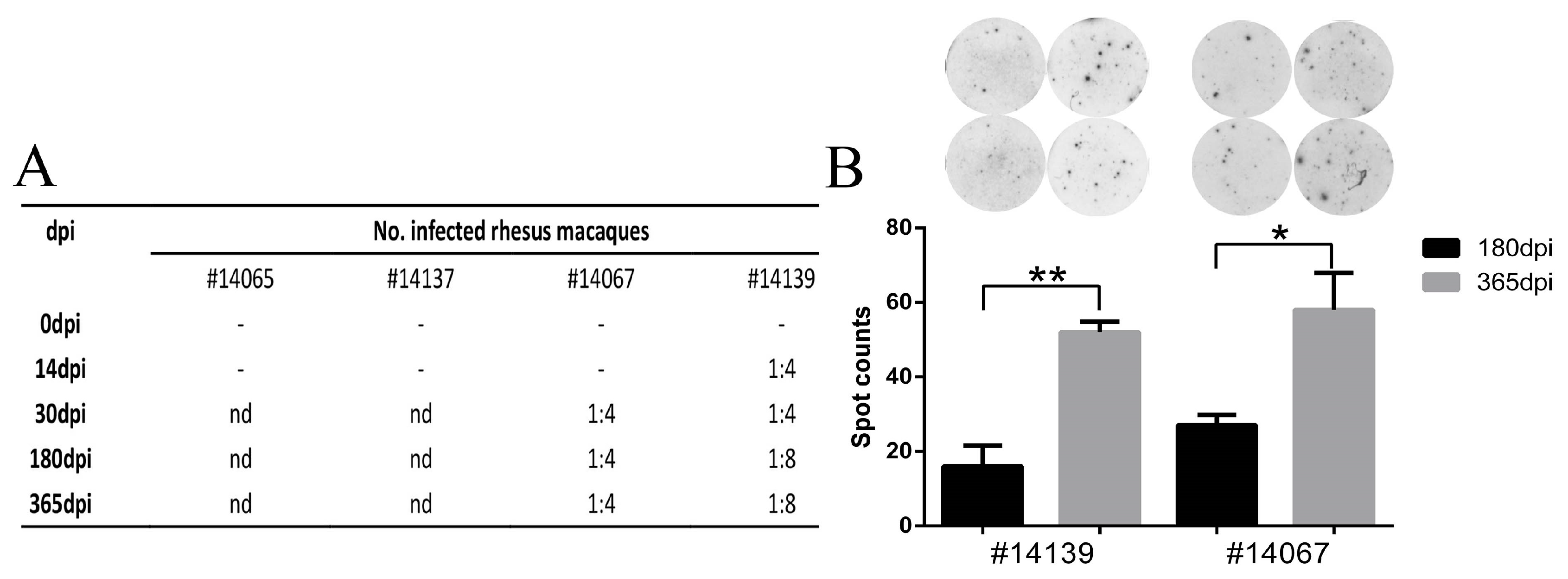
3.5. Latent Viral Infection in Macaques on Day 365 Post-Infection
3.6. HSV1 Infection Is Capable of Inducing a Specific Immune Response in Macaques
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nahmias, A.J.; Lee, F.K.; Beckman-Nahmias, S. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 1990, 69, 19–36. [Google Scholar] [PubMed]
- Groves, M.J. Genital herpes: A review. Am. Fam. Physician 2016, 93, 928–934. [Google Scholar] [PubMed]
- Kaeley, N.; Bansal, S.; Bhatia, R.; Ahmad, S. Herpes simplex encephalitis: An uncommon presentation. J. Clin. Diagn. Res. 2016, 10, OD25–OD26. [Google Scholar] [CrossRef] [PubMed]
- Shivkumar, M.; Lawler, C.; Milho, R.; Stevenson, P.G. Herpes simplex virus type 1 interaction with myeloid cells in vivo. J. Virol. 2016, 90, 8661–8672. [Google Scholar] [CrossRef] [PubMed]
- Kobelt, D.; Lechmann, M.; Steinkasserer, A. The interaction between dendritic cells and herpes simplex virus-1. Curr. Top. Microbiol. Immunol. 2003, 276, 145–161. [Google Scholar] [PubMed]
- Kollias, C.M.; Huneke, R.B.; Wigdahl, B.; Jennings, S.R. Animal models of herpes simplex virus immunity and pathogenesis. J. Neurovirol. 2015, 21, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Laycock, K.A.; Lee, S.F.; Brady, R.H.; Pepose, J.S. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet b radiation. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2741–2746. [Google Scholar]
- Scriba, M.; Tatzber, F. Pathogenesis of herpes simplex virus infections in guinea pigs. Infect. Immun. 1981, 34, 655–661. [Google Scholar] [PubMed]
- Shimomura, Y.; Dudley, J.B.; Gangarosa, L.P., Sr.; Hill, J.M. HSV-1 quantitation from rabbit neural tissues after epinephrine-induced reactivation. Investig. Ophthalmol. Vis. Sci. 1985, 26, 121–125. [Google Scholar]
- Kolb, A.W.; Lee, K.; Larsen, I.; Craven, M.; Brandt, C.R. Quantitative trait locus based virulence determinant mapping of the HSV-1 genome in murine ocular infection: Genes involved in viral regulatory and innate immune networks contribute to virulence. PLoS Pathog. 2016, 12, e1005499. [Google Scholar] [CrossRef] [PubMed]
- Zerboni, L.; Che, X.; Reichelt, M.; Qiao, Y.; Gu, H.; Arvin, A. Herpes simplex virus 1 tropism for human sensory ganglion neurons in the severe combined immunodeficiency mouse model of neuropathogenesis. J. Virol. 2013, 87, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Baghian, A.; Chouljenko, V.N.; Dauvergne, O.; Newmant, M.J.; Baghian, S.; Kousoulas, K.G. Protective immunity against lethal HSV-1 challenge in mice by nucleic acid-based immunisation with herpes simplex virus type-1 genes specifying glycoproteins Gb and Gd. J. Med. Microbiol. 2002, 51, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.W.; Ling, P.; Tung, Y.Y.; Hsu, S.M.; Chen, S.H. In vivo reactivation of latent herpes simplex virus 1 in mice can occur in the brain before occurring in the trigeminal ganglion. J. Virol. 2014, 88, 11264–11270. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Stuart, P.M.; Rogge, M.; Potter, C.; Gupta, N.; Yin, X.T. Recurrent herpetic stromal keratitis in mice, a model for studying human hsk. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Z.; Wang, E.; Yang, R.; Xiao, Y.; Han, H.; Lang, F.; Li, X.; Xia, Y.; Gao, F.; et al. Herpes simplex virus 1 infection of tree shrews differs from that of mice in the severity of acute infection and viral transcription in the peripheral nervous system. J. Virol. 2016, 90, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Deisboeck, T.S.; Wakimoto, H.; Nestler, U.; Louis, D.N.; Sehgal, P.K.; Simon, M.; Chiocca, E.A.; Hochberg, F.H. Development of a novel non-human primate model for preclinical gene vector safety studies. Determining the effects of intracerebral HSV-1 inoculation in the common marmoset: A comparative study. Gene Ther. 2003, 10, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, P.; Hunter, W.D.; Martuza, R.L.; Rabkin, S.D. Attenuated, replication-competent herpes simplex virus type 1 mutant g207: Safety evaluation in mice. J. Virol. 2000, 74, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Newsome, J.T.; Rabkin, S.D.; McGeagh, K.; Mahoney, D.; Nielsen, P.; Todo, T.; Martuza, R.L. Preclinical safety evaluation of g207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates. Hum. Gene Ther. 2001, 12, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Institute for Laboratory Animal Research. Guidance for the Description of Animal Research in Scientific Publications; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Ministry of Science and Technology of the People’s Republic of China. The Guidance to experimental animal welfare and ethical treatment. Available online: http://www.most.gov.cn/fggw/zfwj/zfwj2006/200609/t20060930_54389.htm (accessed on 17 November 2016).
- Pizzi, M. Sampling variation of the fifty percent end-point, determined by the reed-muench (behrens) method. Hum. Biol. 1950, 22, 151–190. [Google Scholar] [PubMed]
- Ryncarz, A.J.; Goddard, J.; Wald, A.; Huang, M.L.; Roizman, B.; Corey, L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J. Clin. Microbiol. 1999, 37, 1941–1947. [Google Scholar] [PubMed]
- Lee, S.; Ives, A.M.; Bertke, A.S. Herpes simplex virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease. J. Virol. 2015, 89, 8383–8391. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.; Lee, D.; Stucky, J.; Chiu, Y.L.; Rubin, A.; Horton, H.; McElrath, M.J. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for hiv vaccine trials. J. Immunol. Methods 2007, 322, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Immune Epitope Database and Analysis Resource. Available online: http://www.immuneepitope.org/ (accessed on 17 November 2016).
- Shimeld, C.; Dyson, H.; Lewkowicz-Moss, S.; Hill, T.J.; Blyth, W.A.; Easty, D.L. Spread of HSV-1 to the mouse eye after inoculation in the skin of the snout requires an intact nerve supply to the inoculation site. Curr. Eye Res. 1987, 6, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, G.D.; Mayo, D.R.; Lucia, H.L.; Landry, M.L. Genital herpes: Pathogenesis and chemotherapy in the guinea pig model. Rev. Infect. Dis. 1984, 6, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Berman, E.J.; Hill, J.M. Spontaneous ocular shedding of hsv-1 in latently infected rabbits. Investig. Ophthalmol. Vis. Sci. 1985, 26, 587–590. [Google Scholar]
- Shivkumar, M.; Milho, R.; May, J.S.; Nicoll, M.P.; Efstathiou, S.; Stevenson, P.G. Herpes simplex virus 1 targets the murine olfactory neuroepithelium for host entry. J. Virol. 2013, 87, 10477–10488. [Google Scholar] [CrossRef] [PubMed]
- Mojadadi, S.; Jamali, A.; Khansarinejad, B.; Soleimanjahi, H.; Bamdad, T. Acute morphine administration reduces cell-mediated immunity and induces reactivation of latent herpes simplex virus type 1 in balb/c mice. Cell. Mol. Immunol. 2009, 6, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Bowen, G.N.; Zhou, S.; Cerny, A.; Zacharia, A.; Knipe, D.M.; Finberg, R.W.; Kurt-Jones, E.A. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J. Virol. 2012, 86, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yamaguchi, M.; Goto, T.; Okamoto, S.; Ohashi, Y. Experimental corneal endotheliitis in rabbit. Investig. Ophthalmol. Vis. Sci. 2000, 41, 377–385. [Google Scholar]
- Liu, T.; Tang, Q.; Hendricks, R.L. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 1996, 70, 264–271. [Google Scholar] [PubMed]
- Bradshaw, M.J.; Venkatesan, A. Herpes simplex virus-1 encephalitis in adults: Pathophysiology, diagnosis, and management. Neurotherapeutics 2016, 13, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Meyding-Lamade, U.K.; Oberlinner, C.; Rau, P.R.; Seyfer, S.; Heiland, S.; Sellner, J.; Wildemann, B.T.; Lamade, W.R. Experimental herpes simplex virus encephalitis: A combination therapy of acyclovir and glucocorticoids reduces long-term magnetic resonance imaging abnormalities. J. Neurovirol. 2003, 9, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Remeijer, L.; Osterhaus, A.; Verjans, G. Human herpes simplex virus keratitis: The pathogenesis revisited. Ocul. Immunol. Inflamm. 2004, 12, 255–285. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.O.; Rabson, A.S.; Yee, C.L.; Tralka, T.S. Herpesvirus hominis: Isolation from human trigeminal ganglion. Science 1972, 178, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.V.; Kulkarni, S.S. Herpes simplex virus: The interplay between HSV, host, and HIV-1. Viral Immunol. 2015, 28, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Shiflett, L.A.; Linderman, J.A.; Mohr, I.; Wilson, A.C. Using homogeneous primary neuron cultures to study fundamental aspects of HSV-1 latency and reactivation. Methods Mol. Biol. 2014, 1144, 167–179. [Google Scholar]
- Evans, C.M.; Kudesia, G.; McKendrick, M. Management of herpesvirus infections. Int. J. Antimicrob. Agents 2013, 42, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.; Mohr, I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol. 2012, 20, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.K.; Coen, D.M. Herpes simplex viruses: Mechanisms of DNA replication. Cold Spring Harb. Perspect. Biol. 2012, 4, a013011. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, E.C. Current and potential therapies for the treatment of herpesvirus infections. Prog. Drug Res. 2001, 60, 185–228. [Google Scholar]
- Dosa, S.; Castellanos, K.; Bacsa, S.; Gagyi, E.; Kovacs, S.K.; Valyi-Nagy, K.; Shukla, D.; Dermody, T.S.; Valyi-Nagy, T. Chronic progressive deficits in neuron size, density and number in the trigeminal ganglia of mice latently infected with herpes simplex virus. Brain Pathol. 2011, 21, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Nolan, N.M.; McFerrin, H.E.; Clement, C.; Foster, T.P.; Halford, W.P.; Kousoulas, K.G.; Lukiw, W.J.; Thompson, H.W.; Stern, E.M.; et al. HSV-1 latent rabbits shed viral DNA into their saliva. Virol. J. 2012, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Brans, R.; Eriksson, E.; Yao, F. Immunization with a dominant-negative recombinant HSV type 1 protects against HSV-1 skin disease in guinea pigs. J. Investig. Dermatol. 2008, 128, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Katzin, D.S.; Connor, J.D.; Wilson, L.A.; Sexton, R.S. Experimental herpes simplex infection in the owl monkey. Proc. Soc. Exp. Biol. Med. 1967, 125, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Melendez, L.V.; Espana, C.; Hunt, R.D.; Daniel, M.D.; Garcia, F.G. Natural herpes simplex infection in the owl monkey (aotus trivirgatus). Lab. Anim. Care 1969, 19, 38–45. [Google Scholar] [PubMed]
- Hunter, W.D.; Martuza, R.L.; Feigenbaum, F.; Todo, T.; Mineta, T.; Yazaki, T.; Toda, M.; Newsome, J.T.; Platenberg, R.C.; Manz, H.J.; et al. Attenuated, replication-competent herpes simplex virus type 1 mutant g207: Safety evaluation of intracerebral injection in nonhuman primates. J. Virol. 1999, 73, 6319–6326. [Google Scholar]
- Luy, J. Ethical and legal aspects of animal experiments on non-human primates. DTW Dtsch. Tierarztliche Wochenschr. 2007, 114, 81–85. [Google Scholar]
- Meignier, B.; Martin, B.; Whitley, R.J.; Roizman, B. In vivo behavior of genetically engineered herpes simplex viruses r7017 and r7020. Ii. Studies in immunocompetent and immunosuppressed owl monkeys (aotus trivirgatus). J. Infect. Dis. 1990, 162, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lauten, M.; Guttel, C.; Hartel, C.; Erdlenbruch, B. Herpes simplex virus reactivation and disease during treatment for childhood acute lymphoblastic leukemia. Klin. Padiatrie 2014, 226, 188–189. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Cai, H.; Xu, X.; Feng, M.; Wang, L.; Liao, Y.; Zhang, Y.; He, Z.; Yang, F.; Yu, W.; et al. The Characteristics of Herpes Simplex Virus Type 1 Infection in Rhesus Macaques and the Associated Pathological Features. Viruses 2017, 9, 26. https://doi.org/10.3390/v9020026
Fan S, Cai H, Xu X, Feng M, Wang L, Liao Y, Zhang Y, He Z, Yang F, Yu W, et al. The Characteristics of Herpes Simplex Virus Type 1 Infection in Rhesus Macaques and the Associated Pathological Features. Viruses. 2017; 9(2):26. https://doi.org/10.3390/v9020026
Chicago/Turabian StyleFan, Shengtao, Hongzhi Cai, Xingli Xu, Min Feng, Lichun Wang, Yun Liao, Ying Zhang, Zhanlong He, Fengmei Yang, Wenhai Yu, and et al. 2017. "The Characteristics of Herpes Simplex Virus Type 1 Infection in Rhesus Macaques and the Associated Pathological Features" Viruses 9, no. 2: 26. https://doi.org/10.3390/v9020026



