Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium
Abstract
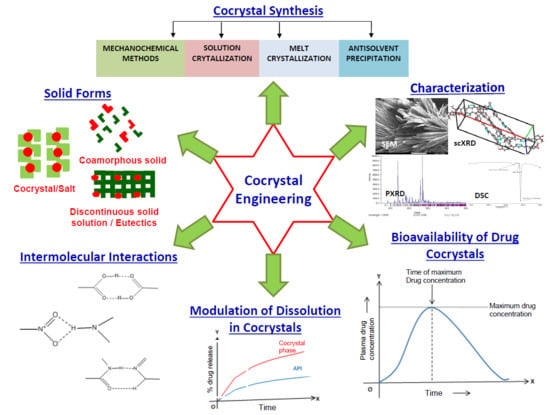
:1. Introduction
2. Types of Solid Forms Obtained from Cocrystallization
2.1. Cocrystals
2.2. Eutectics
2.3. Solid Solutions
2.4. Coamorphous Solids
2.5. Salts
2.6. Salt-Cocrystal Continuum
3. Factors Determining Cocrystallization
3.1. ∆pKa Rule
3.2. Hydrogen Bond Donors and Acceptors
- Mostly all good proton donors (such as –COOH, –NH4+) and acceptors (such as –OH, –NH3) are utilized in hydrogen bonding.
- Six-membered ring intramolecular hydrogen bonds (such as C-H…O) are formed first in preference to intermolecular hydrogen bonds (such as N-H…O and O-H…O)
- The best proton donors and acceptors available after intramolecular hydrogenbond formation then participate in intermolecular hydrogen bonds
- All acidic hydrogen atoms are included in hydrogen bonding in the crystal structure
3.3. Molecular Recognition Points
3.4. Flexibility of Synthon-Forming Functional Groups
3.5. Carbon Chain Length of Dicarboxylic Acid Coformers
3.6. Effect of Solvents
4. Screening Methods for Cocrystals
4.1. Computational Methods
4.2. Experimental Methods
4.2.1. Differential Scanning Calorimetric (DSC) Analysis
4.2.2. Phase Diagrams
5. Synthesis of Cocrystals
5.1. Batch Processes
5.2. Continuous Production of Cocrystals
- Batchwise variation in cocrystals quality can be rectified by means of continuous production by adopting a Quality-based Design (QbD) approach
- Continuous production of cocrystals is less labor intensive when compared to batch mode
- As compared to batch process, the maintenance of uniform particle distribution throughout the entire production, monitoring of quality of cocrystals by Process Analytical Technology (PAT) and maintaining consistency in the quality of products obtained is easier for continuous production
6. Characterization of Cocrystals
6.1. Structural Analysis
6.2. Thermal Analysis
6.3. Spectroscopic Analysis
6.4. Morphological Characterization
6.5. Drug Release Testing
7. Influence of Intermolecular Interactions on Cocrystallization
7.1. Hydrogen Bonding
7.2. Halogen Bonding
7.3. Ionic Interactions
7.4. Π…Π Stacking Interactions
7.5. Vanderwaal’s Interactions
8. Ternary and Quaternary Cocrystals
8.1. Approaches for Designing Ternary/Quaternary Cocrystals
- Hydrogen bond formation take place in a hierarchical manner (best donor forms hydrogen with the best acceptor, the second-best donor with the second-best acceptor) [103]
- A small number of specific intermolecular interactions such as hydrogen-bonding interactions can contribute to larger stabilization energy of the molecular crystals [269]
8.2. Advantages of Ternary/Quaternary Cocrystals
8.3. Disadvantages of Ternary/Quaternary Cocrystals
9. Polymorphism in Cocrystals
9.1. Synthon Polymorphism
9.2. Concomitant Polymorphism
9.3. Conformational Polymorphism
9.4. Packing Polymorphism
9.5. Pseudopolymorphism
9.6 Impact of Cocrystal Polymorphism on Solid-State Properties of API
10. Solubility and Dissolution Enhancement by Cocrystals
10.1. Cocrystal Solubilization
10.1.1. Phase Solubility Diagram (PSD)
10.1.2. Factors Influencing Solubility of Cocrystals
10.2. Dissolution of Cocrystals
- (i)
- (ii)
- Transformation of the amorphous or nanocrystalline drug clusters into a stable form through formation of a metastable phase by adopting Ostwald’s Law of Stages
- (iii)
10.2.1. Factors Influencing Dissolution of Cocrystals
10.2.2. Cocrystals with Low Dissolution Rates
11. In Vitro and In Vivo Studies
12. Challenges and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Edward, K.H.; Li, D. Drug Like Properties: Concept, Structure, Design and Methods, from ADME to Toxicity Optimization; Solubility Elsevier: New York, NY, USA, 2008; p. 56. [Google Scholar]
- Butler, J.M.; Dressman, J.B. The Developability Classification System: Application of Biopharmaceutics Concepts to Formulation Development. J. Pharm. Sci. 2010, 99, 4940–4954. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Terada, K. Rate- and Extent-Limiting Factors of Oral Drug Absorption: Theory and Applications. J. Pharm. Sci. 2015, 104, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Ronak, S.; Stephen, T. Predicting and Selecting Formulations for Drug Discovery and Early Development. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/341313-Predicting-and-Selecting-Formulations-for-Drug-Discovery-and-Early-Development/ (accessed on 12 July 2018).
- Lipert, M.P.; Roy, L.; Childs, S.L.; Rodriguez-Hornedo, N. Cocrystal solubilization in biorelevant media and its prediction from drug solubilization. J. Pharm. Sci. 2015, 104, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Kandi, P.; Lingireddy, S.R. Formulation of a Danazol Cocrystal with Controlled Supersaturation Plays an Essential Role in Improving Bioavailability. Mol. Pharm. 2013, 10, 3112–3127. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Good, D.J.; Rodriguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Torchillin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Soni, M.; Kumar, S.; Gupta, G.D. Solubility enhancement-eminent role in poorly soluble drugs. Res. J. Pharm. Technol. 2009, 2, 220–224. [Google Scholar]
- Ter Horst, J.H.; Deij, M.A.; Cains, P.W. Discovering New Co-Crystals. Cryst. Growth Des. 2009, 9, 1531–1537. [Google Scholar] [CrossRef]
- Huang, L.; Tong, W. Impact of solid state properties on developability assessment of drug candidates. Adv. Drug Deliv. Rev. 2004, 56, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Trask, A.V. An Overview of Pharmaceutical Cocrystals as Intellectual Property. Mol. Pharm. 2007, 4, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Saikia, B.; Sarma, B. Regulation of π···π Stacking Interactions in Small Molecule Cocrystals and/or Salts for Physiochemical Property Modulation. Cryst. Growth Des. 2018, 18, 1448–1458. [Google Scholar] [CrossRef]
- Fatima, Z.; Srivastava, D.; Kaur, C.D. Multicomponent Pharmaceutical Cocrystals: A Novel Approach for Combination Therapy. Mini Rev. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Thakuria, R.; Sarma, B. Drug-Drug and Drug-Nutraceutical Cocrystal/Salt as Alternative Medicine for Combination Therapy: A Crystal Engineering Approach. Crystals 2018, 8, 101. [Google Scholar] [CrossRef]
- Multidrug Co-Crystals Leading to Improved and Effective Therapeutics in Drug Development. Available online: https://sussexdrugdiscovery.wordpress.com/2016/04/25/multidrug-co-crystals-leading-to-improved-and-effective-therapeutics-in-drug-development/ (accessed on 25 October 2017).
- Valproate Information. Available online: https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm192645.htm (accessed on 6 June 2018).
- Goud, N.R.; Suresh, K.; Sanphui, P.; Nangia, A. Fast dissolving eutectic compositions of curcumin. Int. J. Pharm. 2012, 439, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Cherukuvada, S.; Nangia, A. Fast dissolving eutectic compositions of two anti-tubercular drugs. CrystEngComm. 2012, 14, 2579–2588. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Crystal Engineering of Curcumin with Salicylic Acid and Hydroxyquinol as Coformers. Cryst. Growth Des. 2017, 17, 3974–3988. [Google Scholar] [CrossRef]
- Skieneh, J.M.; Sathisaran, I.; Dalvi, S.V.; Rohani, S. Co-amorphous form of Curcumin-Folic acid dihydrate with increased dissolution rate. Cryst. Growth Des. 2017, 17, 6273–6280. [Google Scholar] [CrossRef]
- Haneef, J.; Chadha, R. Drug-Drug Multicomponent Solid Forms: Cocrystal, Coamorphous and Eutectic of Three Poorly Soluble Antihypertensive Drugs Using Mechanochemical Approach. AAPS PharmSciTech. 2017, 18, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Bhanoth, S.; Nangia, A. New polymorphs of curcumin. Chem. Commun. 2011, 47, 5013–5015. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Mannava, M.K.C.; Nangia, A. A novel curcumin-artemisinin coamorphous solid: Physical properties and pharmacokinetic profile. RSC Adv. 2014, 4, 58357–58361. [Google Scholar] [CrossRef]
- Pang, W.; Lv, J.; Du, S.; Wang, J.; Wang, J.; Zeng, Y. Preparation of Curcumin−Piperazine Coamorphous Phase and Fluorescence Spectroscopic and Density Functional Theory Simulation Studies on the Interaction with Bovine Serum Albumin. Mol. Pharm. 2017, 14, 3013–3024. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, K.; Laitinen, R.; Grohganz, H.; Gordon, K.C.; Strachan, C.; Rades, T. Coamorphous Drug Systems: Enhanced Physical Stability and Dissolution Rate of Indomethacin and Naproxen. Mol. Pharm. 2011, 8, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T.; Lobmann, K.; Rades, T.; Grohganz, H. Improving Co-Amorphous Drug Formulations by the Addition of theHighly Water Soluble Amino Acid, Proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gniado, K.; Erxleben, A.; McArdle, P. Mechanochemical Reaction of Sulfathiazole with Carboxylic Acids: Formation of a Cocrystal, a Salt, and Coamorphous Solids. Cryst. Growth Des. 2013, 14, 803–813. [Google Scholar] [CrossRef]
- Bi, Y.; Xiao, D.; Ren, S.; Bi, S.; Wang, J.; Li, F. The Binary System of Ibuprofen-Nicotinamide UnderNanoscale Confinement: From Cocrystal to Coamorphous State. J. Pharm. Sci. 2017, 106, 3150–3155. [Google Scholar] [CrossRef] [PubMed]
- Cherukuvada, S.; Nangia, A. Eutectics as improved pharmaceutical materials: Design, properties and characterization. Chem. Commun. 2014, 50, 906–923. [Google Scholar] [CrossRef] [PubMed]
- Cheney, M.L.; Shan, N.; Healey, E.R.; Hanna, M.; Wojtas, L.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal Engineering Case Study of Lamotrigine. Cryst. Growth Des. 2010, 10, 394–405. [Google Scholar] [CrossRef]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khankari, R.K.; Grant, D.J.W. Pharmaceutical hydrates. Thermochim. Acta 1995, 248, 61–79. [Google Scholar] [CrossRef]
- Yamamoto, N.; Taga, T.; Machida, K. Structure of mixed crystals of benzoic acid and p-fluorobenzoic acid, and their energy evaluation by empirical potential functions. Acta Cryst. 1989, 45, 162–167. [Google Scholar] [CrossRef]
- Nath, N.K.; Saha, B.K.; Nangia, A. Isostructural polymorphs of triiodophloroglucinoland triiodoresorcinol. New J. Chem. 2008, 32, 1693–1701. [Google Scholar] [CrossRef]
- Chakraborty, S.; Desiraju, G.R. C−H···F Hydrogen Bonds in Solid Solutions of Benzoic Acid and 4-Fluorobenzoic acid. Cryst. Growth Des. 2018, 18, 3607–3615. [Google Scholar] [CrossRef]
- Elder, D.P.; Holm, R.; de Diego, H.L. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Blagden, N.; Coles, S.J.; Berry, D.J. Pharmaceutical co-crystals—Are we there yet? CrystEngComm. 2014, 16, 5753–5761. [Google Scholar] [CrossRef]
- Stahly, G.P. A survey of cocrystals reported prior to 2000. Cryst. Growth Des. 2009, 9, 4212–4229. [Google Scholar] [CrossRef]
- Sarma, B.; Chen, J.; Hsi, H.; Myerson, A.S. Solid forms of pharmaceuticals: Polymorphs, salts and cocrystals. Korean J. Chem. Eng. 2011, 28, 315–322. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Grothe, E.; Meekes, H.; Vlieg, E.; ter Horst, J.H.; de Gelder, R. Solvates, Salts, and Cocrystals: A proposal for a Feasible Classification System. Cryst. Growth Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef] [Green Version]
- Zachary, B. FDA to Reclassify Pharmaceutical Co-Crystals. Available online: http://www.raps.org/Regulatory-Focus/News/2016/08/16/25611/FDA-to-Reclassify-Pharmaceutical-Co-Crystals/ (accessed on 26 October 2017).
- Gadade, D.D.; Pekamwar, S.S. Pharmaceutical Cocrystals: Regulatory and Strategic Aspects, Design and Development. Adv. Pharm. Bull. 2016, 6, 479–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US Food and Drug Administration. Guidance for Industry: Regulatory Classification of Pharmaceutical Co-Crystals; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2013.
- Reflection Paper on the Use of Cocrystals and Other Solid State Forms of Active Substances in Medicinal Products. Available online: http://docplayer.net/76383282-Reflection-paper-on-the-use-of-cocrystals-of-active-substances-in-medicinal-products.html (accessed on 28 July 2018).
- Stoler, E.; Warner, J.C. Non-Covalent Derivatives: Cocrystals and Eutectics. Molecules 2015, 20, 14833–14848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohler, F. Untersuchungen über das Chinon. Ann. Chem. Pharm. 1844, 51, 145–163. [Google Scholar] [CrossRef]
- CSD; Version 5.34, ConQuest 1.11; Cambridge Crystallographic Data Centre: Cambridge, UK, 2008.
- Etter, M.C. Hydrogen Bonds as Design Elements in Organic Chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Row, T.N.G. Comprehending the Formation of Eutectics and Cocrystals in Terms of Design and Their Structural Interrelationships. Cryst. Growth Des. 2014, 14, 4187–4198. [Google Scholar] [CrossRef]
- Bevill, M.J.; Vlahova, P.I.; Smit, J.P. Polymorphic Cocrystals of Nutraceutical Compound p-Coumaric Acid with Nicotinamide: Characterization, Relative Solid-State Stability, and Conversion to Alternate Stoichiometries. Cryst. Growth Des. 2014, 14, 1438–1448. [Google Scholar] [CrossRef]
- Childs, S.L.; Chyall, L.J.; Dunlap, J.T.; Smolenskaya, V.N.; Stahly, B.C.; Stahly, G.P. Crystal Engineering Approach to Forming Cocrystals of Amine Hydrochlorides with Organic Acids. Molecular Complexes of Fluoxetine Hydrochloride with Benzoic, Succinic, and Fumaric Acids. J. Am. Chem. Soc. 2004, 126, 13335–13342. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.P.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’Donell, E.; Park, A. Use of a Glutaric Acid Cocrystal to Improve Oral Bioavailability of a Low Solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.K.; Bak, A. Physicochemical Properties of Pharmaceutical Co-crystals: A Case Study of Ten AMG 517 Co-crystals. Cryst. Growth Des. 2008, 8, 3856–3862. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Lu, T. Simultaneously enhancing the solubility and permeability of acyclovir by crystal engineering approach. CrystEngComm 2013, 15, 6457–6460. [Google Scholar] [CrossRef]
- Masuda, T.; Yoshihashi, Y.; Yonemochi, E.; Fujii, K.; Uekusa, H.; Terada, K. Cocrystallization and amorphization induced by drug–excipient interaction improves the physical properties of acyclovir. Int. J. Pharm. 2012, 422, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.; Rodriguez-Hornedo, N.; Suryanarayanan, R. A rapid thermal method for cocrystal screening. CrystEngComm 2008, 10, 665–668. [Google Scholar] [CrossRef]
- Bethune, S.J.; Huang, N.; Jayasankar, A.; Rodriguez-Hornedo, N. Understanding and Predicting the Effect of Cocrystal Components and pH on Cocrystal Solubility. Cryst. Growth Des. 2009, 9, 3976–3988. [Google Scholar] [CrossRef]
- Childs, S.L.; Rodriguez-Hornedo, N.; Reddy, L.S.; Jayasankar, A.; Maheshwari, C.; McCausland, L.; Shipplett, R.; Stahly, B.C. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals ofcarbamazepine. CrystEngComm 2008, 10, 856–864. [Google Scholar] [CrossRef]
- Moradiya, H.G.; Islam, M.T.; Halsey, S.; Maniruzzaman, M.; Chowdhry, B.Z.; Snowden, M.J.; Douroumis, D. Continuous cocrystallisation of carbamazepine and trans-cinnamic acidviamelt extrusion processing. CrystEngComm 2014, 16, 3573–3583. [Google Scholar] [CrossRef]
- Salan, J.; Anderson, S.R.; Am, E.D.J. A Method to Produce and Scale-Up Cocrystals and Salts via Resonant Acoustic Mixing. U.S. Patent EP 2845852 A1, 11 March 2015. [Google Scholar]
- Drozd, K.V.; Manin, A.N.; Churakov, A.V.; Perlovich, G.L. Novel drug–drug cocrystals of carbamazepine with para-aminosalicylic acid: Screening, crystal structures and comparative study of carbamazepine cocrystal formation thermodynamics. CrystEngComm 2017, 19, 4273–4286. [Google Scholar] [CrossRef]
- Shewale, S.; Shete, A.S.; Doijad, R.C.; Kadam, S.S.; Patil, V.A.; Yadav, A.V. Formulation and Solid State Characterization of Nicotinamide-based Co-crystals of Fenofibrate. Ind. J. Pharm. Sci. 2015, 77, 328–334. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Geng, N.; Lu, T. Improving the Solubility of Agomelatine via Cocrystals. Cryst. Growth Des. 2012, 12, 2226–2233. [Google Scholar] [CrossRef]
- Seaton, C.C.; Parkin, A. Making Benzamide Cocrystals with Benzoic Acids: The Influence of Chemical Structure. Cryst. Growth Des. 2011, 11, 1502–1511. [Google Scholar] [CrossRef]
- Da Silva, C.C.P.; Pepino, R.O.; de Melo, C.C.; Tenorio, J.C.; Ellena, J. Controlled Synthesis of New 5-Fluorocytosine Cocrystals Based on the pKa Rule. Cryst. Growth Des. 2014, 14, 4383–4393. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Nangia, A. Fast Dissolving Curcumin Cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Chow, S.F.; Shi, L.; Ng, W.W.; Leung, K.H.Y.; Nagapudi, K.; Sun, C.C.; Chow, A.H.L. Kinetic Entrapment of a Hidden Curcumin Cocrystal with Phloroglucinol. Cryst. Growth Des. 2014, 14, 5079–5089. [Google Scholar] [CrossRef]
- Su, H.; He, H.; Tian, Y.; Zhao, N.; Sun, F.; Zhang, X.; Jiang, Q.; Zhu, G. Syntheses and characterizations of two curcumin-based cocrystals. Inorg. Chem. Commun. 2015, 55, 92–95. [Google Scholar] [CrossRef]
- Moradiya, H.G.; Islam, M.T.; Scoutaris, N.; Halsey, S.A.; Chowdhry, B.Z.; Douroumis, D. Continuous Manufacturing of High Quality Pharmaceutical Cocrystals Integrated with Process Analytical Tools for In-Line Process Control. Cryst. Growth Des. 2016, 6, 3425–3434. [Google Scholar] [CrossRef] [Green Version]
- Daurte, I.; Temtem, M.; Cil, M.; Gaspar, F. Overcoming poor bioavailability through amorphous solid dispersions. Ind. Pharm. 2011, 30, 4–6. [Google Scholar]
- Fenofibrate Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022118s005lbl.pdf (accessed on 9 July 2018).
- Faeges, M. Stable Suspensions of Acetyl Salicylic Acid. U.S. Patent US3316150A, 25 April 1967. [Google Scholar]
- Chadha, K.; Karan, M.; Chadha, R.; Bhalla, Y.; Vasisht, K. Is Failure of Cocrystallization Actually a Failure? Eutectic Formation in Cocrystal Screening of Hesperetin. J. Pharm. Sci. 2017, 106, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Sangster, J. Phase Diagrams and Thermodynamic Properties of Binary Systems of Drugs. J. Phys. Chem. Ref. Data 1999, 28, 889–930. [Google Scholar] [CrossRef]
- Gorniak, A.; Karolewicz, B.; Zurawska-Plaksej, E.; Pluta, J. Thermal, spectroscopic, and dissolution studies of the simvastatin–acetylsalicylic acid mixtures. J. Therm. Anal. Calorim. 2013, 111, 2125–2132. [Google Scholar] [CrossRef]
- Ganduri, R.; Cherukuvada, S.; Row, T.N.G. Multicomponent Adducts of Pyridoxine: An Evaluation of the Formation of Eutectics and Molecular Salts. Cryst. Growth Des. 2015, 15, 3474–3480. [Google Scholar] [CrossRef]
- Haneef, J.; Chadha, R. Antioxidant-Based Eutectics of Irbesartan: Viable Multicomponent Forms for the Management of Hypertension. AAPS PharmSciTech 2017. [Google Scholar] [CrossRef] [PubMed]
- Oliviera, M.A.; Peterson, M.L.; Klein, D. Continuously Substituted Solid Solutions of Organic Co-Crystals. Cryst. Growth Des. 2008, 8, 4487–4493. [Google Scholar] [CrossRef]
- Chieng, N.; Aaltonen, J.; Saville, D.; Rades, T. Physical characterization and stability of amorphous indomethacin and ranitidine hydrochloride binary systems prepared by mechanical activation. Eur. J. Pharm. Biopharm. 2009, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Lobmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Reutzel-Edens, S.M.; Zografi, G. Coamorphous Active Pharmaceutical Ingredient—Small Molecule Mixtures: Considerations in the Choice of Coformers for Enhancing Dissolution and Oral Bioavailability. J. Pharm. Sci. 2018, 107, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Shayanfar, A.; Ghavimi, H.; Hamishekhar, H.; Jouyban, A. Coamorphous Atorvastatin Calcium to Improve its Physicochemical and Pharmacokinetic Properties. J. Pharm. Pharm. Sci. 2013, 16, 577–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannava, M.K.C.; Suresh, K.; Bommaka, M.K.; Konga, D.B.; Nangia, A. Curcumin-Artemisinin Coamorphous Solid: Xenograft Model Preclinical Study. Pharmaceutics 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.H.; Grant, D.J.W. Handbook of Experimental Pharmacology: Stereochemical Aspects of Drug Action and Disposition; Eichelbaum, M., Testa, B., Somogyi, A., Eds.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Aakeroy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or Salt: Does It Really Matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M.J. Chapter 7. A Procedure for Salt Selection and Optimization. In Handbook of Pharmaceutical Salts: Properties, Selection, and Use; Stahl, P.H., Wermuth, C.G., Eds.; Wiley-VCH: Weinheim, Germany, 2002; pp. 163–164. [Google Scholar]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt-Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Black, S.N.; Collier, E.A.; Davey, R.J.; Roberts, R.J. Structure, Solubility, Screening, and Synthesis of Molecular Salts. J. Pharm. Sci. 2007, 96, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Jacobs, A.; Noa, F.M.A. Hybrid Salt–Cocrystal Solvate: p-Coumaric Acid and Quinine System. J. Chem. Crystallogr. 2014, 44, 57–62. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Tape and layer structuresin cocrystals of some di- and tricarboxylic acids with 4,4’-bipyridines and isonicotinamide. From binary to ternary cocrystals. CrystEngComm 2005, 7, 551–562. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug. Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shattock, T.R.; Arora, K.K.; Vishweshwar, P.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Carboxylic Acid⋯Pyridine Hydrogen Bonds in Cocrystals that also Contain a Hydroxyl Moiety. Cryst. Growth Des. 2008, 8, 4533–4545. [Google Scholar] [CrossRef]
- Rajput, L.; Banik, M.; Yarava, J.R.; Joseph, S.; Pandey, M.K.; Nishiyama, Y.; Desiraju, G.R. Exploring the salt–cocrystal continuum with solidstate NMR using natural-abundance samples: Implications for crystal engineering. IUCrJ 2017, 4, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, S.; Mannava, M.K.C.; Khandavilli, U.B.R.; Allu, S.; Nangia, A. Soluble Salts and Cocrystals of Clotrimazole. Cryst. Growth Des. 2015, 15, 2493–2504. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Polymorphism in cocrystals: A review and assessment of its significance. CrystEngComm 2014, 16, 3451–3465. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen bond patterns of organic compunds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Donohue, J. The Hydrogen Bond in Organic Crystals. J. Phys. Chem. 1952, 56, 502–510. [Google Scholar] [CrossRef]
- Almarsson, O.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines? Chem. Commun. 2004, 1889–1896, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J. General methods for the construction of complex molecules. Pure Appl. Chem. 1967, 14, 30–37. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Beatty, A.M.; Helfrich, B.A.; Nieuwenhuyzen, M. Do Polymorphic Compounds Make Good Cocrystallizing Agents? A Structural Case Study that Demonstrates the Importance of Synthon Flexibility. Cryst. Growth Des. 2003, 3, 159–165. [Google Scholar] [CrossRef]
- Shevchenko, A.; Miroshnyk, I.; Pietila, L.; Haarala, J.; Salmia, J.; Sinervo, K.; Mirza, S.; Veen, B.; Kolehmainen, E.; Ylirussi, J. Diversity in Itraconazole Cocrystals with Aliphatic Dicarboxylic Acids of Varying Chain Length. Cryst. Growth Des. 2013, 13, 4877–4884. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.; Ulrich, J. Formation of Salicylic Acid/4,4′-Dipyridyl Cocrystals Based on the Ternary Phase Diagram. Chem. Eng. Technol. 2015, 38, 1073–1080. [Google Scholar] [CrossRef]
- Robertson, C.C.; Wright, J.S.; Carrington, E.J.; Perutz, R.N.; Hunter, C.A.; Brammer, L. Hydrogen bonding vs. Halogen bonding: The solvent decides. Chem. Sci. 2017, 8, 5392–5398. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.; Karamertzanis, P.G.; Welch, G.W.A.; Price, S.L. Can the Formation of Pharmaceutical Cocrystals Be Computationally Predicted? I. Comparison of Lattice Energies. Cryst. Growth Des. 2009, 9, 442–453. [Google Scholar] [CrossRef]
- Kuleshova, L.N.; Hofmann, D.W.M.; Boese, R. Lattice energy calculation—A quick tool for screening of cocrystals and estimation of relative solubility. Case of flavonoids. Chem. Phys. Lett. 2013, 564, 26–32. [Google Scholar] [CrossRef]
- Chan, H.C.S.; Kendrick, J.; Neumann, M.A.; Leusen, F.J.J. Towards ab initio screening of co-crystal formation through lattice energy calculations and crystal structure prediction of nicotinamide, isonicotinamide, picolinamide and paracetamol multi-component crystals. CrystEngComm 2013, 15, 3799–3807. [Google Scholar] [CrossRef]
- Karamertzanis, P.G.; Kazantsev, A.V.; Issa, N.; Welch, G.W.A.; Adjiman, C.S.; Pantelides, C.C.; Price, S.L. Can the Formation of Pharmaceutical Cocrystals Be Computationally Predicted? 2. Crystal Structure Prediction. J. Chem. Theory Comput. 2009, 5, 1432–1448. [Google Scholar] [CrossRef] [PubMed]
- Grecu, T.; Hunter, C.A.; Gardiner, E.J.; McCabe, J.F. Validation of a Computational Cocrystal Prediction Tool; Comparison of Virtual and Experimental Cocrystal Screening Results. Cryst. Growth Des. 2014, 14, 165–171. [Google Scholar] [CrossRef]
- Grecu, T.; Adams, H.; Hunter, C.A.; McCabe, J.F.; Portell, A.; Prohens, R. Virtual Screening Identifies New Cocrystals of Nalidixic Acid. Cryst. Growth Des. 2014, 14, 1749–1755. [Google Scholar] [CrossRef]
- Grecu, T.; Prohens, R.; McCabe, J.F.; Carrington, E.J.; Wright, J.S.; Brammer, L.; Hunter, C.A. Cocrystals of spironolactone and griseofulvin based on an in silico screening method. Cryst. Growth Des. 2017, 19, 3592–3599. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.A.; Feeder, N.; Furlow, M.; Galek, P.T.A.; Groom, C.R.; Pidcock, E. Knowledge-based approaches to co-crystal design. CrystEngComm 2014, 16, 5839–5848. [Google Scholar] [CrossRef]
- Delori, A.; Galek, P.T.A.; Pidcock, E.; Patni, M.; Jones, W. Knowledge-based hydrogen bond prediction and the synthesis of salts and cocrystals of the anti-malarial drug pyrimethamine with various drug and GRAS molecules. CrystEngComm 2013, 15, 2916–2928. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S.P. Hansen solubility parameter as a tool to predict cocrystal formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational coformer or solvent selection for pharmaceutical cocrystallization or desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef] [PubMed]
- Abramov, Y.A. Virtual hydrate screening and coformer selection for improved relative humidity stability. CrystEngComm 2015, 17, 5216–5224. [Google Scholar] [CrossRef]
- Habgood, M.; Deij, M.A.; Mazurek, J.; Price, S.L.; ter Horst, J.H. Carbamazepine Cocrystallization with Pyridine Carboxamides: Rationalization by Complementary Phase Diagrams and Crystal Energy Landscapes. Cryst. Growth Des. 2010, 10, 903–912. [Google Scholar] [CrossRef]
- Fabian, L. Cambridge Structural Database Analysis of Molecular Complementarity in Cocrystals. Cryst. Growth Des. 2009, 9, 1436–1443. [Google Scholar] [CrossRef]
- Musumeci, D.; Hunter, C.A.; Prohens, R.; Scuderi, S.; McCabe, J.F. Virtual cocrystal sceening. Chem. Sci. 2011, 2, 883–890. [Google Scholar] [CrossRef]
- Cysewski, P. Transferability of cocrystallization propensities between aromatic and heteroaromatic amides. Struct. Chem. 2016, 27, 1403–1412. [Google Scholar] [CrossRef] [Green Version]
- Loschen, C.; Klamt, A. Solubility prediction, solvate and cocrystal screening as tools for rational crystal engineering. J. Pharm. Pharmacol. 2015, 67, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Solomos, M.A.; Mohammadi, C.; Urbelis, J.H.; Koch, E.S.; Osborne, R.; Usala, C.C.; Swift, J.A. Predicting Cocrystallization Based on Heterodimer Energies: The Case of N,N′-Diphenylureas and Triphenylphosphine Oxide. Cryst. Growth Des. 2015, 15, 5068–5074. [Google Scholar] [CrossRef]
- Stepanovs, D.; Jure, M.; Kuleshova, L.N.; Hofmann, D.W.M.; Mishnev, A. Cocrystals of Pentoxifylline: In Silico and Experimental Screening. Cryst. Growth Des. 2015, 15, 3652–3660. [Google Scholar] [CrossRef]
- Cysewski, P. In silico screening of dicarboxylic acids for cocrystallization with phenylpiperazine derivatives based on both cocrystallization propensity and solubility advantage. J. Mol. Model. 2017, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P. Heat of formation distributions of components involved in bi-component cocrystals and simple binary eutectic mixtures. New J. Chem. 2016, 40, 187–194. [Google Scholar] [CrossRef]
- Newman, A. Specialized Solid Form Screening Techniques. Org. Process Res. Dev. 2013, 17, 457–471. [Google Scholar] [CrossRef]
- Meng, F.; Li, Y.; Liu, X.; Li, B.; Wang, L. Single-Crystal Structures and Typical Hydrogen-Bonding Motifs of Supramolecular Cocrystals Containing 1,4-Di(1H-imidazol-1-yl) benzene. Cryst. Growth Des. 2015, 15, 4518–4525. [Google Scholar] [CrossRef]
- Thomas, R.; Gopalan, R.S.; Kulkarni, G.U.; Rao, C.N.R. Hydrogen bonding patterns in the cocrystals of 5-nitrouracil with several donor and acceptor molecules. Beilstein J. Org. Chem. 2005. [Google Scholar] [CrossRef] [PubMed]
- Mohana, M.; Muthiah, P.T.; McMillen, C.D. Supramolecular hydrogen-bonding patterns in 1:1 cocrystals of 5-fluorouracil with 4-methylbenzoic acid and 3-nitrobenzoic acid. Acta Cryst. 2017, 73, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Oswald, I.D.H.; Motherwell, W.D.S.; Parsons, S. Formation of quinol co-crystals with hydrogen-bond acceptors. Acta Cryst. 2005, 61, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Weyna, D.R.; Cheney, M.L.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M.J. Crystal engineering of multiple-component organic solids: Pharmaceutical cocrystals of tadalafil with persistent hydrogen bonding motifs. CrystEngComm 2012, 14, 2377–2380. [Google Scholar] [CrossRef]
- Kamali, N.; Aljohani, M.; McArdle, P.; Erxleben, A. Hydrogen Bonding Networks and Solid-State Conversions in Benzamidinium Salts. Cryst. Growth Des. 2015, 15, 3905–3916. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Hirakura, Y.; Yuda, M.; Teramura, T.; Terada, K. Detection of cocrystal formation based on binary phase diagrams using thermal analysis. Pharm. Res. 2013, 30, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Hirakura, Y.; Yuda, M.; Terada, K. Coformer Screening Using Thermal Analysis Based on Binary Phase Diagrams. Pharm. Res. 2014, 31, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Saganowska, P.; Weselowski, M. DSC as a screening tool for rapid co-crystal detection in binary mixtures of benzodiazepines with co-formers. J. Therm. Anal. Calorim. 2017. [Google Scholar] [CrossRef]
- Craye, G.; Lobmann, K.; Grohganz, H.; Rades, T.; Laitinen, R. Characterization of Amorphous and Co-Amorphous Simvastatin Formulations Prepared by Spray Drying. Molecules 2015, 20, 21532–21548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Harasimowicz, M.T.; de Villiers, M.M.; Yu, L. Cocrystals of Nicotinamide and (R)-Mandelic Acid in Many Ratios with Anomalous Formation Properties. J. Am. Chem. Soc. 2013, 135, 18981–18989. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, J.; Lu, T. Thermodynamics and preliminary pharmaceutical characterization of a melatonin–pimelic acid cocrystal prepared by a melt crystallization method. CrystEngComm 2015, 17, 612–620. [Google Scholar] [CrossRef]
- Chiarella, R.A.; Davey, R.J.; Peterson, M.L. Making Co-crystals-The Utility of Ternary Phase Diagrams. Cryst. Growth Des. 2007, 7, 1223–1226. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Rasmuson, A.C. Thermodynamics and crystallization of a theophylline–salicylic acid cocrystal. CrystEngComm 2015, 17, 4125–4135. [Google Scholar] [CrossRef]
- Sun, X.; Yin, Q.; Ding, S.; Shen, Z.; Bao, Y.; Gong, J.; Hou, B.; Hao, H.; Wang, Y.; Wang, J.; et al. Solid−Liquid Phase Equilibrium and Ternary Phase Diagrams of Ibuprofen−Nicotinamide Cocrystals in Ethanol and Ethanol/Water Mixtures at (298.15 and 313.15) K. J. Chem. Eng. Data 2015, 60, 1166–1172. [Google Scholar] [CrossRef]
- Good, D.J.; Rodriguez-Hornedo, N. Cocrystal Eutectic Constants and Prediction of Solubility Behavior. Cryst. Growth Des. 2010, 10, 1028–1032. [Google Scholar] [CrossRef]
- Lange, L.; Lehmkemper, K.; Sadowski, G. Predicting the Aqueous Solubility of Pharmaceutical Cocrystals as a Function of pH and Temperature. Cryst. Growth Des. 2016, 16, 2726–2740. [Google Scholar] [CrossRef]
- Karki, S.; Friscic, T.; Jones, W.; Motherwell, W.D.S. Screening for Pharmaceutical Cocrystal Hydrates via Neat and Liquid-Assisted Grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, K.; Davey, R.; Cross, W. How does grinding produce co-crystals? Insights from the case of benzophenone and diphenylamine. CrystEngComm 2007, 9, 732–734. [Google Scholar] [CrossRef]
- Friscic, T. New opportunities for materials synthesis using mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- Weyna, D.R.; Shattock, T.; Vishweshwar, P.; Zaworotko, M.J. Synthesis and Structural Characterization of Cocrystals and Pharmaceutical Cocrystals: Mechanochemistry vs Slow Evaporation from Solution. Cryst. Growth Des. 2009, 9, 1106–1123. [Google Scholar] [CrossRef]
- Aher, S.; Dhumal, R.; Mahadik, K.; Paradkar, A.; York, P. Ultrasound assisted cocrystallization from solution (USSC) containing a non-congruently soluble cocrystal component pair: Caffeine/maleic acid. Eur. J. Pharm. Sci. 2010, 41, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Aher, S.; Dhumal, R.; Mahadik, K.; Ketolainen, J.; Paradkar, A. Effect of cocrystallization techniques on compressional properties of caffeine/oxalic acid 2:1 cocrystal. Pharm. Dev. Technol. 2013, 18, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Takata, N.; Shiraki, K.; Takano, R.; Hayashi, Y.; Terada, K. Cocrystal Screening of Stanolone and Mestanolone Using Slurry Crystallization. Cryst. Growth Des. 2008, 8, 3032–3037. [Google Scholar] [CrossRef]
- Bucar, D.; Henry, R.F.; Lou, X.; Duerst, R.W.; MacGillivray, L.R.; Zhang, G.G.Z. Cocrystals of Caffeine and Hydroxybenzoic Acids Composed of Multiple Supramolecular Heterosynthons: Screening via Solution-Mediated Phase Transformation and Structural Characterization. Cryst. Growth Des. 2009, 9, 1932–1943. [Google Scholar] [CrossRef]
- Jayasankar, A.; Good, D.J.; Rodriguez-Hornedo, N. Mechanisms by Which Moisture Generates Cocrystals. Mol. Pharm. 2007, 4, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Good, D.; Miranda, C.; Rodriguez-Hornedo, N. Dependence of cocrystal formation and thermodynamic stability on moisture sorption by amorphous polymer. CrystEngComm 2011, 13, 1181–1189. [Google Scholar] [CrossRef]
- Seo, J.; Hwang, K.; Lee, S.; Kim, D.; Park, E. Preparation and characterization of adefovir dipivoxil–stearic acid cocrystal with enhanced physicochemical properties. Pharm. Dev. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pando, C.; Cabanas, A.; Cuadra, I.A. Preparation of pharmaceutical co-crystals through sustainable processes using supercritical carbon dioxide: A review. RSC Adv. 2016, 6, 71134–71150. [Google Scholar] [CrossRef]
- Ter Horst, J.H.; Cains, P.W. Co-Crystal Polymorphs from a Solvent-Mediated Transformation. Cryst. Growth Des. 2008, 8, 2537–2542. [Google Scholar] [CrossRef]
- Friscic, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Trask, A.V.; Jones, W. Crystal Engineering of Organic Cocrystals by the Solid-State Grinding Approach. In Organic Solid State Reactions. Topics in Current Chemistry; Toda, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 254, pp. 41–70. [Google Scholar]
- Tilborg, A.; Michaux, C.; Norberg, B.; Wouters, J. Advantages of cocrystallization in the field of solid-state pharmaceutical chemistry: L-Proline and MnCl2. Eur. J. Med. Chem. 2010, 45, 3511–3517. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Bassi, P.S.; Chadha, S.L. Kinetics of reaction between naphthalene and picric acid in the solid state. J. Phys. Chem. 1962, 66, 2707–2708. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Bassi, P.S.; Chadha, S.L. Mechanism of the reaction between hydrocarbons and picric acid in the solid state. J. Phys. Chem. 1963, 67, 2569–2573. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, N.B. Solid-State Reactions between Picric acid and Naphthols. J. Phys. Chem. 1966, 70, 3315–3324. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, N.B. Solid-State Reactivity of Picric Acid and Substituted Hydrocarbons. J. Phys. Chem. 1968, 72, 4446–4449. [Google Scholar] [CrossRef]
- Rehder, S.; Klukkert, M.; Lobmann, K.A.M.; Strachan, C.J.; Sakmann, A.; Gordon, K.; Rades, T.; Leopold, C.S. Investigation of the Formation Process of Two Piracetam Cocrystals during Grinding. Pharmaceutics 2011, 3, 706–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhalaweh, A.; George, S.; Basavoju, S.; Childs, S.L.; Rizvi, S.A.A.; Velaga, S.P. Pharmaceutical cocrystals of nitrofurantoin: Screening, characterization and crystal structure analysis. CrystEngComm 2012, 14, 5078–5088. [Google Scholar] [CrossRef]
- Kuroda, R.; Higashiguchi, K.; Hasebe, S.; Imai, Y. Crystal to crystal transformation in the solid state. CrystEngComm 2004, 6, 464–468. [Google Scholar] [CrossRef]
- Ji, C.; Hoffman, M.C.; Mehta, M.A. Catalytic Effect of Solvent Vapors on the Spontaneous Formation of Caffeine−Malonic Acid Cocrystal. CrystEngComm 2017, 17, 1456–1459. [Google Scholar] [CrossRef]
- Ruecroft, G.; Hipkiss, D.; Ly, T.; Maxted, N.; Cains, P.W. Sonocrystallization: The use of ultrasound for improved industrial crystallization. Org. Process Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Mougin-Andres, P.M.; Stahly, B.C. Screening for Solid Forms by Ultrasound Crystallization and Cocrystallization Using Ultrasound. U.S. Patent US20110251426A1, 13 October 2011. [Google Scholar]
- Morrison, H.; Mrozek-Morrison, M.; Toschi, J.; Luu, V.; Tan, H.; Daurio, D. High Throughput Bench-Top Co-crystal Screening via a Floating Foam Rack/Sonic Bath Method. Org. Process. Res. Dev. 2013, 17, 533–539. [Google Scholar] [CrossRef]
- Ross, S.A.; Lamprou, D.A.; Douroumis, D. Engineering and manufacturing of pharmaceutical co-crystals: A review of solvent-free manufacturing technologies. Chem. Commun. 2016, 52, 8772–8786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daurio, D.; Nagapudi, K.; Li, L.; Quan, P.; Nunez, F.A. Application of twin screw extrusion to the manufacture of cocrystals: Scale-up of AMG 517-sorbic acid cocrystal production. Faraday Discuss. 2014, 170, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.B.; Thipparaboina, R.; Yadav, B.; Shastri, N.R. Continuous manufacturing of co-crystals: Challenges and prospects. Drug Deliv. Transl. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Thipparaboina, R.; Kumar, D.; Chavan, R.B.; Shastri, N.R. Multidrug co-crystals: Towards the development of effective therapeutic hybrids. Drug Discov. Today 2016. [Google Scholar] [CrossRef] [PubMed]
- Daurio, D.; Medina, C.; Saw, R.; Nagapudi, K.; Alvarez-Nunez, F. Application of Twin Screw Extrusion in the Manufacture of Cocrystals, Part I: Four Case Studies. Pharmaceutics 2011, 3, 582–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yu, T.; Tian, Y.; McCoy, C.P.; Jones, D.S.; Andrews, G.P. Mechanochemical Synthesis of Pharmaceutical Cocrystal Suspensions via Hot Melt Extrusion: Feasibility Studies and Physicochemical Characterization. Mol. Pharm. 2016, 13, 3054–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, C.; Wood, C.; Kelly, A.L.; Gough, T.; Blagden, N.; Paradkar, A. Stoichiometric Control of Co-Crystal Formation by Solvent Free Continuous Co-Crystallization (SFCC). Cryst. Growth Des. 2015, 15, 5648–5651. [Google Scholar] [CrossRef] [Green Version]
- Alhalaweh, A.; Velaga, S.P. Formation of Cocrystals from Stoichiometric Solutions of Incongruently Saturating Systems by Spray Drying. Cryst. Growth Des. 2010, 10, 3302–3305. [Google Scholar] [CrossRef]
- Spitzer, D.; Risse, B.; Schnell, F.; Pichot, V.; Klaumunzer, M.; Schaefer, M.R. Continuous engineering of nano-cocrystals for medical and energetic applications. Sci. Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- Korde, S.; Pagire, S.; Pan, H.; Seaton, C.; Kelly, A.; Chen, Y.; Wang, Q.; Coates, P.; Paradkar, A. Continuous Manufacturing of Cocrystals Using Solid State Shear Milling Technology. Cryst. Growth Des. 2018, 18, 2297–2304. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Velaga, S.P.; Matos, H.A.; de Azevedo, E.G. Formation of indomethacin–saccharin cocrystals using supercritical fluid technology. Eur. J. Pharm. Sci. 2009, 38, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; Rodrigues, M.A.; Velaga, S.P.; Fernandes, A.C.; Matos, H.A.; de Azevedo, E.G. Screening for pharmaceutical cocrystals using the supercritical fluid enhanced atomization process. J. Supercrit. Fluids 2010, 53, 156–164. [Google Scholar] [CrossRef]
- Fucke, K.; Myz, S.A.; Shakhtshneider, T.P.; Boldyreva, E.V.; Griesser, U.J. How good are the crystallization methods for co-crystals? A comparative study of piroxicam. New J. Chem. 2012, 36, 1969–1977. [Google Scholar] [CrossRef]
- Seefeldt, K.; Miller, J.; Alvarez-Nunez, F.; Rodriguez-Hornedo, N. Crystallization pathways and kinetics of carbamazepine–nicotinamide cocrystals from the amorphous state byin situthermomicroscopy, spectroscopy, and calorimetry studies. J. Pharm. Sci. 2007, 96, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.E.; Miskimmin, C.K.G.; Albadarin, A.B.; Walker, G.; James, S.L. Organic synthesis by Twin Screw Extrusion (TSE): Continuous, scalable and solvent-free. Green Chem. 2017, 19, 1507–1518. [Google Scholar] [CrossRef]
- Ober, C.A.; Gupta, R.B. Formation of Itraconazole-Succinic Acid Cocrystals by Gas Antisolvent Cocrystallization. AAPS PharmSciTech 2012, 13, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.A.; Montgomery, S.E.; Gupta, R.B. Formation of itraconazole/l-malic acid cocrystals by gas antisolvent cocrystallization. Powder Technol. 2013, 236, 122–131. [Google Scholar] [CrossRef]
- Imchalee, R.; Charoenchaitrakool, M. Gas anti-solvent processing of a new sulfamethoxazole-l-malic acid cocrystal. J. Ind. Eng. Chem. 2015, 25, 12–15. [Google Scholar] [CrossRef]
- Tiago, J.M.; Padrela, L.; Rodrigues, M.A.; Matos, H.A.; Almeida, A.J.; de Azevedo, E.G. Single-Step Co-crystallization and Lipid Dispersion by Supercritical Enhanced Atomization. Cryst. Growth Des. 2013, 13, 4940–4947. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Tiago, J.; Velaga, S.P.; Matos, H.A.; de Azevedo, E.G. Tuning physicochemical properties of theophylline by cocrystallization using the supercritical fluid enhanced atomization technique. J. Supercrit. Fluids 2014, 86, 129–136. [Google Scholar] [CrossRef]
- Mullers, K.C.; Paisana, M.; Wahl, M.A. Simultaneous Formation and Micronization of Pharmaceutical Cocrystals by Rapid Expansion of Supercritical Solutions (RESS). Pharm. Res. 2015, 32, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.E.; Eriksson, L.; Westdahl, M. TREOR, a semi-exhaustive trial-and-error powder indexing program for all symmetries. J. Appl. Cryst. 1985, 18, 367–370. [Google Scholar] [CrossRef]
- Visser, J.W. A fully automatic program for finding the unit cell from powder data. J. Appl. Cryst. 1969, 2, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Zlokazov, V.B. MRIAAU—A program for autoindexing multiphase polycrystals. J. Appl. Cryst. 1992, 25, 69–72. [Google Scholar] [CrossRef]
- David, W.I.F.; Shankland, K.; van de Streek, J.; Pidcock, E.; Motherwell, W.D.S.; Cole, J.C. DASH: A program for crystal structure determination from powder diffraction data. J. Appl. Cryst. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- Bortolloti, M.; Lonardelli, I. ReX.Cell: A user-friendly program for powder diffraction indexing. J. Appl. Cryst. 2013, 46, 259–261. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Cryst. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. EXPO software for solving crystal structures by powder diffraction data: Methods and application. Cryst. Res. Technol. 2015. [Google Scholar] [CrossRef]
- International Union of Crystallography, Crystallographic Software List. Available online: https://www.iucr.org/resources/other-directories/software?result_42405_result_page=X (accessed on 10 May 2018).
- Sanphui, P.; Bolla, G.; Nangia, A.; Chernyshev, V. Acemetacin cocrystals and salts: Structure solution from powder X-ray data and form selection of the piperazine salt. IUCrJ 2014, 1, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Chadha, K.; Karan, M.; Bhalla, Y.; Chadha, R.; Khullar, S.; Mandal, S.; Vasisht, K. Cocrystals of Hesperetin: Structural, Pharmacokinetic, and Pharmacodynamic Evaluation. Cryst. Growth Des. 2017, 5, 2386–2405. [Google Scholar] [CrossRef]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friscic, T.; et al. Applying Hot-Stage Microscopy to Co-Crystal Screening: A Study of Nicotinamide with Seven Active Pharmaceutical Ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef] [Green Version]
- Leksic, E.; Pavlovic, G.; Mestrovic, E. Cocrystals of Lamotrigine Based on Coformers Involving Carbonyl Group Discovered by Hot-Stage Microscopy and DSC Screening. Cryst. Growth Des. 2012, 12, 1847–1858. [Google Scholar] [CrossRef]
- Lu, J.; Rohani, S. Preparation and Characterization of Theophylline−Nicotinamide Cocrystal. Cryst. Growth Des. 2009, 13, 1269–1275. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Xu, B.; Sun, B. A cocrystal strategy for the precipitation of liquid 2,3-dimethyl pyrazine with hydroxyl substituted benzoic acid and a Hirshfeld surfaces analysis of them. CrystEngComm 2012, 14, 6860–6868. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Polymorphism and phase transformations of a cocrystal of nicotinamide and pimelic acid. CrystEngComm 2012, 14, 8193–8198. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Dimorphs of a 1:1 cocrystal of ethenzamide and saccharin: Solid-state grinding methods result in metastable polymorph. CrystEngComm 2009, 11, 889–895. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Conformational and enantiotropic polymorphism of a 1:1 cocrystal involving ethenzamide and ehylmalonic acid. CrystEngComm 2010, 12, 3691–3697. [Google Scholar] [CrossRef]
- Zhang, S.; Rasmuson, A.C. Thermodynamics and Crystallization of the Theophylline−Glutaric Acid Cocrystal. Cryst. Growth Des. 2013, 13, 1153–1161. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, M.; Yang, L.; Li, X.; Cong, X.; Liu, S.; Cai, B. Fourier transform near-infrared spectroscopy used for purity determination of rhein-l-arginine cocrystal (argirein). Anal. Sci. 2013, 29, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Vogt, F.G.; Clawson, J.S.; Strohmeier, M.; Edwards, A.J.; Pham, T.N.; Watson, S.A. Solid-State NMR Analysis of Organic Cocrystals and Complexes. Cryst. Growth Des. 2009, 9, 921–937. [Google Scholar] [CrossRef]
- Maruyoshi, K.; Iuga, D.; Antzutkin, O.N.; Alhalaweh, A.; Velaga, S.P.; Brown, S.P. Identifying the intermolecular hydrogen-bonding supramolecular synthons in an indomethacin–nicotinamide cocrystal by solid-state NMR. Chem. Commun. 2012, 48, 10844–10846. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Matzger, A.J. Influence of Coformer Stoichiometric Ratio on Pharmaceutical Cocrystal Dissolution: Three Cocrystals of Carbamazepine/4-Aminobenzoic Acid. Mol. Pharm. 2016, 13, 990–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vangala, V.R.; Chow, P.S.; Schreyer, M.; Lau, G.; Tan, R.B.H. Thermal and in Situ X-ray Diffraction Analysis of a Dimorphic Co-Crystal, 1:1 Caffeine–Glutaric Acid. Cryst. Growth Des. 2016, 16, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Robeyns, K.; Leyssens, T. Crystallizing Ionic Cocrystals: Structural Characteristics, Thermal Behavior, and Crystallization Development of a Piracetam-CaCl2 Cocrystallization Process. Cryst. Growth Des. 2018, 18, 3215–3221. [Google Scholar] [CrossRef]
- Proffen, T.; Page, K.L.; McLain, S.E.; Clausen, B.; Darling, T.W.; Tencate, J.A.; Lee, S.Y.; Ustundag, E. Atomic pair distribution function analysis of materials containing crystalline and amorphous phases. Z. Kristallogr. 2005, 220, 1002–1008. [Google Scholar] [CrossRef]
- Charron, D.M.; Ajito, K.; Kim, J.; Ueno, Y. Chemical Mapping of Pharmaceutical Cocrystals Using Terahertz Spectroscopic Imaging. Anal. Chem. 2013, 85, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hisada, H.; Koide, T.; Carriere, J.; Heyler, R.; Fukami, T. In Situ Monitoring of Crystalline Transformation of Carbamazepine Using Probe-Type Low-Frequency Raman Spectroscopy. Org. Process Res. Dev. 2017, 21, 262–265. [Google Scholar] [CrossRef]
- Lee, M.; Chun, N.; Kim, M.; Kim, P.; Song, K.; Choi, G.J. In Situ Monitoring of Antisolvent Cocrystallization by Combining Near-Infrared and Raman Spectroscopies. Cryst. Growth Des. 2015, 15, 4385–4393. [Google Scholar] [CrossRef]
- Rodriguez-Hornedo, N.; Nehm, S.J.; Seefeldt, K.F.; Pagan-Torres, Y.; Falkiewicz, C.J. Reaction Crystallization of Pharmaceutical Molecular Complexes. Mol. Pharm. 2006, 3, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Yang, X.; Liang, R.; Zhao, J.; Li, S.; Yan, D. Molecular cocrystals of diphenyloxazole with tunable fluorescence, up-conversion emission and dielectric properties. CrystEngComm 2016, 18, 240–249. [Google Scholar] [CrossRef]
- Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM281764.pdf (accessed on 13 July 2018).
- Peltonen, L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-cocrystals in the pharmaceutical industry. Adv. Drug Deliv. Rev. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Devi, V.K.; Clara, D.; Malviya, N.; Ganguly, S.; Desiraju, G.R. Cocrystals of Hydrochlorothiazide: Solubility and Diffusion/Permeability Enhancements through Drug−Coformer Interactions. Mol. Pharm. 2015, 12, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Remenar, J.; MacPhee, M.; Lynn-Peterson, M.; Lynn-Morissette, S.; Almarsson, O. CIS-Itraconazole Crystalline Forms and Related Processes, Pharmaceutical Compositions and Methods. U.S. Patent US7078526B2, 18 July 2006. [Google Scholar]
- Zaworotko, M.J. Polymorphism in co-crystals. In Proceedings of the IQPC Pharmaceutical Cocrystals Conference, Amsterdam, The Netherlands, 26–27 September 2006. [Google Scholar]
- Glasstone, S. Textbook of Physical Chemistry; McMillan: London, UK, 1940; p. 389. [Google Scholar]
- Dunitz, J.D. Crystal and co-crystal: A second opinion. CrystEngComm 2003, 5, 506. [Google Scholar] [CrossRef]
- Lara-Ochoa, F.; Espinosa-Perez, G. Cocrystals Definitions. Supramol. Chem. 2007, 19, 553–557. [Google Scholar] [CrossRef]
- Bis, J.A.; Vishweshwar, P.; Weyna, D.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Hydroxyl⋯Pyridine Hydrogen Bonds in Cocrystals That Contain a Cyano Acceptor. Mol. Pharm. 2007, 4, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Kavuru, P.; Aboarayes, D.; Arora, K.K.; Clarke, H.D.; Kennedy, A.; Marshall, L.; Ong, T.T.; Perman, J.; Pujari, T.; Wojtas, L.; et al. Hierarchy of Supramolecular Synthons: Persistent Hydrogen Bonds between Carboxylates and Weakly Acidic Hydroxyl Moieties in Cocrystals of Zwitterions. Cryst. Growth Des. 2010, 10, 3568–3584. [Google Scholar] [CrossRef]
- Sarma, B.; Saikia, B. Hydrogen bond synthon competition in the stabilization of theophylline cocrystals. CrystEngComm 2014, 16, 4753–4765. [Google Scholar] [CrossRef]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Novick, S.E.; Janda, K.C.; Klemperer, W. HFClF: Structure and Bonding. J. Chem. Phys. 1976, 65, 5115–5121. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. Non-Conventional Hydrogen Bonds. Chem. Soc. Rev. 1998, 27, 163–170. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Choquesillo-Lazarte, D.; Nemec, V.; Cincic, D. Halogen bonded cocrystals of active pharmaceutical ingredients: Pyrazinamide, lidocaine and pentoxifylline in combination with haloperfluorinated compounds. CrystEngComm 2017, 19, 5293–5299. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Lampronti, G.I.; Maini, L.; Turrina, A. Ionic Co-crystals of Organic Molecules with Metal Halides: A New Prospect in the Solid Formulation of Active Pharmaceutical Ingredients. Cryst. Growth Des. 2011, 11, 5621–5627. [Google Scholar] [CrossRef]
- Perumalla, S.R.; Sun, C.C. Improved solid-state stability of salts by cocrystallization between conjugate acid–base pairs. CrystEngComm 2013, 15, 5756–5759. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Maini, L.; Prosperi, S.; Gobetto, R.; Chierotti, M.R. From unexpected reactions to a new family of ionic co-crystals: The case of barbituric acid with alkali bromides and caesium iodide. Chem. Commun. 2010, 46, 7715–7717. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Grepioni, F.; Maini, L.; Capucci, D.; Nanna, S.; Wouters, J.; Aerts, L.; Quere, L. Combining piracetam and lithium salts: Ionic co-crystals andco-drugs? Chem. Commun. 2012, 48, 8219–8221. [Google Scholar] [CrossRef] [PubMed]
- Grepioni, F.; Wouters, J.; Braga, D.; Nanna, S.; Fours, B.; Coquerel, G.; Longfils, G.; Rome, S.; Aerts, L.; Quere, L. Ionic co-crystals of racetams: Solid-state properties enhancement of neutral active pharmaceutical ingredients via addition of Mg2+ and Ca2+ chlorides. CrystEngComm 2014, 16, 5887–5896. [Google Scholar] [CrossRef]
- Smith, A.J.; Kim, S.; Duggirala, N.K.; Jin, J.; Wojtas, L.; Ehrhart, J.; Giunta, B.; Tan, J.; Zaworotko, M.J.; Shytle, R.D. Improving Lithium Therapeutics by Crystal Engineering of Novel Ionic Cocrystals. Mol. Pharm. 2013, 10, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
- Santra, R.; Ghosh, N.; Biradha, K. Crystal engineering with acid andpyridine heteromeric synthon: Neutral and ionic co-crystals. New J. Chem. 2008, 32, 1673–1676. [Google Scholar] [CrossRef]
- Buist, A.R.; Kennedy, A.R. Ionic Cocrystals of Pharmaceutical Compounds: Sodium Complexes of Carbamazepine. Cryst. Growth Des. 2014, 14, 6508–6513. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Desiraju, G.R. Using structural modularity in cocrystals to engineer properties: Elasticity. Chem. Commun. 2016, 52, 7676–7679. [Google Scholar] [CrossRef] [PubMed]
- Sangtani, E.; Sahu, S.K.; Thorat, S.H.; Gawade, R.L.; Jha, K.K.; Munshi, P.; Gonnade, R.G. Furosemide Cocrysals with Pyridines: An Interesting Case of Color Cocrystal Polymorphism. Cryst. Growth Des. 2015, 15, 5858–5872. [Google Scholar] [CrossRef]
- Sangtani, E.; Mandal, S.K.; Sreelakshmi, A.S.; Munshi, P.; Gonnade, R.G. Salts and Cocrystals of Furosemide wih Pyridines: Differences in π-Stacking and Color Polymorphism. Cryst. Growth Des. 2017, 17, 3071–3087. [Google Scholar] [CrossRef]
- Chalmers, M.J.; Wang, Y.; Novick, S.; Sato, M.; Bryant, H.U.; Montrose-Rafizdeh, C.; Griffin, P.R.; Dodge, J.A. Hydrophobic Interactions Improve Selectivity to ERα for Benzothiophene SERMs. ACS Med. Chem. Lett. 2012, 3, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Schamme, B.; Couvrat, N.; Tognetti, V.; Delbreilh, L.; Dupray, V.; Dargent, E.; Coquerel, G. Investigation of Drug–Excipient Interactions in Biclotymol Amorphous Solid Dispersions. Mol. Pharm. 2018, 15, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Song, Y.; Liu, Y.; Li, Y.; Wu, Z.; Yan, C. Drug-Bridge-Drug Ternary Cocrystallization Strategy for Antituberculosis Drugs Combination. Cryst. Growth Des. 2018, 18, 1283–1286. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Novel solid forms of the anti-tuberculosis drug: Ternary and polymorphic cocrystals. CrystEngComm 2013, 15, 5877–5887. [Google Scholar] [CrossRef]
- Mir, N.A.; Dubey, R.; Desiraju, G.R. Four- and five-component molecular solids: Crystal engineering strategies based on structural inequivalence. IUCrJ 2016, 3, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Adsmond, D.A.; Sinha, A.S.; Khandavilli, U.B.R.; Maguire, A.R.; Lawrence, S.E. Design and Synthesis of Ternary Cocrystals Using Carboxyphenols and Two Complementary Acceptor Compounds. Cryst. Growth Des. 2016, 16, 59–69. [Google Scholar] [CrossRef]
- Dubey, R.; Mir, N.A.; Desiraju, G.R. Quaternary cocrystals: Combinatorial synthetic strategies based on long-range synthon Aufbau modules (LSAM). IUCrJ 2016, 3, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Aakeroy, C.B.; Beatty, A.M.; Helfrich, B.A. “Total Synthesis” Supramolecular Style: Design and Hydrogen-Bond-Directed Assembly of Ternary Supermolecules. Angew. Chem. Int. Ed. 2001, 40, 3240–3242. [Google Scholar] [CrossRef] [Green Version]
- Bolla, G.; Nangia, A. Binary and ternary cocrystals of sulfa drug acetazolamide with pyridine carboxamides and cyclic amides. IUCrJ 2016, 3, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Tothadi, S.; Sanphui, P.; Desiraju, G.R. Obtaining Synthon Modularity in Ternary Cocrystals with Hydrogen Bonds and Halogen Bonds. Cryst. Growth Des. 2014, 14, 5293–5302. [Google Scholar] [CrossRef]
- Dauber, P.; Hagler, A.T. Crystal Packing, Hydrogen Bonding, and the Effect of Crystal Forces on Molecular Conformation. Acc. Chem. Res. 1980, 13, 105–112. [Google Scholar] [CrossRef]
- Kitaigorodskii, A. Organic Chemical Crystallography; Consultants Bureau: New York, NY, USA, 1961. [Google Scholar]
- Dubey, R.; Desiraju, G.R. Combinatorial Crystal Synthesis: Structural Landscape of Phloroglucinol:1,2-bis(4-pyridyl)ethylene and Phloroglucinol:Phenazine. Angew. Chem. Int. Ed. 2014, 53, 13178–13182. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Desiraju, G.R. Combinatorial selection of molecular conformations and supramolecular synthons in quercetin cocrystal landscapes: A route to ternary solids. IUCrJ 2015, 2, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pyrazinamide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/pyrazinamide#section=Solubility (accessed on 22 May 2018).
- Isoniazid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/isoniazid#section=Solubility (accessed on 22 May 2018).
- Wang, J.; Ye, C.; Zhu, B.; Zhou, C.; Mei, X. Pharmaceutical cocrystals of the anti-tuberculosis drug pyrazinamide with dicarboxylic and tricarboxylic acids. CrystEngComm 2015, 17, 747–752. [Google Scholar] [CrossRef]
- Sarcevica, I.; Orola, L.; Veidis, M.V.; Podjava, A.; Belyakov, S. Crystal and Molecular Structure and Stability of Isoniazid Cocrystals with Selected Carboxylic Acids. Cryst. Growth Des. 2013, 13, 1082–1090. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Nangia, A. Ternary and quaternary co-crystals of 1,3-cis,5-cis-cyclohexanetricarboxylic acid and 4,4′-bipyridines. New J. Chem. 2008, 32, 800–807. [Google Scholar] [CrossRef]
- Tilborg, A.; Leyssens, T.; Norberg, B.; Wouters, J. Structural Study of Prolinium/Fumaric Acid Zwitterionic Cocrystals: Focus on Hydrogen-Bonding Pattern Involving Zwitterionic (Ionic) Heterosynthons. Cryst. Growth Des. 2013, 13, 2373–2389. [Google Scholar] [CrossRef]
- Cheung, E.Y.; Kitchin, S.J.; Harris, K.D.M.; Imai, Y.; Tajima, N.; Kuroda, R. Direct Structure Determination of a Multicomponent Molecular Crystal Prepared by a Solid-State Grinding Procedure. J. Am. Chem. Soc. 2003, 125, 14658–14659. [Google Scholar] [CrossRef] [PubMed]
- Allu, S.; Bolla, G.; Tothadi, S.; Nangia, A. Supramolecular Synthons in Bumetanide Cocrystals and Ternary Products. Cryst. Growth Des. 2017, 17, 4225–4236. [Google Scholar] [CrossRef]
- ANDAs: Pharmaceutical Solid Polymorphism. Available online: https://www.fda.gov/downloads/Drugs/Guidances/UCM072866.pdf (accessed on 24 October 2017).
- Datta, S.; Grant, D.J.W. Crystal structures of drugs: Advances in determination, rediction and engineering. Nat. Rev. Drug Discov. 2004, 3, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Stephenson, G.A.; Mitchell, C.A.; Bunnell, C.A.; Snorek, S.V.; Bowyer, J.J.; Borchardt, T.B.; Stowell, J.G.; Byrn, S.R. Thermochemistry and Conformational polymorphism of a Hexamorphic Crystal System. J. Am. Chem. Soc. 2000, 122, 585–591. [Google Scholar] [CrossRef]
- Sun, C.; Grant, D.J.W. Influence of Crystal Structure on the Tableting Properties of Sulfamerazine Polymorphs. Pharm. Res. 2001, 18, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Desiraju, G.R. Synthon polymorphism and pseudopolymorphism in co-crystals. The 4,4′-bipyridine–4-hydroxybenzoic acid structural landscape. Chem. Commun. 2011, 47, 4090–4092. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Tothadi, S.; Chakraborty, S.; Ganguly, S.; Desiraju, G.R. Synthon identification in co-crystals and polymorphs with IR spectroscopy. Primary amides as a case study. CrystEngComm 2013, 15, 4640–4654. [Google Scholar] [CrossRef]
- Kaur, R.; Perumal, S.S.R.R.; Bhattacharyya, A.J.; Yashonath, S.; Row, T.N.G. Structural Insights into Proton Conduction in Gallic Acid–Isoniazid Cocrystals. Cryst. Growth Des. 2014, 14, 423–426. [Google Scholar] [CrossRef]
- Kaur, R.; Cherukuvada, S.; Managutti, P.B.; Row, T.N.G. A gallic acid–succinimide co-crystal landscape: Polymorphism, pseudopolymorphism, variable stoichiometry co-crystals and concomitant growth of non-solvated and solvated co-crystals. CrystEngComm 2016, 18, 3191–3203. [Google Scholar] [CrossRef]
- Ghosh, S.; Bag, P.P.; Reddy, C.M. Co-Crystals of Sulfamethazine with Some Carboxylic Acids and Amides: Co-Former Assisted Tautomerism in an Active Pharmaceutical Ingredient and Hydrogen Bond Competition Study. Cryst. Growth Des. 2011, 11, 3489–3503. [Google Scholar] [CrossRef]
- Sreekanth, B.R.; Vishweshwar, P.; Vyas, K. Supramolecular synthon polymorphism in 2:1 co-crystal of 4-hydroxybenzoic acid and 2,3,5,6-tetramethylpyrazine. Chem. Commun. 2007, 2375–2377. [Google Scholar] [CrossRef]
- Sanphui, P.; Babu, N.J.; Nangia, A. Temozolomide Cocrystals with Carboxamide Coformers. Cryst. Growth Des. 2013, 13, 2208–2219. [Google Scholar] [CrossRef]
- Goud, N.R.; Nangia, A. Synthon polymorphs of sulfacetamide–acetamidecocrystal based on N–H⋯O=S and N–H⋯O=C hydrogen bonding. CrystEngComm 2013, 15, 7456–7461. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Lu, T. Synthon polymorphs of 1:1 co-crystal of 5-fluorouracil and 4-hydroxybenzoic acid: Their relative stability and solvent polarity dependence of grinding outcomes. CrystEngComm 2014, 16, 6450–6458. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Polymorphs and solvates of a cocrystal involving an analgesic drug, ethenzamide, and 3,5-dinitrobenzoic acid. Cryst. Growth Des. 2010, 10, 2229–2238. [Google Scholar] [CrossRef]
- Ueto, T.; Takata, N.; Muroyama, N.; Nedu, A.; Sasaki, A.; Tanida, S.; Terada, K. Polymorphs and a Hydrate of Furosemide–Nicotinamide 1:1 Cocrystal. Cryst. Growth Des. 2012, 12, 485–494. [Google Scholar] [CrossRef]
- Tothadi, S.; Desiraju, G.R. Synthon Modularity in 4-Hydroxybenzamide–Dicarboxylic Acid Cocrystals. Cryst. Growth Des. 2012, 12, 6188–6198. [Google Scholar] [CrossRef]
- Porter, W.W., III; Elie, S.C.; Matzger, A.J. Polymorphism in Carbamazepine Cocrystals. Cryst. Growth Des. 2008, 8, 14–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, N.J.; Reddy, L.S.; Aitipamula, S.; Nangia, A. Polymorphs and polymorphic cocrystals of temozolomide. Chem. Asian J. 2008, 3, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guzei, I.A.; de Villiers, M.M.; Yu, L.; Krzyzaniak, J.F. Formation Enthalpies and Polymorphs of Nicotinamide–R-Mandelic Acid Co-Crystals. Cryst. Growth Des. 2012, 12, 4090–4097. [Google Scholar] [CrossRef]
- Nanubolu, J.B.; Ravikumar, K. Designing a new cocrystal of olanzapine drug and observation of concomitant polymorphism in a ternary cocrystal system. CrystEngComm 2017, 19, 355–366. [Google Scholar] [CrossRef]
- Shattock, T.R.; Vishweshwar, P.; Wang, Z.; Zaworotko, M.J. 18-Fold Interpretation and Concomitant Polymorphism in the 2:3 Co-Crystal of Trimesic Acid and 1,2-bis(4-pyridyl)ethane. Cryst. Growth Des. 2005, 5, 2046–2049. [Google Scholar] [CrossRef]
- Bis, J.A.; Vishweshwar, P.; Middleton, R.A.; Zaworotko, M.J. Concomitant and Conformational Polymorphism, Conformational Isomorphism, and Phase Relationships in 4-Cyanopyridine-4,4′-biphenol Cocrystals. Cryst. Growth Des. 2006, 6, 1048–1053. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Pharmaceutical Cocrystallization: Engineering a Remedy for Caffeine Hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Bolla, G.; Mittapalli, S.; Nangia, A. Celecoxib cocrystal polymorphs with cyclic amides: Synthons of a sulfonamide drug with carboxamide coformers. CrystEngComm 2014, 16, 24–27. [Google Scholar] [CrossRef]
- Skovsgaard, S.; Bond, A.D. Co-crystallisationofbenzoic acid derivatives with N-containing bases in solution and by mechanicalgrinding: Stoichiometric variants, polymorphism and twinning. CrystEngComm 2009, 11, 444–453. [Google Scholar] [CrossRef]
- Tothadi, S. Polymorphism in cocrystals of urea: 4,4′-bipyridine and salicylic acid: 4,4′-bipyridine. CrystEngComm 2014, 16, 7587–7597. [Google Scholar] [CrossRef]
- Surov, A.O.; Manin, A.N.; Voronin, A.P.; Churakov, A.V.; Perlovich, G.L.; Vener, M.V. Weak Interactions Cause Packing Polymorphism in Pharmaceutical Two-Component Crystals. The Case Study of the Salicylamide Cocrystal. Cryst. Growth Des. 2017, 17, 1425–1437. [Google Scholar] [CrossRef]
- CSD; Version 5.34, ConQuest 1.15; Cambridge Crystallographic Data Centre: Cambridge, UK, 2012; February 2013 Update.
- Mukherjee, A.; Desiraju, G.R. Combinatorial Exploration of the Structural Landscape of Acid−Pyridine Cocrystals. Cryst. Growth Des. 2014, 14, 1375–1385. [Google Scholar] [CrossRef]
- Pagire, S.K.; Jadav, N.; Vangala, V.R.; Whiteside, B.; Paradkar, A. Thermodynamic Investigation of Carbamazepine-SaccharinCo-crystal Polymorphs. J. Pharm. Sci. 2017, 106, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Heidrich, A.; Greiser, A.; Benemann, S.; Rademann, K.; Emmerling, F. Polymorphism of Mechanochemically Synthesized Cocrystals: A Case Study. Cryst. Growth Des. 2016, 16, 1701–1707. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Scott, R.L. The Solubility of Nonelectrolytes; Reinhold Publishing: New York, NY, USA, 1950. [Google Scholar]
- Rao, V.M.; Sanghvi, R.; Zhu, H. Solubility of pharmaceutical solids. In Developing Solid Oral Dosage Forms. Pharmaceutical Theory and Practice; Qiu, Y., Chen, Y., Zhang, G.G.Z., Eds.; Academic Press: New York, NY, USA, 2009; pp. 3–24. [Google Scholar]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodriguez-Hornedo, N. Pharmaceutical cocrystals and poorly water soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D.; Conradi, R.A. Predictive relationships in the water solubility of salts of a nonsteroidal anti-inflammatory drug. J. Pharm. Sci. 1985, 74, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, C.; Andre, V.; Reddy, S.; Roy, L.; Duarte, T.; Rodriguez-Hornedo, N. Tailoring aqueous solubility of a highly soluble compound via cocrystallization: Effect of coformer ionization, pHmax and solute-solvent interactions. CrystEngComm 2012, 14, 4801–4811. [Google Scholar] [CrossRef]
- Kuminek, G.; Cao, F.; Rocha, A.B.O.; Cardoso, S.G.; Rodriguez-Hornedo, N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipert, M.P.; Rodriguez-Hornedo, N. Cocrystal Transition Points: Role of Cocrystal Solubility, Drug Solubility, and Solubilizing Agents. Mol. Pharm. 2015, 12, 3535–3546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.M.; Rodriguez-Hornedo, N. Cocrystals Mitigate Negative Effects of High pH on Solubility and Dissolution of a Basic Drug. Cryst. Growth Des. 2018, 18, 1358–1366. [Google Scholar] [CrossRef]
- Remenar, J.F.; Morissette, S.L.; Peterson, M.L.; Moulton, B.; MacPhee, J.M.; Guzman, H.R.; Almarsson, O. Crystal engineering of novel cocrystals of a triazole drug with 1,4-dicarboxylic acids. J. Am. Chem. Soc. 2003, 125, 8456–8457. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.S.; Bethune, S.J.; Kampf, J.W.; Rodriguez-Hornedo, N. Cocrystals and salts of gabapentin: pH dependent cocrystal stability and solubility. Cryst. Growth Des. 2009, 9, 378–385. [Google Scholar] [CrossRef]
- Shikhar, A.; Bommana, M.M.; Gupta, S.S.; Squillante, E. Formulation development of Carbamazepine–Nicotinamide co-crystals complexed with -cyclodextrin using supercritical fluid process. J. Supercrit. Fluids 2011, 55, 1070–1078. [Google Scholar] [CrossRef]
- Guzman, H.R.; Tawa, M.; Zhang, Z.; Ratanabanangkoon, P.; Shaw, P.; Gardner, C.L.; Chen, H.; Moreau, J.-P.; Almarsson, O.; Remenar, J.F. Combined use of crystalline salt forms and precipitation inhibitors to improve oral absorption of celecoxib from solid oral formulations. J. Pharm. Sci. 2007, 96, 2686–2702. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, M. Role of Solvents in Improvement of Dissolution Rate of Drugs: Crystal Habit and Crystal Agglomeration. Adv. Pharm. Bull. 2015, 5, 13–18. [Google Scholar] [PubMed]
- Serrano, D.R.; O’Connell, P.; Paluch, K.J.; Walsh, D.; Healy, A.M. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J. Pharm. Pharmacol. 2016, 68, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Amidon, G.L.; Rodriguez-Hornedo, N.; Amidon, G.E. Mechanistic Analysis of Cocrystal Dissolution as a Function of pH and Micellar Solubilization. Mol. Pharm. 2016, 13, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.R.; Khan, R.A.; Nangia, A. Modulating the solubility of sulfacetamide by means of cocrystals. CrystEngComm 2014, 16, 5859–5869. [Google Scholar] [CrossRef]
- Gately, S.T.; Triezenberg, S.J. Solid Forms of Curcumin. U.S. Patent WO 2012138907A2, 11 October 2012. [Google Scholar]
- Kaur, R.; Cavanagh, K.L.; Rodriguez-Hornedo, N.; Matzger, A.J. Multidrug Cocrystal of Anticonvulsants; Influence of Strong Intermolecular Interactions on Physicochemical Properties. Cryst. Growth Des. 2017, 17, 5012–5016. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Ferretti, V.; Bertolasi, V.; Pavan, B.; Monari, A.; Pastore, M. From Physical Mixtures to Co-Crystals: How the Coformers Can Modify Solubility and Biological Activity of Carbamazepine. Mol. Pharm. 2018, 15, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Arjmand, F.; Khan, R.A.; Alsalme, A.; Ahmad, M.; Tabassum, S. Biological evaluation of dinuclear copper complex/dichloroacetic acid cocrystal against human breast cancer: Design, synthesis, characterization, DFT studies and cytotoxicity assays. RSC Adv. 2017, 7, 47920–47932. [Google Scholar] [CrossRef]
- Saha, R.; Sengupta, S.; Dey, S.K.; Steele, I.M.; Bhattacharyya, A.; Biswas, S.; Kumar, S. A pharmaceutical cocrystal with potential anticancer activity. RSC Adv. 2014, 4, 49070–49078. [Google Scholar] [CrossRef]









































| Property | Eutectic Phase | Cocrystal | Solid Solution | Reference(s) |
|---|---|---|---|---|
| Structural similarity of the parent molecules | Similar or dissimilar | Similar or dissimilar | Similar | [55] |
| Isomorphous or non-isomorphous | Isomorphous/non-isomorphous | Isomorphous/non-isomorphous | Isomorphous | [34,55] |
| Melting point of the solid formed | Lower melting than the parent components | Mostly in between the melting points of parent molecules but may also be higher or lower than the parent molecules | Exhibits a solidus-liquidus melting behavior | [34,55] |
| Binary phase diagram | ‘V’-shaped curve | ‘W’-shaped curve | Unary phase diagram | [34,55] |
| Intermolecular interactions | Short-range and weaker non-covalent adhesive interactions | Stronger and non-covalent adhesive interactions (hydrogen bonding, halogen bonding, Π-Π interactions, etc.) | Stronger and non-covalent cohesive interactions | [34,55] |
| Arrangement of molecules in crystal lattice | Molecules are randomly arranged | Molecules are well organized and well-packed | Molecules are well organized | [34,55] |
| Predominant thermodynamic force of the system | Entropy | Enthalpy | Enthalpy | [34,55] |
| Crystal structure of the solid formed | No significant change from the parent components | Characterized by a new crystal phase formation and hence possess a new crystal structure | Characterized by a new crystal phase formation and therefore possess a new crystal structure | [34,55] |
| Thermodynamic stability of the solid formed | Less stable | More stable than eutectics | More stable than eutectics | [34] |
| Name of the API | Therapeutic Use of the API | Binary/Ternary System | Name of the Coformer | Stoichiometric Ratio of the Cocrystal | Preparation Method | Comments on Dissolution Behavior | Reference(s) |
|---|---|---|---|---|---|---|---|
| Fluoxetine Hydrochloride | Antidepressant drug | Binary | Benzoic acid, | 1:1 | Slow evaporation | Fluoxetine HCl-Succinic acid (2:1) and Fluoxetine HCl-Fumaric acid (2:1) cocrystals exhibited enhanced intrinsic dissolution rate than raw Fluoxetine HCl while Fluoxetine HCl-Benzoic acid (1:1) and Fluoxetine HCl-Fumaric acid (2:1) cocrystals showed lower powder dissolution rate than raw Fluoxetine HCl | [57] |
| Succinic acid, | 2:1 | ||||||
| Fumaric acid | 2:1 | ||||||
| 2-[4-(4-chloro-2-fluorophenoxy)phenyl]pyrimidine-4-carboxamide | Sodium channel blocker | Binary | Glutaric acid | 1:1 | Solution crystallization | The cocrystal phase showed 18 times increased intrinsic dissolution rate than the commercial API | [58] |
| AMG 517 | A transient receptor potential vanilloid 1 antagonist (TRPV1) | Binary | Benzoic acid, trans-cinnamic acid, 2,5-dihydroxybenzoic acid, glutaric acid, glycolic acid, trans-2-hexanoic acid, 2-hydroxycaproic acid, l(+)-lactic acid, sorbic acid, l(+)-tartaric acid | 1:1 | Slow cooling | The cocrystals showed enhanced dissolution in Fasted Simulated Intestinal Fluid (FaSIF) (characterized by bell-shaped profile) than raw AMG 517 | [59] |
| Acyclovir | A guanosine analogue antiviral drug | Binary | Fumaric acid, Glutaric acid | 1:1.5, 1:1 | Reaction crystallization method | Solubility and permeability of the cocrystals were higher than that of raw acyclovir | [60] |
| Tartaric acid | 1:1 | Solution crystallization and Solvent-drop grinding | Dissolution rate of the cocrystals were faster than anhydrous acyclovir | [61] | |||
| Carbamazepine | Anticonvulsant drug | Binary | Salicylic acid | 1:1 | Slurry method and High-Throughput Screening methods HTS Evaporative experiments, Sonic slurry experiments, Grinding experiments and Reaction crystallization Hot-Melt Extrusion Resonant Acoustic mixing Liquid-assisted grinding, slurry conversion and solution crystallization methods | Not reported | [62,63] |
| Maleic acid or maleinic acid, | 1:1 | Not reported | [64] | ||||
| Salicylic acid and few other carboxylic acids | 1:1 | The extruded cocrystals showed faster dissolution rates than the solvent-crystallized cocrystals and raw carbamazepine | [65] | ||||
| Saccharin | 1:1 | ||||||
| 4,4′-Bipyridine p-aminosalicylic acid | 1:1, 2:1:1 cocrystal hydrate and 2:1:1 cocrystal solvate with methanol as solvent | Not reported | [66] | ||||
| Carbamazepine- p-aminosalicylic acid (1:1) cocrystal showed enhanced dissolution than raw carbamazepine | [67] | ||||||
| Fenofibrate | Helps to lower cholesterol and fat level | Binary | Nicotinamide | 1:1 | Solution crystallization and Solvent-drop grinding | Cocrystals showed enhanced dissolution than raw nicotinamide | [68] |
| Agomelatine | Antidepressant | Binary | Urea, glycolic acid, isonicotinamide and methyl-4-hydroxybenzoate | 1:1 | Agomelatine-urea (1:1) and agomelatine-glycolic acid (1:1) cocrystal was prepared by solution crystallization whereas agomelatine-isonicotinamide (1:1) and agomelatine-methyl-4-hydroxybenzoate (1:1) cocrystal was prepared by melting and recrystallizing in solvent | Cocrystals showed enhanced powder dissolution rate than raw agomelatine | [69] |
| Benzamide | Neuroleptics and antipsychotics | Binary | Salicylic acid, 3,5-dinitrobenzoic acid, 3-nitrobenzoic acid and 4-hydroxy 3-nitrobenzoic acid | 1:1 | Solvent-assisted grinding and solvent evaporation | Not reported | [70] |
| Fluorocytosine | Antitumor agent | Binary | Adipic acid, succinic acid, terephthalic acid, benzoic acid and malic acid | 1:1 | Solution crystallization | Not reported | [71] |
| Curcumin | Anticancer, antimalarial and antibacterial compound | Binary | Resorcinol, Pyrogallol | 1:1 | Solution crystallization and liquid- | The cocrystals exhibited faster dissolution than raw curcumin. Curcumin-pyrogallol cocrystals showed enhanced dissolution than curcumin-resorcinol cocrystal and raw curcumin | [72] |
| Phloroglucinol | 1:1 | assisted grinding | Curcumin-phloroglucinol cocrystal showed lower dissolution than raw curcumin | [73] | |||
| Hydroxyquinol | 1:1 and 1:2 | Rotary evaporation method Solution crystallization and Solid-state grinding | Curcumin-hydroxyquinol cocrystals showed enhanced dissolution than raw curcumin. Curcumin-hydroxyquinol (1:2) cocrystal exhibited enhanced dissolution than curcumin-hydroxyquinol (1:1) cocrystal and raw curcumin | [24] | |||
| 4,4′-Bipyridine-N,N′-dioxide | 1:1 | Solution crystallization | Not reported | [74] | |||
| Indomethacin | Non-Steroidal Anti-Inflammatory Drug (NSAID) | Binary | Saccharin | 1:1 | Twin screw extrusion | Indomethacin-saccharin cocrystal showed enhanced dissolution raw indomethacin | [75] |
| API | Therapeutic Use of API | Coformers Studied | Binary/Ternary System | Mole Fraction of the Coformer | Preparation Method | Comments on Dissolution Behavior | Reference(s) |
|---|---|---|---|---|---|---|---|
| Curcumin (Form 1) | Anticancer, antimalarial and antibacterial compound | Nicotinamide, | Binary | 0.67 | Solid-State Grinding | All the eutectics showed enhanced dissolution than raw curcumin | [22] |
| Hydroquinone, | Binary | 0.5 | Solid-State Grinding | [22] | |||
| p-hydroxybenzoic acid, | Binary | 0.5 | Solid-State Grinding | [22] | |||
| Tartaric acid, | Binary | 0.5 | Solid-State Grinding | [22] | |||
| Ferulic acid, | Binary | 0.5 | Solid-State Grinding | [22] | |||
| Salicylic acid, | Binary | 0.67 | Solid-State Grinding | [24] | |||
| Suberic acid | Binary | 0.8 | Liquid-Assisted Grinding | Not reported | [25] | ||
| Pyrazinamide | Anti-tuberculosis drug | Isoniazid, | Binary | 0.5 | Solid-state grinding | Showed faster dissolution than raw pyrazinamide | [34] |
| Isoniazid + Fumaric acid, | Ternary | 0.5 | Solid-state grinding | ||||
| Isoniazid + Succinic acid, | Ternary | 0.5 | Solid-state grinding | ||||
| Succinamide | Nitrogen supplement in fresh water algae cultivation | Isonicotinamide, | Binary | 0.25, 0.5, 0.75 | Solid-state grinding | Not reported | [56] |
| Isoniazid, | Binary | 0.25, 0.5, 0.75 | Solid-state grinding | Not reported | |||
| Fluoxetine HCl | Binary | 0.25, 0.5, 0.75 | Solid-state grinding | Not reported | |||
| Succinamic acid | Antipruritic and anti-infective agent | Isoniazid, | Binary | 0.25, 0.5, 0.75 | Solid-state grinding | Not reported | [55] |
| Fluoxetine HCl | Binary | 0.25, 0.5, 0.75 | Solid-state grinding | Not reported | |||
| Hesperetin | Antioxidant, Anticancer and cardioprotective agent | Theophylline, | Binary | 0.4 | Solvent-drop-assisted solid-state grinding | Hesperetin eutectics showed 2 to 4 times faster dissolution than raw hesperetin | [79] |
| Adenine, | Binary | 0.67 | |||||
| Gallic acid, | Binary | 0.6 | |||||
| Theobromine | Binary | 2:1 | |||||
| Phenazone | Analgesic, NSAID drug | Phenylbutazone, | Binary | 0.550 | Not reported | Not reported | [80] |
| Phenacetin, | Binary | 0.420 | Not reported | Not reported | |||
| Urea | Binary | 0.5 | Not reported | Not reported | |||
| Sulfadiazine | Antibiotic | Trimethoprim | Binary | 0.737 | Not reported | Not reported | [80] |
| Aminophenazone | Analgesic, anti-inflammatory andantipyretic drug | 4-aminophenazone, | Binary | 0.5 | Not reported | Not reported | [80] |
| Phenacetin, | Binary | 0.320 | Not reported | Not reported | |||
| Phenylbutazone, | Binary | 0.5 | Not reported | Not reported | |||
| Phenazone, | Binary | 0.463 | Not reported | Not reported | |||
| Etofylline, | Binary | 0.142 | Not reported | Not reported | |||
| Acetanilide | Analgesic and antipyretic compound | Phenacitin | Binary | 0.337 | Not reported | Not reported | [80] |
| Phenacitin | NSAID | Phenobarbital | Binary | 0.340 | Not reported | Not reported | [80] |
| Paracetamol | Treatment of pain and fever | Phenobarbital | Binary | 0.450 | Not reported | Not reported | [80] |
| Aspirin | Treatment of pain, fever andinflammation | Phenobarbital | Binary | 0.327 | Not reported | Not reported | [80] |
| 2-3 parts glycerin or propylene glycol (w/v) | Binary | Not reported | Proper mixing by melting and followed by allowing to cool to room temperature | Not reported | [78] | ||
| Simvastatin | Binary | 66.6% w/w | Grinding | The eutectic mixture exhibited enhanced dissolution than raw drug | [81] | ||
| Sulfanilamide | Antibacterial agent | Phenylbutazone, | Binary | 0.867 | Not reported | Not reported | [80] |
| Benzocaine, | 0.867 | ||||||
| 4-aminobenzoic acid | 0.404 | ||||||
| Caffeine | CNS Stimulant | Sulfathiazole, | Binary | 0.601 | Not reported | Not reported | [80] |
| Paracetamol | 0.619 | ||||||
| Sulfathiazole | Antibiotic | Benzocaine, | Binary | 0.936 | Not reported | Not reported | [80] |
| 4-aminobenzoic acid | 0.574 | ||||||
| Khellin | Vasodilator | Sulfapyridine, | Binary | 0.296 | Not reported | Not reported | [80] |
| Nicotinic acid | 0.194 | ||||||
| 2-nitroaniline | - | 4-aminobenzoic acid | Binary | 0.052 | Not reported | Not reported | [80] |
| Estradiol benzoate | Hormonal therapeutic agent for menopausal symptoms | Estradiol phenyl propionate | Binary | 0.837 | Not reported | Not reported | [80] |
| Pyridoxine | Vitamin B6 | Isoniazid, Nicotinic acid | Binary | 0.2, 0.25 | Liquid-Assisted Grinding | Not reported | [82] |
| Irbesartan | Antihypertensive drug | Syringic acid | Binary | 50/50% w/w | Solid-state grinding | Irbesartan eutectic mixtures exhibited 2- to 3-fold enhancement in intrinsic dissolution rate | [83] |
| Nicotinic acid | Binary | 50/50% w/w | Solid-state grinding | ||||
| Ascorbic acid | Binary | 50/50% w/w | Solid-state grinding |
| Name of the API | Therapeutic Use of API | Binary/Ternary System | Excipient | Reference |
|---|---|---|---|---|
| Triiodoresorcinol (TIR) | Mycoses treatment | Binary | Triiodophloroglucinol (TIG-O) | [39] |
| Triiodophenol (TIP) | Disinfectant | Binary | o-Triiodoresorcinol (TIR-O) | [39] |
| Benzoic acid | Antibacterial agent | Binary | 4-Fluorobenzoic acid | [38,40] |
| Isoicotinamide | Treatment of pellagra (caused due to Niacin deficiency) | Ternary | Succinic acid and fumaric acid | [84] |
| API | Therapeutic Use of API | Coformer | Coamorphous Solid Stoichiometry | Preparation Method | Comments on Dissolution Behavior | Reference(s) |
|---|---|---|---|---|---|---|
| Curcumin (Form 1) | Anticancer compound | Artemisinin | 1:1 | Rotavaporization | The coamorphous solid exhibited 2.6 times faster dissolution than raw curcumin | [28] |
| Piperazine | 1:2 | Ethanol-assisted Grinding | Curcumin-piperazine coamorphous phase showed lower dissolution than raw curcumin at temperature above Tg of the coamorphous solid and exhibited higher dissolution than raw curcumin at a temperature below Tg | [29] | ||
| Folic Acid Dihydrate | 1:1 | Liquid-Assisted Grinding | The coamorphous phase showed 4 times higher dissolution than raw curcumin | [25] | ||
| Indomethacin | Non-steroidal Anti-Inflammatory Drug (NSAID) | Naproxen | 1:2, 1:1 and 2:1 (1:1 was the stable form) | Quench cooling | The coamorphous solids exhibited increased intrinsic dissolution rate and 1:1 form showed a synchronized release | [30] |
| Naproxen | Non-steroidal Anti-Inflammatory Drug (NSAID) | Tryptophan+Proline | 1:1:1 | Ball milling | Coamorphous phase showed increased intrinsic dissolution rate than the crystalline naproxen | [31] |
| Arginine+Proline | 1:1:1 | Ball milling | Coamorphous phase showed increased intrinsic dissolution rate than the crystalline naproxen | |||
| Atorvastatin calcium | Lipid-lowering agent and in treatment of cardiovascular diseases | Nicotinamide | 1:1 | Solvent evaporation | Exhibited increased intrinsic dissolution rate than the raw drug | [88] |
| Sulfathiazole | Short-acting sulfonamide antibiotic | l-Tartaric acid | 1:1 | Co-milling | Not reported | [32] |
| Citric acid | 1:1 | Co-milling | Not reported | |||
| Ibuprofen | NSAID | Nicotinamide | 50% wt./wt. | Loading of mixtures into nanopores of mesoporous silica microspheres | The nanoconfined coamorphous solid showed enhanced dissolution than raw ibuprofen | [33] |
| Computational Screening Technique | Investigated Drug-Coformer Pair | Comments | Reference |
|---|---|---|---|
| Lattice energy prediction | 6 cocrystals of caffeine, 8 cocrystals of succinic acid and 12 cocrystals of 4-aminobenzoic acid | Lattice energies of the cocrystals were compared with thesum of lattice energies of individual crystal components | [112] |
| Lattice energy calculation | Cocrystals of flavonoids | Flexcryst program was used to determine the relative stability of flavonoid cocrystals stored in Cambridge Structural Database (CSD) w.r.t pure crystals and a comparative analysis was made | [113] |
| Virtual screening tool based on gas phase molecular electrostatic potential surfaces (MEPS) | Diclofenac with 22 coformers, | SSIPs (surface site interaction points) and the interaction site pairing energies of different solid forms (ΔE) determines the stability of the solid forms and thereby enables ranking of Cocrystal Formers based on the calculated probability of cocrystal formation. | [116] |
| Piracetam with 29 coformers, | |||
| Pyrazine carboxamide with 45 coformers, | |||
| Acetazolamide with 36 coformers, | |||
| Indomethacin with 57 coformers, | |||
| a drug candidate with 28 coformers, | |||
| Furosemide with 28 coformers, | |||
| Nalidixic acid with 22 coformers and Paracetamol with 37 coformers | |||
| Virtual screening tool based on Functional group interaction energy calculations | Calculations were carried out for Nalidixic acid with 310 coformers | This methodology utilizes surface | [117] |
| 44 Successful pairs were chosen for experimental studies | site interaction points (SSIPs) calculated from the ab initio MEPS of the isolated molecule in the gas phase | ||
| Virtual screening tool based on gas phase MEPS | Spironolactone and Griseofulvin | Molecular electrostatic potential surfaces enable assession of molecular complementarity between two cocrystal components for determining the cocrystal forming ability | [118] |
| Virtual screening tool | Several coformers for combination with caffeine ad carbamazepine (identified from literature review) | Interaction site pairing energies of different solid forms were calculated w.r.t pure components and ranking is provided | [126] |
| Virtual cocrystal screening tool | Isonicotinamide with 97 different coformers | Miscibility affinities of liquid components under supercooledconditions were used as a key parameter to model the cocrystallization propensities | [127] |
| COSMO-RS (Conductor-like Screening Model for Real Solvents) | Tyrosine kinase inhibitor axitinib, thiophanate-methyl and thiophanate-ethyl benzimidazole fungicides were studied for their solubility behavior using COSMO-RS | Ranking is primarily based on the screening of coformers for API solubility improvement based on calculation of excess enthalpy, Hex, between an API-coformer mixture relative to the pure components | [122] |
| COSMO-RS (Conductor-like Screening Model for Real Solvents) | - | COSMO-RS enables screening of coformers having good solubility with suitable solvents for forming cocrystals | [128] |
| COSMO-RS | Wide range of pharmaceutical compounds | Virtual coformer screening based on the API-coformer miscibility was performed to screen coformerto produce cocrystals with good Relative Humidity (RH) stability | [123] |
| Prediction of Hansen Solubility Parameter | Indomethacin with thirty different coformers | Utilization of group contribution method | [121] |
| Calculation of energy difference | N,N′-Diphenylureas and Triphenylphosphine Oxide | Relative ΔΔEint between heterodimers and homodimers served as a good predictor of cocrystal formation in the investigated system | [129] |
| Free energy calculation | Pentoxifylline with coformers, aspirin, salicylic acid, and benzoic acid | Flexcrystprogram was used to calculatethe free energy of experimental and hypothetical crystal structures and then correlated with each other | [130] |
| In silico screening | Phenylpiperazine derivatives anddicarboxylic acids | Computed values for the mixing enthalpies and solubility advantage factors determine the cocrystallization propensities | [131] |
| Ab initio screening | Nicotinamide, isonicotinamide, picolinamide and two paracetamol cocrystals were screened for their stability | Lattice energy calculations | [114] |
| Heat of formation distribution of components | 492 pairs | Calculation of Heat of formation (Hf) values | [132] |
| Fabian approach | 974 cocrystal structures were investigated by Fabian approach | Calculation ofQuantitative Structure-Activity Relationship (QSAR) molecular descriptors of cocrystallizing components | [125] |
| Utilization ofcomputed crystal energy landscapes | Carbamazepine (CBZ)-Nicotinamide (NCT), | Computed crystal energy landscapes of the drug-coformer pairs were compared and analyzed with the binary and ternary phase diagrams | [124] |
| Carbamazepine (CBZ)-Isonicotinamide (INA), | |||
| Carbamazepine (CBZ)-Benzamide (BNZ) and Carbamazepine (CBZ)—Picolinamide (PA) | |||
| Knowledge-based approach | Meloxicam cocrystal and Artemisinin cocrystal (pairs reported in literature were used for the study) | Molecular complementarity, hydrogen bond motifs and multicomponent hydrogenbond propensities were compared and analyzed | [119] |
| Knowledge-based hydrogen bond prediction | Pyrimethamine drug with carbamazepine, theophylline, aspirin, α-ketoglutaric acid, saccharin, p-coumaric acid, succinimide and L-isoleucine as coformers | Hydrogen bond propensity calculations were carried out to detect the formation of new molecular adducts | [120] |
| Multistage lattice energy minimization methodology | Cocrystal of 4-aminobenzoic acid with 2,2′-bipyridine and 4-nitrophenylacetic acid as coformers | Involves three important steps: (1) Modelling of intermolecular electrostatic interactions with atomic charges, (2) Interpolation of deformation energies and (3) Enhancement of accuracy | [115] |
| Cocrystallization Technique (in Batch Mode) | Principle | Reference(s) |
|---|---|---|
| Solid-State Grinding (SSG)/Neat Grinding | Low molecular weight coformer molecules diffuse into the crystal lattice of API molecules, forming intermediate phases such as eutectic or amorphous phase which further lead to a new cocrystal phase | [151] |
| Liquid-Assisted Grinding (LAG) | Addition of solvent molecules during grinding of API-coformer mixture facilitates diffusion of low molecular weight coformer molecules into the crystal lattice of an API | [151] |
| Ion and Liquid-Assisted Grinding (ILAG) | Grinding of multiple components along with liquid phase facilitates diffusion of liquid phase into the solid-state thereby facilitating mobility of molecules and exposing the hidden molecules to the surface | [153] |
| Slow evaporation | Preparing a supersaturated solution and allowing solvent to evaporate slowly at ambient conditions induces primary nucleation, thereby leading to slow growth of crystals | [154] |
| Ultrasound-assisted cocrystallization | The cavitation energy of ultrasonic waves induces primary nucleation of particles and leads to attainment of supersaturation for cocrystal growth | [155] |
| Solvent-Mediated Phase Transformation (SMPT) | Keeping the binary mixture of API and coformer into a solvent/solvent mixture for a long-time enables phase transformation to a new phase (cocrystal) or conversion from one polymorphic form to another due to the activity of solvent molecules and their nature of interaction with solute molecules over a period | [163] |
| Cocrystal formation from moisture | Uptake of moisture by a solid mixture, dissolution of reactant molecules in the mixture, attaining supersaturation leading to cocrystal nucleation and growth are the primary steps involved in cocrystal generation from moisture | [159] |
| Liquid Anti-Solvent precipitation (LAS) | Supersaturation of molecules (drug + coformer mixture) is attained by mixing of solvent and anti-solvent. This results in increase in supersaturation and nucleation rate and thereby facilitates production of sub-micron cocrystal particles | [161] |
| Gas Anti-Solvent precipitation (GAS) | In GAS process, gaseous phase is used as anti-solvent instead of a liquid to obtain a cocrystal from a solution of API and coformer from a liquid solution | [162] |
| Type of Grinding | Mechanism of Solid Phase Formation | API | Therapeutic Use of the API | Coformer | Reference(s) |
|---|---|---|---|---|---|
| Co-milling | Diffusion of coformer molecules into benzoquinone crystal lattice | Benzoquinone | Antioxidant and anti-inflammatory compound | Diols | [173] |
| Solid-State Grinding/Neat grinding | Formation of submerged eutectic | Benzophenone | Antioxidant and anti-inflammatory compound | Diphenylamine | [152] |
| Solid-State Grinding/Neat grinding | Vapor diffusion of naphthalene molecules into the crystal lattice of picric acid | Picric acid | Antiseptic | Naphthalene | [167] |
| Solid-State Grinding/Neat grinding | Vapor diffusion of naphthalene molecules into the crystal lattice of picric acid | Picric acid | Antiseptic | Naphthalene | [168] |
| Solid-State Grinding/Neat grinding | Inclusion of water (from the reactant molecules) in the crystal generate a cocrystal hydrate | Theophylline hydrate | Chronic Obstructive Pulmonary Disease (COPD) and Asthma Treatment | Anhydrous citric acid | [151] |
| Solid-State Grinding/Neat grinding | Inclusion of water (from the reactant molecules) in the crystal lattice to generate a cocrystal hydrate | Anhydrous Theophylline | Chronic Obstructive Pulmonary Disease (COPD) and Asthma Treatment | Citric acid monohydrate | [151] |
| Solid-State Grinding/Neat grinding | Diffusion of anhydrous citric acid into the crystal lattice of anhydrous theophylline results in anhydrous cocrystal | Anhydrous Theophylline | Chronic Obstructive Pulmonary Disease (COPD) and Asthma Treatment | Anhydrous citric acid | [151] |
| Solid-State Grinding/Neat grinding | No solid-state reaction | Caffeine | Stimulant | Anhydrous Citric acid | [151] |
| Solid-State Grinding/Neat grinding | No solid-state reaction | Caffeine | Stimulant | Citric acid monohydrate | [151] |
| Solid-State Grinding/Neat grinding | The water molecules in the reactant molecule facilitated formation of ‘Caffeine citrate’ cocrystal on grinding | Caffeine hydrate | Stimulant | Anhydrous citric acid | [151] |
| Solid-State Grinding/Neat grinding | The water molecules in the reactant molecules facilitated formation of ‘Caffeine citrate’ cocrystal on grinding | Caffeine hydrate | Stimulant | Citric acid monohydrate | [151] |
| Solid-State Grinding/Neat grinding | Solvent vapors of acetone and methanol has catalytic effect on Caffeine-malonic acid cocrystal ground mixture and generates cocrystal | Caffeine | Stimulant | Malonic acid | [174] |
| Solid-State Grinding/Neat grinding | Inclusion of water (from the reactant molecules) in the crystal lattice to generate a cocrystal hydrate | Theophylline monohydrate | Chronic Obstructive Pulmonary Disease (COPD)and Asthma Treatment | Citric acid monohydrate | [151] |
| Liquid-assisted grinding | An intermediate amorphous solid phase having high molecular mobility facilitates cocrystallization | Piracetam | Neuroprotective and anticonvulsant drug | Tartaric acid, Citric acid | [171] |
| Liquid-assisted grinding with water | Inclusion of water as solvent during grinding induces cocrystal hydrate formation | Anhydrous Theophylline | Chronic Obstructive Pulmonary Disease (COPD) and Asthma Treatment | Anhydrous citric acid | [151] |
| Liquid-assisted grinding with water | Caffeine hydrate acted as an intermediate phase for cocrystal formation | Caffeine | Stimulant | Citric acid | [151] |
| Liquid-assisted grinding | ‘Caffeine citrate’ cocrystal formation | Caffeine | Stimulant | Citric acid | [151] |
| Production Method | Principle | API | Therapeutic Use of the API | Coformer | API-Coformer Stoichiometric Ratio | Comments on the Dissolution Behavior | Reference |
|---|---|---|---|---|---|---|---|
| Twin Screw Extrusion (TSE)/Melt extrusion (ME) processing /Hot-Melt Extrusion (HME) | Raw materials were fed through abarrel containing one or more rotary screws towards a die undercontrolled conditions. The frictional force created between thescrew and the barrel at high temperatures enables good mixing and melting of reactants, reduction in its particle size and thereby result into cocrystals | Indomethacin | Non-Steroidal Anti-Inflammatory Drug (NSAID) | Saccharin | 1:1 | During powder dissolution studies, 60–70% cocrystals dissolved in first 60 min w.r.t 30% of raw indomethacin | [75] |
| Caffeine | Stimulant | Oxalic acid | 2:1 | Not reported | [183] | ||
| Caffeine | Stimulant | Maleic acid | 1:1 and 2:1 | Not reported | [185] | ||
| Carbamazepine | Anticonvulsant | Saccharin | 1:1 | Not reported | [183] | ||
| Nicotinamide | Vitamin of B3 | Trans-cinnamic acid | 1:1 | Not reported | [183] | ||
| Theophylline | COPD and Asthma treatment | Citric acid | 1:1 | Not reported | [183] | ||
| AMG-517 | TRPV antagonist | Sorbic acid | 1:1 | Not reported | [180] | ||
| Carbamazepine | Anticonvulsant | Trans-cinnamic acid | 1:1 | The extruded cocrystals exhibited faster dissolution rates than raw carbamazepine and the cocrystals produced by conventional methods | [65] | ||
| Carbamazepine | Anticonvulsant | Saccharin | 1:1 | TSE processed cocrystals (at temperatures of 120 °C, 135 °C, and 140 °C) at different RPM (5 and 10 RPM) exhibited increased dissolution rates than raw carbamazepine | [65] | ||
| Ibuprofen | NSAID | Isonicotinamide with Xylitol as carrier | 1:1 | Hot-Melt Extruded cocrystals showed enhanced dissolution rates than raw ibuprofen | [184] | ||
| Solid-State Shear Milling (S3M) Technology | The applied shear force in S3M for milling along with the polymeric aid facilitates efficient milling due to generation of high stress fields for grinding | Carbamazepine | Anticonvulsant | Salicylic acid | 1:1 | Carbamazepine-salicylic acid cocrystals prepared by continuous process exhibited higher dissolution than the cocrystals prepared by batch process. Nearly 95% of drug was dissolved in case of cocrystal samples treated with PolyEthylene Oxide (PEO) and nearly 90% of drug was dissolved in case of cocrystal samples without PEO treatment | [188] |
| Spray Drying | A technique in which dry cocrystal powders are obtained from a solution or a suspension by evaporating the solvent very rapidly in a fraction of a second by passing a hot air stream | Carbamazepine | Anticonvulsant | Glutaric acid | 1:1 | Not reported | [186] |
| Theophylline | COPD and Asthma treatment | Nicotinamide | 1:1 | Not reported | [186] | ||
| Urea | Organic carbamide | Succinic acid | 1:1 | Not reported | [186] | ||
| Caffeine | Stimulant | Glutaric acid | 1:1 | Not reported | [186] | ||
| Caffeine | Stimulant | Oxalic acid | 2:1 | Not reported | [186] | ||
| Indomethacin | Non-Steroidal Anti-Inflammatory Drug (NSAID) | Nicotinamide | 1:1 | Not reported | [186] | ||
| Spray Flash evaporation process for preparation of nanococrystals | Subjecting the flashing superheated liquids to a sudden pressure drop followed by atomization in atomization chamber yields nano-sized cocrystals | Caffeine | Stimulant | Oxalic acid | 2:1 | Not reported | [187] |
| Caffeine | Stimulant | Glutaric acid | 1:1 | ||||
| TNT-20 | - | CL20 | 1:1 | ||||
| HMX | - | CL20 | 1:1 |
| API | Inorganic Ions Used | Counterions Used | Cocrystallization Process | Enhancement in Dissolution Rate | Reference(s) |
|---|---|---|---|---|---|
| Nicotinamide (NCT) | CaCl2 | Nil | Liquid-assisted grinding and slow evaporation in Ethanol | Exhibited lower dissolution than raw NCT | [248] |
| Piracetam (PRT) | CaCl2 | Nil | Liquid-assisted grinding and slow evaporation in Ethanol | Exhibited lower dissolution than raw PRT | [248] |
| Piracetam (PRT) | CaCl2 | Nil | Liquid-assisted grinding using Methanol and slow evaporation | Not reported | [223] |
| Barbituric acid (BBA) | KBr | Nil | Kneading, vapor digestion and crystallization in Methanol (MeOH) | Not reported | [250] |
| Barbituric acid (BBA) | LiBr | Nil | Grinding | Not reported | [250] |
| Barbituric acid (BBA) | NaBr | Nil | Kneading, vapor digestion and crystallization in MeOH | Not reported | [250] |
| Barbituric acid (BBA) | RbBr | Nil | Kneading, vapor digestion, crystallization in MeOH, grinding and crystallization in Ethanol (EtOH) | Exhibited higher dissolution than raw BA | [250] |
| Barbituric acid (BBA) | CsBr | Nil | Grinding and crystallization in EtOH | Exhibited higher dissolution than raw BA | [250] |
| Barbituric acid (BBA) | CsI | Nil | Grinding and crystallization in EtOH | Exhibited higher dissolution than raw BA | [250] |
| Brivaracetam (BRV) | MgCl2 6H2O and CaCl2 | Nil | Kneading and crystallization | Not reported | [252] |
| Seletracetam (SEL) | MgCl2 6H2O and CaCl2 | Nil | Kneading and crystallization | Not reported | [252] |
| l-Proline (PRO) | Lithium salicylate | Nil | Crystallization in deionized water | Not reported | [253] |
| l-Proline (PRO) | Lithium hydroxide | Nicotinic acid | Crystallization in deionized water | Not reported | [253] |
| Piracetam (PIR) | LiCl | Nil | Kneading at different RH conditions and crystallization | There was no significant difference in the Intrinsic Dissolution Rate (IDR) of raw PIR and the ionic cocrystal | [251] |
| Piracetam (PIR) | LiBr | Nil | Kneading and crystallization | There was no significant difference in the IDR of raw PIR and the ionic cocrystal | [251] |
| Trimesic acid (H3TMA) | 2,6-bis(4-pyridylmethylene)cyclohexanone | Nil | Crystallization | Not reported | [254] |
| Carbamazepine (CBZ) | Sodium iodide (NaI) | Acetyl chloride | Crystallization in methanol | Not reported | [255] |
| Sodium iodide (NaI) and Hydrobromic acid (HBr) | Acridinium I2X species | Crystallization in methanol | Not reported | [255] | |
| Benzoic acid (BA) | Phenoxy acetic acid | Nil | Slow evaporation technique | Not reported | [249] |
| API | Coformer | Solubility Trend w.r.t pH | Reference(s) | ||
|---|---|---|---|---|---|
| Name of the API | Ionizing Nature of API | Name of the Coformer | Ionizing Nature of the Coformer | ||
| Carbamazepine | Non-ionizable | Succinic acid | Diprotic acid | Increase in solubility | [64] |
| Carbamazepine | Non-ionizable | 4-aminobenzoic acid hydrate | Monoprotic acid | Increase in solubility | [63] |
| Ketoconazole | Weak basic | Adipic acid | Diprotic acid | U-shaped trend in which solubility reached minimum with an increase in pH | [319] |
| Ketoconazole | Weak basic | Fumaric acid | Diprotic acid | U-shaped trend in which solubility reached minimum with an increase in pH | [319] |
| Ketoconazole | Weak basic | Succinic acid | Diprotic acid | U-shaped trend in which solubility reached minimum with an increase in pH | [319] |
| Itraconazole | Basic | l-Tartaric acid | Acidic | U-shaped trend in which solubility reached minimum with an increase in pH (minimum solubilityoccurred in the pH range which is equivalent to the differencebetween two pKa values) | [320] |
| Gabapentin-lactam | Non-ionizable | Gentisic acid | Acidic | Increase in solubility | [316] |
| Gabapentin-lactam | Non-ionizable | Benzoic acid | Acidic | Increase in solubility | [316] |
| Gabapentin-lactam | Non-ionizable | 4-aminobenzoic acid | Monoprotic acid | U-shaped trend in which solubility reached minimum with an increase in pH | [316] |
| Gabapentin-lactam | Non-ionizable | 4-hydroxybenzoic acid | Acidic | Increase in solubility | [316] |
| Gabapentin-lactam | Non-ionizable | Fumaric acid | Diprotic acid | Increase in solubility | [316] |
| Gabapentin | Zwitterionic | 3-hydroxybenzoic acid | Acidic | U-shaped trend leading to increase in solubility | [321] |
| S. No | Cocrystal Polymorph | Preparation Method | Crystal Habit | Morphology | Dissolution Level in Deionized Water | Reference |
|---|---|---|---|---|---|---|
| 1 | I | Liquid-assisted co-milling | - | - | Highest | [325] |
| 2 | II | Solvent evaporation with Ethanol | I | Large prismatic crystals | Higher | |
| 3 | II | Solvent evaporation with Acetone | II | Large plate-like crystals | Higher | |
| 4 | II | Solvent evaporation with Ethanol followed by dry milling | III | Small cube-like crystals | Higher | |
| 5 | II | Spray drying | IV | Microspheres | Lower |
| API | Nature of the Solid Phase | Medical Use of the API | Coformer | API-Coformer Stoichiometric Ratio | Cocrystallization Technique | Pharmacokinetic/Pharmacodynamic/Bioavailability Studies | Reference(s) |
|---|---|---|---|---|---|---|---|
| 2-[4-(4-chloro-2-fluorophenoxy)phenyl]pyrimidine-4-carboxamide | Cocrystal | Sodium channel blocker | Glutaric acid | 1:1 | Solution crystallization | Oral administration of cocrystals and raw drug to dog indicated thatthe cocrystal increased plasma AUC (plasma Area-Under-the-Curve) values by three times than the raw drug | [58] |
| Curcumin (Form I) | Cocrystal | Anticancer agent | Pyrogallol | 1:1 | Liquid-assisted grinding | Showed improved pharmacokinetic profile than raw curcumin and did not show toxic effects even at 10 times higher concentrations (at 2000 mg/kg). Curcumin-pyrogallol cocrystal exhibited a bioavailability of 200 mg/kg oral dose in xenograft model | [89] |
| Curcumin (Form I) | Coamorphous solid | Anticancer agent | Artemisinin | 1:1 | Rotavaporization | The coamorphous phase exhibited greater dissolution and pharmacokinetic profile than raw curcumin. It also showed higher bioavailability and therapeutic effect than raw curcumin. The coamorphous solid was non-toxic even at a dose of 10 times higher dose at 2000 mg/kg in xenograft model | [89] |
| Hesperetin | Cocrystal | Antioxidant molecule | Picolinic acid | 1:1 | Liquid-assisted grinding and solvent evaporation | Showed 20% enhancement in antioxidant activity, 30% hemolysis decrement, 72% inflammation inhibition and exhibited relative bioavailability of 1.36 w.r.t raw hesperetin | [79] |
| Hesperetin | Cocrystal | Antioxidant molecule | Nicotinamide | 1:1 | Liquid-assisted grinding and solvent evaporation | Showed 30% enhancement in antioxidant activity, 40% hemolysis decrement, 79% inflammation inhibition and exhibited relative bioavailability of 1.57 w.r.t raw hesperetin | [79] |
| Hesperetin | Cocrystal | Antioxidant molecule | Caffeine | 1:1 | Liquid-assisted grinding and solvent evaporation | Showed 50% enhancement in antioxidant activity, 60% hemolysis decrement, 87% inflammation inhibition and exhibited relative bioavailability of 1.60 w.r.t raw hesperetin | [79] |
| Hesperetin | Eutectic | Antioxidant molecule | Theophylline | 1:1.5 | Liquid-assisted cogrinding | Showed 30% increment in antioxidant activity w.r.t raw hesperetin and exhibited 2 times greater anti-hemolytic activity than raw hesperetin | [79] |
| Hesperetin | Eutectic | Antioxidant molecule | Adenine | 2:1 | Liquid-assisted cogrinding | Showed decreased antioxidant activity w.r.t raw hesperetin and exhibited 1.5 times greater anti-hemolytic activity than raw hesperetin | [79] |
| Hesperetin | Eutectic | Antioxidant molecule | Gallic acid | 1.5:1 | Liquid-assisted cogrinding | Showed 50% increment in antioxidant activity w.r.t raw hesperetin and exhibited 2.5 times greater anti-hemolytic activity than raw hesperetin | [79] |
| Hesperetin | Eutectic | Antioxidant molecule | Theobromine | 2:1 | Liquid-assisted cogrinding | Showed 30% increment in antioxidant activity w.r.t raw hesperetin and exhibited 2 times greater anti-hemolytic activity than raw hesperetin | [79] |
| Carbamazepine | Cocrystal | Anticonvulsant | Vanillic acid | 1:1 | Slow evaporation | Molecular aggregates formed as a result ofthe dissolution of physical mixture reduced the integrity of intestinal cell monolayer ofNCM460 intestinal cells whereas the molecular aggregates which resulted from dissolution of cocrystal phase maintained the integrity of intestinal cell monolayer of NCM460 intestinal cells | [330] |
| Carbamazepine | Cocrystal | Anticonvulsant | 4-Nitropyridine-N-oxide | 1:1 | Slow evaporation | Molecular aggregates formed as a result of the dissolution of physical mixtureand cocrystal phase maintained the integrity of intestinal cell monolayer ofNCM460 intestinal cells | [330] |
| Carbamazepine | Cocrystal | Anticonvulsant | Succinic acid | 1:1 | Slow evaporation | Molecular aggregates formed as a result of the dissolution of physical mixture reduced the integrity of intestinal cell monolayer ofNCM460 intestinal cells whereas the molecular aggregates formed from dissolution of cocrystal phase maintained the integrity of intestinal cell monolayer ofNCM460 intestinal cells | [330] |
| Dichloroacetic acid | Cocrystal | Anticonvulsant | Cu2(valdien)2 | 1:1 | Controlled evaporation | Exhibited in vitro cytotoxicity on MCF-7 cancer cell lines | [331] |
| Quinoxaline | Cocrystal | Anticancer agent | Diacetylmonoxime and 3-thiosemicarbano-butan-2-oneoxime (TSBO) | 1:1:1 | Slow cooling of boiled solution | The cocrystal phase followed mitochondrial mediated cell death pathway in lung cancer cells, A549 by means of activating caspase 9 and Bax. It also exhibited anticancer activity on breast cancer (MCF-7) cell lines | [332] |
| Irbesartan | Eutectic | Antioxidant | Syringic acid | 1:1 | Solid-state grinding | In vivo pharmacokinetic profile of the irbesartan-syringic acid eutectic mixture showed 1.5-fold improvement w.r.t raw irbesartan | [84] |
| Irbesartan | Eutectic | Antioxidant | Nicotinic acid | 1:1 | Solid-state grinding | In vivo pharmacokinetic profile of the irbesartan-nicotinic acid eutectic mixture showed 1.6-fold improvement w.r.t raw irbesartan | [84] |
| Irbesartan | Eutectic | Antioxidant | Ascorbic acid | 1:1 | Solid-state grinding | In vivo pharmacokinetic profile of the irbesartan-ascorbic acid eutectic mixture showed 2-fold improvement w.r.t raw irbesartan | [84] |
| Atorvastatin calcium | Coamorphous solid | Lipid-lowering agent, for treatment of cardiovascular diseases | Nicotinamide | 1:1 | Solvent evaporation | Atorvastatin calcium-nicotinamide coamorphous phase showed improved pharmacokinetic profile in rats than raw atorvastatin calcium | [88] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. https://doi.org/10.3390/pharmaceutics10030108
Sathisaran I, Dalvi SV. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics. 2018; 10(3):108. https://doi.org/10.3390/pharmaceutics10030108
Chicago/Turabian StyleSathisaran, Indumathi, and Sameer Vishvanath Dalvi. 2018. "Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium" Pharmaceutics 10, no. 3: 108. https://doi.org/10.3390/pharmaceutics10030108