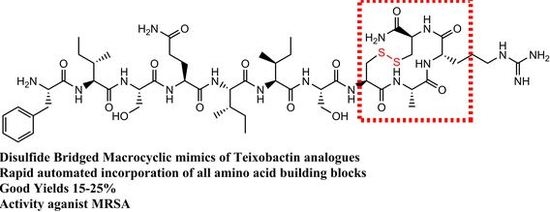
Cysteines and Disulfide-Bridged Macrocyclic Mimics of Teixobactin Analogues and Their Antibacterial Activity Evaluation against Methicillin-Resistant Staphylococcus Aureus (MRSA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Methods
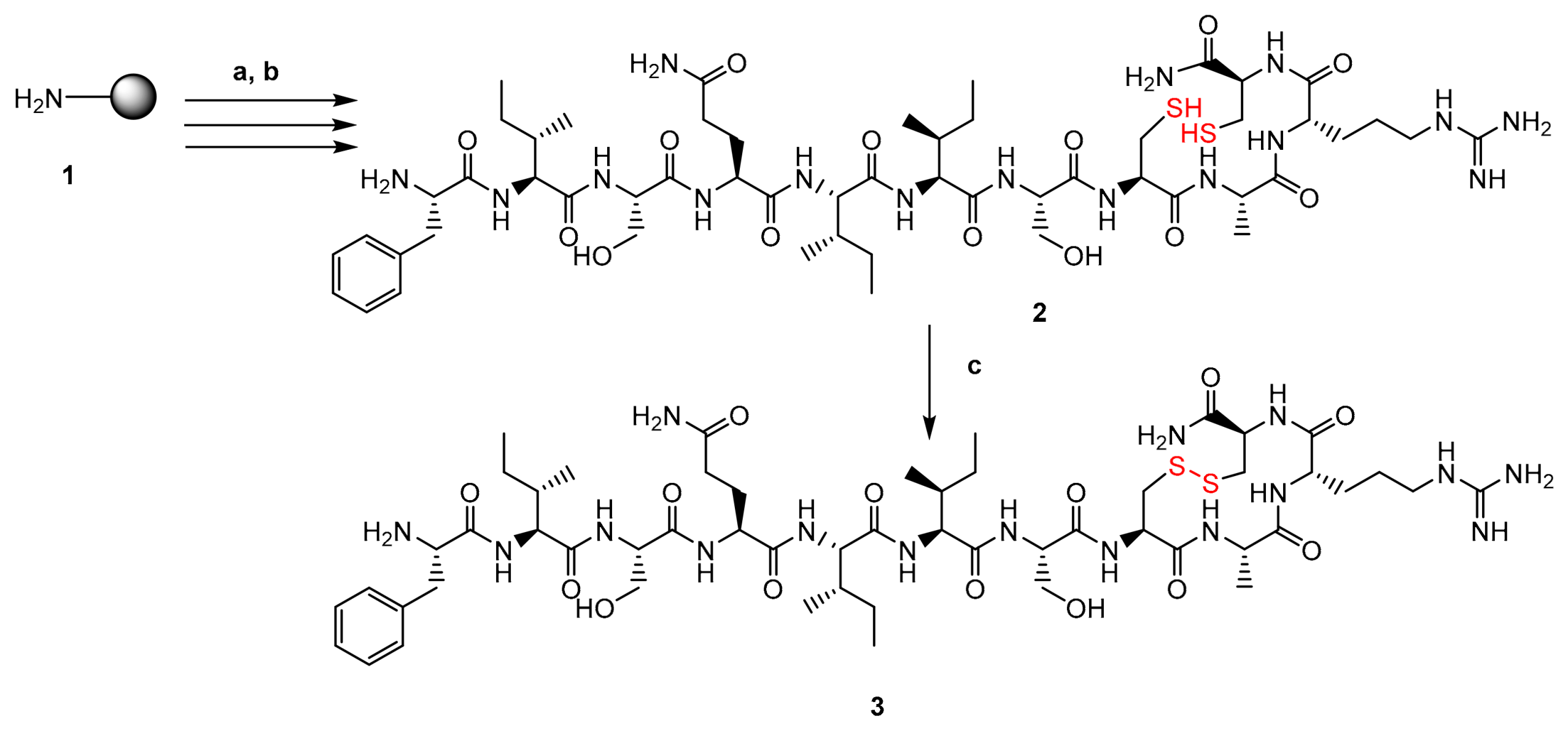
2.3. Synthesis of Teixobactin Analogue 3
2.4. Antibacterial Screening
3. Results and Discussion
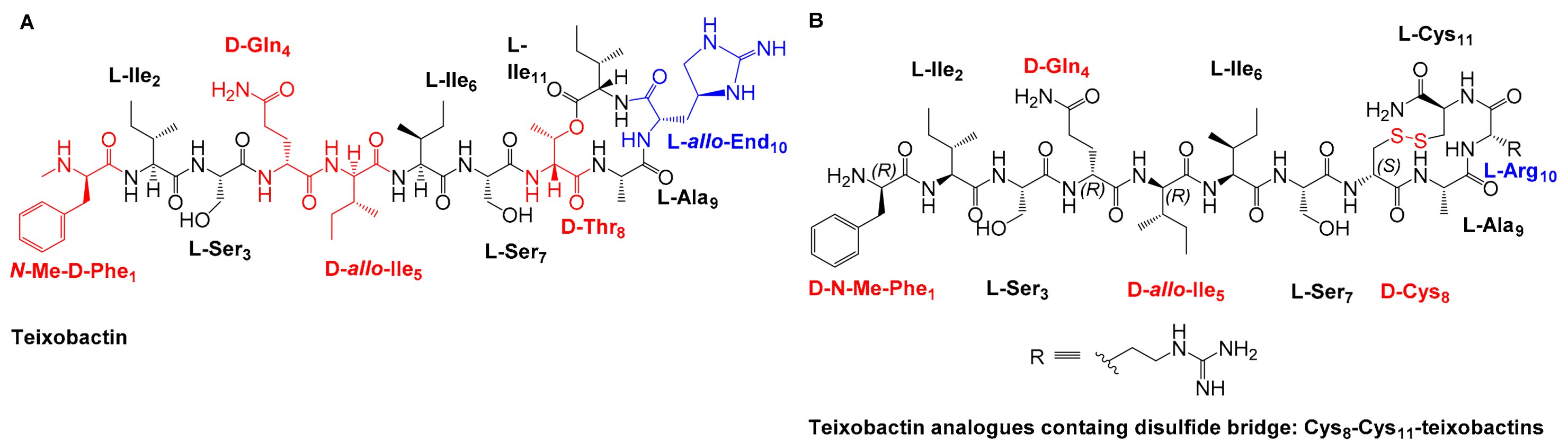
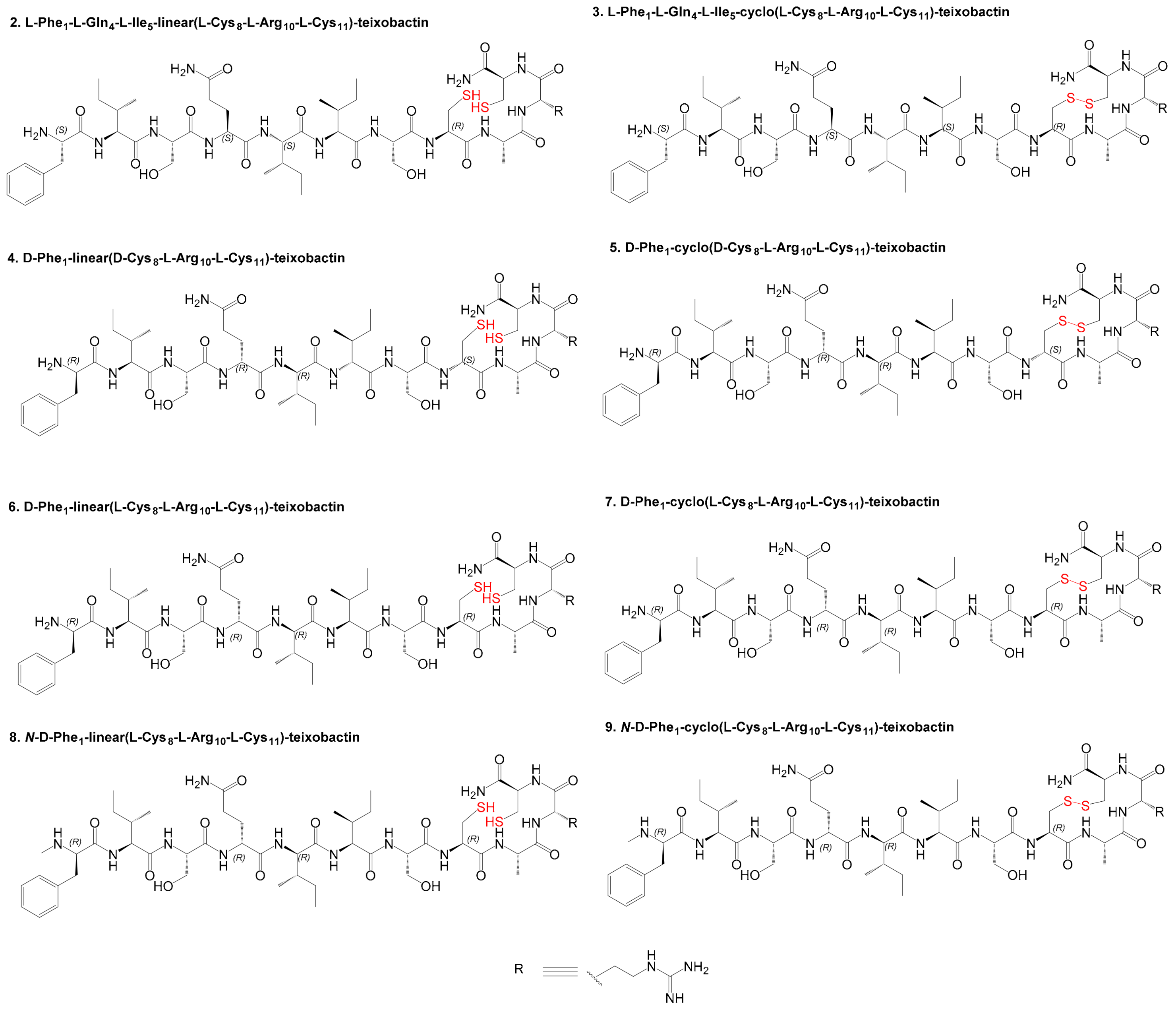
3.1. Design and Synthesis
3.2. Antibacterial Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Review on Antimicrobial Resistance. Available online: http://amr-review.org/ (accessed on 9 September 2018).
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Hughes, D.E.; Epstein, S.; Jones, M.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Giltrap, A.M.; Dowman, L.J.; Nagalingam, G.; Ochoa, J.L.; Linington, R.G.; Britton, W.J.; Payne, R.J. Total Synthesis of Teixobactin. Org. Lett. 2016, 18, 2788–2791. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Sam, I.H.; Po, K.H.L.; Lin, D.; Ghazvini Zadeh, E.H.; Chen, S.; Yuan, Y.; Li, X. Total Synthesis of Teixobactin. Nat. Commun. 2016, 7, 12394. [Google Scholar] [CrossRef] [PubMed]
- Jad, Y.E.; Acosta, G.A.; Naicker, T.; Ramtahal, M.; El-Faham, A.; Govender, T.; Kruger, H.G.; De La Torre, B.G.; Albericio, F. Synthesis and Biological Evaluation of a Teixobactin Analogue. Org. Lett. 2015, 17, 6182–6185. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Iyer, A.; Vincent, C.S.; Van Lysebetten, D.; Prior, S.H.; Madder, A.; Taylor, E.J.; Singh, I. Efficient Total Syntheses and Biological Activities of Two Teixobactin Analogues. Chem. Commun. 2016, 52, 6060–6063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Chen, K.H.; Nowick, J.S. Elucidation of the Teixobactin Pharmacophore. ACS Chem. Biol. 2016, 11, 1823–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, A.; Prior, S.H.; Iyer, A.; Vincent, C.S.; Van Lysebetten, D.; Breukink, E.; Madder, A.; Taylor, E.J.; Singh, I. Defining the Molecular Structure of Teixobactin Analogues and Understanding Their Role in Antibacterial Activities. Chem. Commun. 2017, 53, 2016–2019. [Google Scholar] [CrossRef] [PubMed]
- Abdel Monaim, S.A.H.; Jad, Y.E.; Ramchuran, E.J.; El-Faham, A.; Govender, T.; Kruger, H.G.; de la Torre, B.G.; Albericio, F. Lysine Scanning of Arg10—Teixobactin: Deciphering the Role of Hydrophobic and Hydrophilic Residues. ACS Omega 2016, 1, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Iyer, A.; Lloyd, D.G.; Vincent, C.S.; Prior, S.H.; Madder, A.; Taylor, E.J.; Singh, I. Syntheses of Potent Teixobactin Analogues against Methicillin-Resistant Staphylococcus Aureus (MRSA) through the Replacement of l-Allo-Enduracididine with Its Isosteres. Chem. Commun. 2017, 53, 7788–7791. [Google Scholar] [CrossRef] [PubMed]
- Fiers, W.D.; Craighead, M.; Singh, I. Teixobactin and Its Analogues: A New Hope in Antibiotic Discovery. ACS Infect. Dis. 2017, 3, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Iyer, A.; Prior, S.H.; Lloyd, D.G.; Leng, T.; Charlotte, S.; Palmai-Pallag, T.; Bachrati, C.Z.; Breukink, E.; Madder, A.; et al. Teixobactin Analogues Reveal Enduracididine to Be Non-Essential for Highly Potent Antibacterial Activity and Lipid II Binding. Chem. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, C.E.; Harris, P.W.R.; Ding, X.-B.; Krause, B.; Wright, T.H.; Cook, G.M.; Furkert, D.P.; Brimble, M.A. Synthesis and Biological Evaluation of Novel Teixobactin Analogues. Org. Biomol. Chem. 2017, 15, 8755–8760. [Google Scholar] [CrossRef] [PubMed]
- Girt, G.C.; Mahindra, A.; Al Jabri, Z.J.H.; De Ste Croix, M.; Oggioni, M.R.; Jamieson, A.G. Lipopeptidomimetics Derived from Teixobactin Have Potent Antibacterial Activity against Staphylococcus Aureus. Chem. Commun. 2018, 54, 2767–2770. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Lakshminarayanan, R.; Iyer, A.; Mayandi, V.; Leng Goh, E.T.; Lloyd, D.G.; Chalasani, M.L.S.; Verma, N.K.; Prior, S.H.; Beuerman, R.W.; et al. Design and Syntheses of Highly Potent Teixobactin Analogues against Staphylococcus Aureus, Methicillin-Resistant Staphylococcus Aureus (MRSA), and Vancomycin-Resistant Enterococci (VRE) in Vitro and in Vivo. J. Med. Chem. 2018, 61, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Haag, A.F.; Kerscher, B.; Dall’Angelo, S.; Sani, M.; Longhi, R.; Baloban, M.; Wilson, H.M.; Mergaert, P.; Zanda, M.; Ferguson, G.P. Role of Cysteine Residues and Disulfide Bonds in the Activity of a Legume Root Nodule-Specific, Cysteine-Rich Peptide. J. Biol. Chem. 2012, 287, 10791–10798. [Google Scholar] [CrossRef] [PubMed]
- Ehret-sabatier, L.; Loew, D.; Fehlbaum, P.; Jules, A.H.; Dorsselaer, A.V.; Goyffon, M.; Bulet, P. Protein Chemistry and Structure: Characterization of Novel Cysteine-Rich Antimicrobial Peptides from Scorpion Blood Characterization of Novel Cysteine-Rich Antimicrobial Peptides from Scorpion Blood. J. Biol. Chem. 1996, 271, 29537–29544. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wang, W.; Yang, X.; Yan, X.; Liu, R. A Novel Cysteine-Rich Antimicrobial Peptide from the Mucus of the Snail of Achatina Fulica. Peptides 2013, 39, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.; Roussel, J.; Letellierii, L.; Hetru, C.; Hoffmann, J.A. Structure-Activity Analysis of Thanatin, a 21-Residue Inducible Insect Defense Peptide with Sequence Homology to Frog Skin Antimicrobial Peptides. Biochemistry 1996, 93, 1221–1225. [Google Scholar] [CrossRef]
- Wu, Z.; Hoover, D.M.; Yang, D.; Boulegue, C.; Santamaria, F.; Oppenheim, J.J.; Lubkowski, J.; Lu, W. Engineering Disulfide Bridges to Dissect Antimicrobial and Chemotactic Activities of Human -Defensin 3. Proc. Natl. Acad. Sci. USA 2003, 100, 8880–8885. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.; Indrevoll, B. Regioselective Formation, Using Orthogonal Cysteine Protection, of an Alpha-Conotoxin Dimer Peptide Containing Four Disulfide Bonds. Org. Lett. 2003, 5, 2955–2957. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Du Bois, D.R.; Ziller, J.W.; Nowick, J.S. X-Ray Crystallographic Structure of a Teixobactin Analogue Reveals Key Interactions of the Teixobactin Pharmacophore. Chem. Commun. 2017, 53, 2772–2775. [Google Scholar] [CrossRef] [PubMed]
| Compound No. | MRSA ATCC 33591 | MRSA ATCC 700699 | S. aureus ATCC 29213 |
|---|---|---|---|
| 2 | 128 | >128 | >128 |
| 3 | 32–64 | >128 | >128 |
| 4 | >128 | >128 | >128 |
| 5 | >128 | >128 | >128 |
| 6 | >128 | - | >128 |
| 7 | >128 | - | >128 |
| 8 | >128 | - | >128 |
| 9 | >128 | - | >128 |
| Vancomycin | - | 2 | 4 |
| Leu10-teixobactin12 | 0.25 | 0.25 | 0.062 |
| Teixobactin12 | 0.25 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkawi, R.; Iyer, A.; Parmar, A.; Lloyd, D.G.; Leng Goh, E.T.; Taylor, E.J.; Sarmad, S.; Madder, A.; Lakshminarayanan, R.; Singh, I. Cysteines and Disulfide-Bridged Macrocyclic Mimics of Teixobactin Analogues and Their Antibacterial Activity Evaluation against Methicillin-Resistant Staphylococcus Aureus (MRSA). Pharmaceutics 2018, 10, 183. https://doi.org/10.3390/pharmaceutics10040183
Malkawi R, Iyer A, Parmar A, Lloyd DG, Leng Goh ET, Taylor EJ, Sarmad S, Madder A, Lakshminarayanan R, Singh I. Cysteines and Disulfide-Bridged Macrocyclic Mimics of Teixobactin Analogues and Their Antibacterial Activity Evaluation against Methicillin-Resistant Staphylococcus Aureus (MRSA). Pharmaceutics. 2018; 10(4):183. https://doi.org/10.3390/pharmaceutics10040183
Chicago/Turabian StyleMalkawi, Ruba, Abhishek Iyer, Anish Parmar, Daniel G. Lloyd, Eunice Tze Leng Goh, Edward J. Taylor, Sarir Sarmad, Annemieke Madder, Rajamani Lakshminarayanan, and Ishwar Singh. 2018. "Cysteines and Disulfide-Bridged Macrocyclic Mimics of Teixobactin Analogues and Their Antibacterial Activity Evaluation against Methicillin-Resistant Staphylococcus Aureus (MRSA)" Pharmaceutics 10, no. 4: 183. https://doi.org/10.3390/pharmaceutics10040183
APA StyleMalkawi, R., Iyer, A., Parmar, A., Lloyd, D. G., Leng Goh, E. T., Taylor, E. J., Sarmad, S., Madder, A., Lakshminarayanan, R., & Singh, I. (2018). Cysteines and Disulfide-Bridged Macrocyclic Mimics of Teixobactin Analogues and Their Antibacterial Activity Evaluation against Methicillin-Resistant Staphylococcus Aureus (MRSA). Pharmaceutics, 10(4), 183. https://doi.org/10.3390/pharmaceutics10040183