Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications
Abstract
:1. Introduction
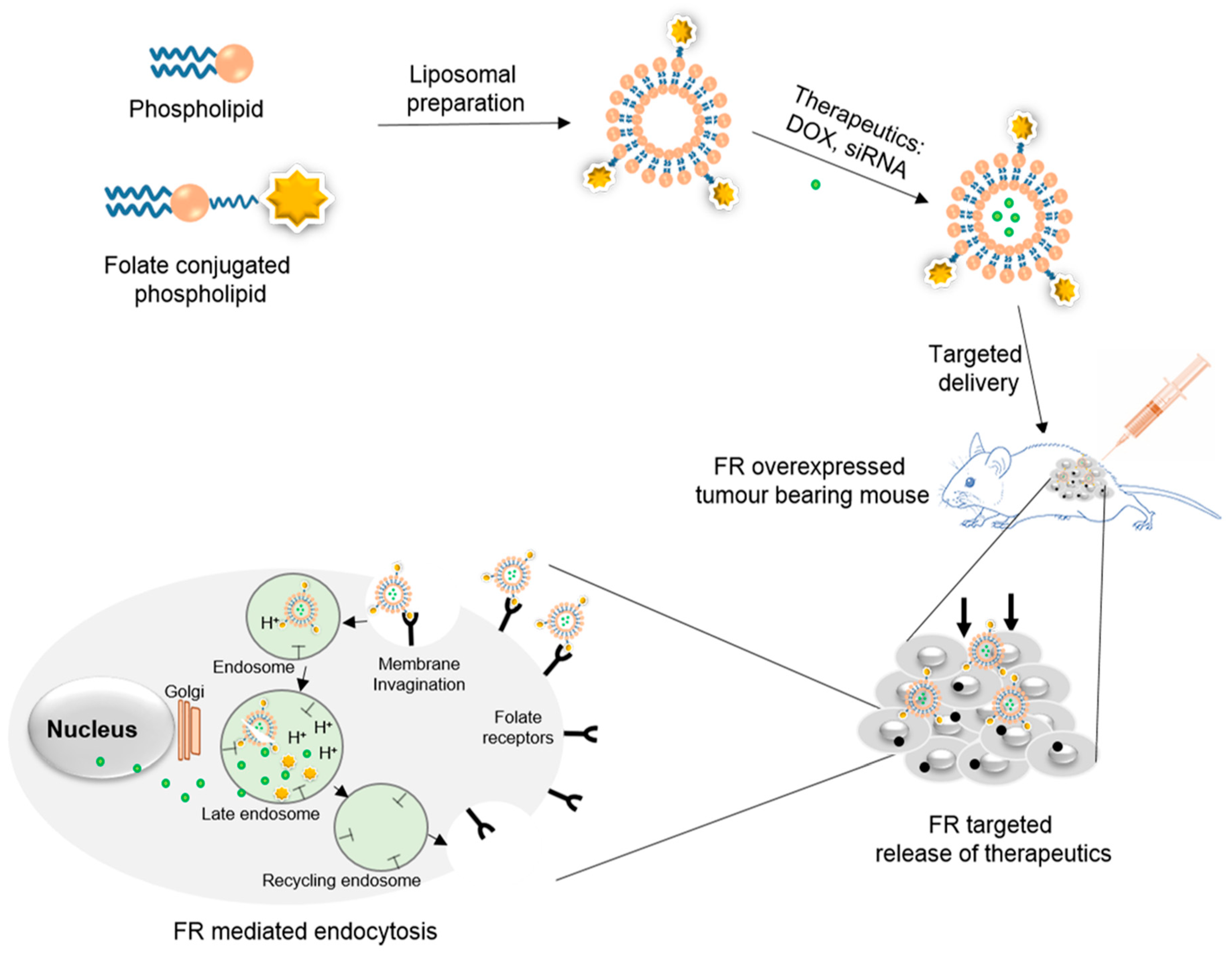
2. Design and Fabrication of Folate-Conjugated Liposomes
2.1. Design and Fabrication of Folate-Conjugated Phospholipids
2.1.1. Un-Cleavable Folate-Conjugated Phospholipids
2.1.2. Acid-Cleavable Folate-Conjugated Phospholipids
2.2. Design and Fabrication of Folate-Conjugated Cholesterol Derivative
2.3. Folate-Conjugated Proteins
3. Biomedical Applications of Folate-Conjugated Liposomes
3.1. Folate-Targeted Liposomes for Anticancer Drug Delivery (Small Hydrophilic/Hydrophobic Molecules)
3.2. Folate-Targeted Liposomes for Gene Delivery
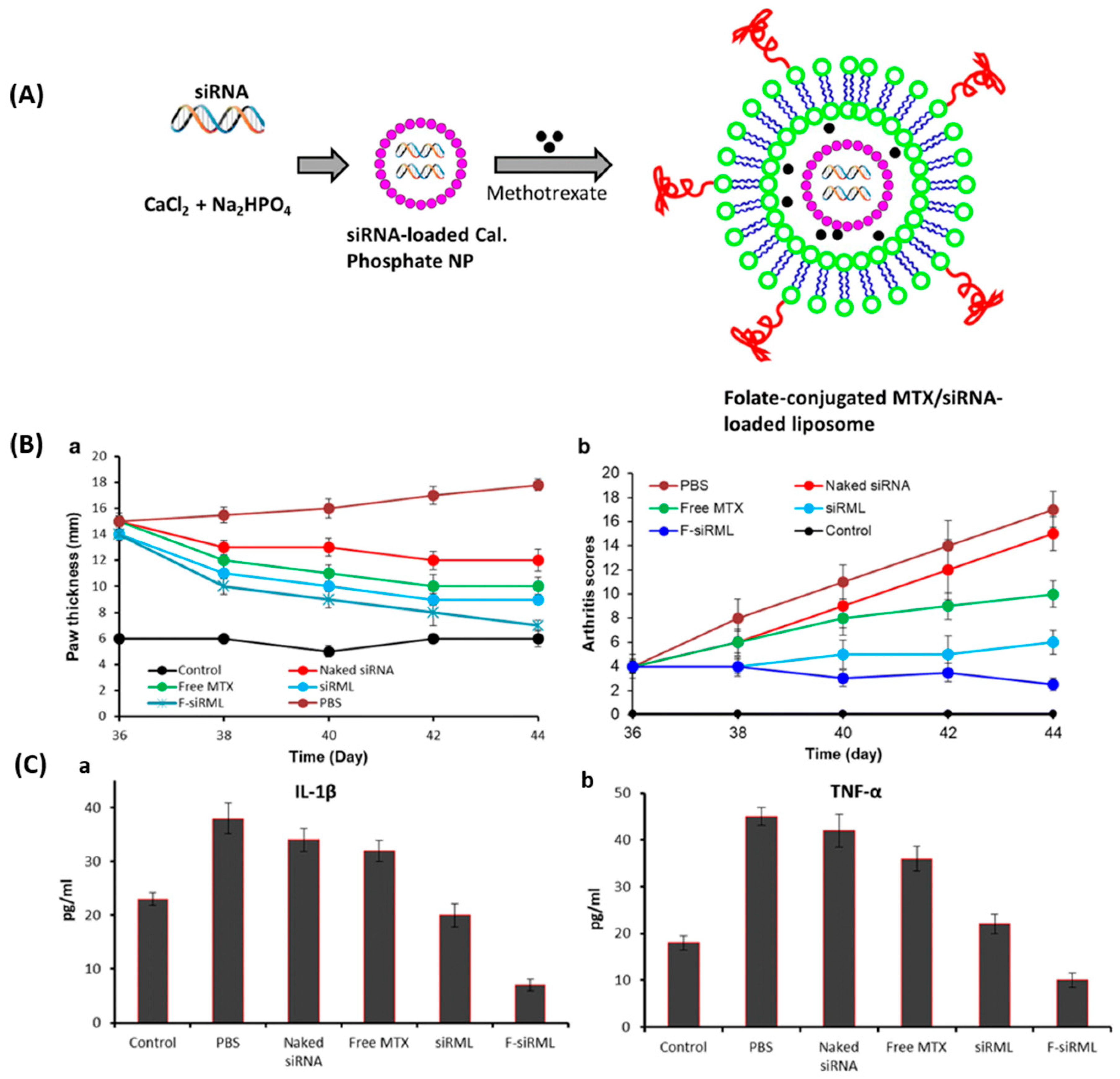
3.3. Folate-Targeted Liposomes for Rheumatoid Arthritis
4. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Antony, A. The biological chemistry of folate receptors. Blood 1992, 79, 2807–2820. [Google Scholar] [PubMed]
- Kamen, B.A.; Capdevila, A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc. Natl. Acad. Sci. USA 1986, 83, 5983–5987. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lacey, S.; Sanders, J.; Rothberg, K.; Anderson, R.; Kamen, B. Complementary DNA for the folate binding protein correctly predicts anchoring to the membrane by glycosyl-phosphatidylinositol. J. Clin. Investig. 1989, 84, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, M.; Marquardt, H.; Duhring, J.L.; Freisheim, J.H. Homologous membrane folate binding proteins in human placenta: Cloning and sequence of a cDNA. Biochemistry 1989, 28, 8249–8254. [Google Scholar] [CrossRef]
- Elnakat, H.; Ratnam, M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 8, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Ross, J.; Wang, X.; Ratnam, M. Identification of a novel folate receptor, a truncated receptor, and receptor type. beta. in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry 1994, 33, 1209–1215. [Google Scholar] [CrossRef]
- Shen, F.; Wu, M.; Ross, J.F.; Miller, D.; Ratnam, M. Folate receptor type. Gamma. Is primarily a secretory protein due to lack of an efficient signal for glycosylphosphatidylinositol modification: Protein characterization and cell type specificity. Biochemistry 1995, 34, 5660–5665. [Google Scholar] [CrossRef]
- Nakashima-Matsushita, N.; Homma, T.; Yu, S.; Matsuda, T.; Sunahara, N.; Nakamura, T.; Tsukano, M.; Ratnam, M.; Matsuyama, T. Selective expression of folate receptor β and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1999, 42, 1609–1616. [Google Scholar] [CrossRef]
- Xia, W.; Hilgenbrink, A.R.; Matteson, E.L.; Lockwood, M.B.; Cheng, J.-X.; Low, P.S. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood 2009, 113, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Xiang, G.; Wu, J.; Lu, Y.; Liu, Z.; Lee, R.J. Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. Int. J. Pharm. 2008, 356, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Spiegelstein, O.; Eudy, J.D.; Finnell, R.H. Identification of two putative novel folate receptor genes in humans and mouse. Gene 2000, 258, 117–125. [Google Scholar] [CrossRef]
- Kamen, B.A.; Caston, J.D. Properties of a folate binding protein (FBP) isolated from porcine kidney. Biochem. Pharmacol. 1986, 35, 2323–2329. [Google Scholar] [CrossRef]
- da Costa, M.; Rothenberg, S.P. Purification and characterization of folate binding proteins from rat placenta. Biochim. Et Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1996, 1292, 23–30. [Google Scholar] [CrossRef]
- Holm, J.; Hansen, S.; Høier-Madsen, M.; Bostad, L. High-affinity folate binding in human choroid plexus. Characterization of radioligand binding, immunoreactivity, molecular heterogeneity and hydrophobic domain of the binding protein. Biochem. J. 1991, 280, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, M.D.; Jallad, K.N.; Lu, J.; Low, P.S.; Ben-Amotz, D. Evaluation of folate conjugate uptake and transport by the choroid plexus of mice. Pharm. Res. 2003, 20, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R.; Kamen, B.A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar] [PubMed]
- Reddy, J.A.; Abburi, C.; Hofland, H.; Howard, S.J.; Vlahov, I.; Wils, P.; Leamon, C.P. Folate-targeted, cationic liposome-mediated gene transfer into disseminated peritoneal tumors. Gene Ther. 2002, 9, 1542. [Google Scholar] [CrossRef]
- Reddy, J.A.; Dean, D.; Kennedy, M.D.; Low, P.S. Optimization of folate-conjugated liposomal vectors for folate receptor-mediated gene therapy. J. Pharma. Sci. 1999, 88, 1112–1118. [Google Scholar] [CrossRef]
- Lee, R.J.; Huang, L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J. Biol. Chem. 1996, 271, 8481–8487. [Google Scholar] [CrossRef]
- Reddy, J.A.; Low, P.S. Enhanced folate receptor mediated gene therapy using a novel pH-sensitive lipid formulation. J. Control. Release 2000, 64, 27–37. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Hattori, Y.M.; Koga, K.; Maitani, Y. Folate-linked lipid-based nanoparticles for synthetic siRNA delivery in KB tumor xenografts. Eur. J. Pharm. Biopharm. 2008, 70, 718–725. [Google Scholar] [CrossRef]
- Goren, D.; Horowitz, A.T.; Tzemach, D.; Tarshish, M.; Zalipsky, S.; Gabizon, A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin. Cancer Res. 2000, 6, 1949–1957. [Google Scholar]
- Ni, S.; Stephenson, S.M.; Lee, R.J. Folate receptor targeted delivery of liposomal daunorubicin into tumor cells. Anticancer Res. 2002, 22, 2131. [Google Scholar]
- Saul, J.M.; Annapragada, A.; Natarajan, J.V.; Bellamkonda, R.V. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J. Control. Release 2003, 92, 49–67. [Google Scholar] [CrossRef]
- Gazzano, E.; Rolando, B.; Chegaev, K.; Salaroglio, I.C.; Kopecka, J.; Pedrini, I.; Saponara, S.; Sorge, M.; Buondonno, I.; Stella, B. Folate-targeted liposomal nitrooxy-doxorubicin: An effective tool against P-glycoprotein-positive and folate receptor-positive tumors. J. Control. Release 2017, 270, 37–52. [Google Scholar] [CrossRef]
- Lof, M.; Fernandes, R.S.; Cmr, O.; Lopes, S.C.; Townsend, D.M.; Cardoso, V.N.; Oliveira, M.C.; Leite, E.A.; Rubello, D.; Alb, D.B. Paclitaxel-loaded folate-coated long circulating and pH-sensitive liposomes as a potential drug delivery system: A biodistribution study. Biomed. Pharmacother. 2018, 97, 489–495. [Google Scholar]
- Mary Jo, T.; Breur, G.J.; Widmer, W.R.; Paulos, C.M.; Le-Cun, X.; Lee Ann, G.; Low, P.S. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 2014, 46, 1947–1955. [Google Scholar]
- Li, L.; An, X.; Yan, X. Folate-polydiacetylene-liposome for tumor targeted drug delivery and fluorescent tracing. Colloids Surf. B Biointerfaces 2015, 134, 235–239. [Google Scholar] [CrossRef]
- Dong, S.; Teo, J.D.W.; Chan, L.Y.; Lee, C.L.K.; Sou, K. Far-Red Fluorescent Liposomes for Folate Receptor-targeted Bioimaging. ACS Appl. Nano Mater. 2018, 1, 1009–1013. [Google Scholar] [CrossRef]
- Stephenson, S.M. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J. Control. Release 2002, 80, 309–319. [Google Scholar]
- Zhao, X.B.; Lee, R.J. Tumor-selective targeted delivery of genes and antisense oligodeoxyribonucleotides via the folate receptor. Adv. Drug Deliv. Rev. 2004, 56, 1193–1204. [Google Scholar] [CrossRef]
- Xia, T.; He, Q.; Shi, K.; Wang, Y.; Yu, Q.; Zhang, L.; Zhang, Q.; Gao, H.; Ma, L.; Liu, J. Losartan Loaded Liposomes Improve the Antitumor Efficacy of Liposomal Paclitaxel Modified with pH Sensitive Peptides by Inhibition of Collagen in Breast Cancer. Pharm. Dev. Technol. 2016, 23, 1–29. [Google Scholar] [CrossRef]
- Hou, S.; Yang, Y.; Zhou, S.; Kuang, X.; Yang, Y.; Gao, H.; Wang, Z.; Liu, H. Novel SS-31 modified liposomes for improved protective efficacy of minocycline against drug-induced hearing loss. Biomater. Sci. 2018, 6, 1627–1635. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Li, X.; Liang, X.; Luo, X. The influence of different long-circulating materials on the pharmacokinetics of liposomal vincristine sulfate. Int. J. Nanomed. 2016, 11, 4187–4197. [Google Scholar]
- Perche, F.; Torchilin, V.P. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J. Drug Deliv. 2013, 2013, 705265. [Google Scholar] [CrossRef]
- Lee, R.J.; Wang, S.; Low, P.S. Measurement of endosome pH following folate receptor-mediated endocytosis. Biochim. Biophys. Acta 1996, 1312, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Antony, A.C.; Kane, M.A.; Portillo, R.M.; Elwood, P.C.; Kolhouse, J.F. Studies of the role of a particulate folate-binding protein in the uptake of 5-methyltetrahydrofolate by cultured human KB cells. J. Biol. Chem. 1985, 260, 14911–14917. [Google Scholar]
- Turek, J.J.; Leamon, C.P.; Low, P.S. Endocytosis of folate-protein conjugates: Ultrastructural localization in KB cells. J. Cell Sci. 1993, 106, 423–430. [Google Scholar]
- Anderson, R.G.; Kamen, B.A.; Rothberg, K.G.; Lacey, S.W. Potocytosis: Sequestration and transport of small molecules by caveolae. Science 1992, 255, 410–412. [Google Scholar] [CrossRef]
- Patel, H.M. Liposomes: A Practical Approach; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Olson, F.; Hunt, C.; Szoka, F.; Vail, W.; Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 1979, 557, 9–23. [Google Scholar] [CrossRef]
- Szoka, F.; Olson, F.; Heath, T.; Vail, W.; Mayhew, E.; Papahadjopoulos, D. Preparation of unilamellar liposomes of intermediate size (0.1–0.2 μm) by a combination of reverse phase evaporation and extrusion through polycarbonate membranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 1980, 601, 559–571. [Google Scholar] [CrossRef]
- Nayar, R.; Hope, M.J.; Cullis, P.R. Generation of large unilamellar vesicles from long-chain saturated phosphatidylcholines by extrusion technique. Biochim. Et Biophys. Acta (BBA) Biomembr. 1989, 986, 200–206. [Google Scholar] [CrossRef]
- Schubert, R.; Wolburg, H.; Schmidt, K.-H.; Roth, H.J. Loading of preformed liposomes with high trapping efficiency by detergent-induced formation of transient membrane holes. Chem. Phys. Lipids 1991, 58, 121–129. [Google Scholar] [CrossRef]
- Chapman, C.J.; Erdahl, W.E.; Taylor, R.W.; Pfeiffer, D.R. Effects of solute concentration on the entrapment of solutes in phospholipid vesicles prepared by freeze-thaw extrusion. Chem. Phys. Lipids 1991, 60, 201–208. [Google Scholar] [CrossRef]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.M.; Takeshita, K.; Subbarao, N.K.; Hu, L.R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Et Biophys. Acta (BBA) Biomembr. 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Amselem, S.; Gabizon, A.; Barenholz, Y. Evaluation of a new extrusion device for the production of stable oligolamellar liposomes in a liter scale. J. Liposome Res. 1989, 1, 287–301. [Google Scholar] [CrossRef]
- Turanek, J. Fast-protein liquid chromatography system as a tool for liposome preparation by the extrusion procedure. Anal. Biochem. 1994, 218, 352–357. [Google Scholar] [CrossRef]
- Schneider, T.; Sachse, A.; Röbling, G.; Brandl, M. Large-scale production of liposomes of defined size by a new continuous high pressure extrusion device. Drug Dev. Ind. Pharm. 1994, 20, 2787–2807. [Google Scholar] [CrossRef]
- Berger, N.; Sachse, A.; Bender, J.; Schubert, R.; Brandl, M. Filter extrusion of liposomes using different devices: Comparison of liposome size, encapsulation efficiency, and process characteristics. Int. J. Pharm. 2001, 223, 55–68. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Middelberg, A.P.; Zhao, C.-X. Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf. B Biointerfaces 2016, 148, 402–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, R.R.; Shao, C.; Omiatek, D.M.; Vreeland, W.N.; DeVoe, D.L. Microfluidic synthesis of PEG-and folate-conjugated liposomes for one-step formation of targeted stealth nanocarriers. Pharm. Res. 2013, 30, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Zook, J.M.; Vreeland, W.N. Effects of temperature, acyl chain length, and flow-rate ratio on liposome formation and size in a microfluidic hydrodynamic focusing device. Soft Matter 2010, 6, 1352–1360. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; DeVoe, D.L.; Locascio, L.E.; Gaitan, M. Microfluidic directed formation of liposomes of controlled size. Langmuir 2007, 23, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Wang, H.; Liu, Y.; Hui, Y.; Sun, Q.; Seth, A.; Wibowo, D.; Chen, D.; Zhao, C.-X. Microfluidic self-assembly of a combinatorial library of single-and dual-ligand liposomes for in vitro and in vivo tumor targeting. Eur. J. Pharm. Biopharm. 2018, 130, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.R.; DeVoe, D.L. High-Throughput Continuous Flow Production of Nanoscale Liposomes by Microfluidic Vertical Flow Focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef] [PubMed]
- Baby, T.; Liu, Y.; Middelberg, A.P.; Zhao, C.-X. Fundamental studies on throughput capacities of hydrodynamic flow-focusing microfluidics for producing monodisperse polymer nanoparticles. Chem. Eng. Sci. 2017, 169, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Ran, R.; Sun, Q.; Baby, T.; Wibowo, D.; Middelberg, A.P.; Zhao, C.-X. Multiphase microfluidic synthesis of micro-and nanostructures for pharmaceutical applications. Chem. Eng. Sci. 2017, 169, 78–96. [Google Scholar] [CrossRef]
- Valencia, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012, 7, 623. [Google Scholar] [CrossRef]
- Anderson, K.E.; Eliot, L.A.; Stevenson, B.R.; Rogers, J.A. Formulation and Evaluation of a Folic Acid Receptor-Targeted Oral Vancomycin Liposomal Dosage Form. Pharm. Res. 2001, 18, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Chiani, M.; Norouzian, D.; Shokrgozar, M.A.; Azadmanesh, K.; Najmafshar, A.; Mehrabi, M.R.; Akbarzadeh, A. Folic acid conjugated nanoliposomes as promising carriers for targeted delivery of bleomycin. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Pan, J.; Sarisozen, C.; Luther, E.; Torchilin, V. Enhanced Cytotoxicity of Folic Acid-Targeted Liposomes Co-Loaded with C6 Ceramide and Doxorubicin: In Vitro Evaluation on HeLa, A2780-ADR and H69-AR Cells. Mol. Pharm. 2015, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, L.; Yang, X.; Fei, W.; Lu, W. Folic acid-conjugated liposomal vincristine for multidrug resistant cancer therapy. Asian J. Pharm. Sci. 2013, 8, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Tong, G.H.; Liu, C.M.; Liu, W.; Yang, S.B.; Liu, W.L.; Yin, T.T.; Zheng, H.J. Preparation and Stability of Folic Acid Liposomes. Food Sci. 2010, 31, 178–182. [Google Scholar]
- Patil, Y.; Shmeeda, H.; Amitay, Y.; Ohana, P.; Kumar, S.; Gabizon, A. Targeting of folate-conjugated liposomes with co-entrapped drugs to prostate cancer cells via prostate-specific membrane antigen (PSMA). Nanomed. Nanotechnol. Biol. Med. 2018, 14, S1549963418300832. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Low, P.S. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim. Et Biophys. Acta Biomembr. 1995, 1233, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Low, P.S. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem. 1994, 269, 3198–3204. [Google Scholar]
- Davis, S.S.; Ilium, L.; Moghimi, S.M.; Davies, M.C.; Porter, C.J.H.; Muir, I.S.; Brindley, A.; Christy, N.M.; Norman, M.E.; Williams, P. Microspheres for targeting drugs to specific body sites. J. Control. Release 1993, 24, 157–163. [Google Scholar] [CrossRef]
- Lee, J.; Martic, P.A.; Tan, J.S. Protein adsorption on pluronic copolymer-coated polystyrene particles. J. Colloid Interface Sci. 1989, 131, 252–266. [Google Scholar] [CrossRef]
- Kumar, P.; Wasim, L.; Chopra, M.; Chhikara, A. Co-delivery of Vorinostat and Etoposide Via Disulfide Cross-Linked Biodegradable Polymeric Nanogels: Synthesis, Characterization, Biodegradation, and Anticancer Activity. AAPS PharmSciTech 2017, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Behl, G.; Sikka, M.; Chhikara, A.; Chopra, M. Poly(ethylene glycol)-co-Methacrylamide-co-Acrylic acid based Nanogels for Delivery of Doxorubicin. J. Biomater. Sci. Polym. Ed. 2016, 27, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, M.; Zhang, N. Folate-targeted docetaxel-lipid-based-nanosuspensions for active-targeted cancer therapy. Int. J. Nanomed. 2012, 7, 3281–3294. [Google Scholar] [Green Version]
- Li, H.; Li, Y.; Ao, H.; Bi, D.; Han, M.; Guo, Y.; Wang, X. Folate-targeting annonaceous acetogenins nanosuspensions: Significantly enhanced antitumor efficacy in HeLa tumor-bearing mice. Drug Deliv. 2018, 25, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nakamura, M.; Takeuchi, N.; Tamaki, K.; Shimizu, S.; Yoshiike, Y.; Taguchi, M.; Ohno, H.; Ozaki, K.; Onishi, H. Effect of cationic lipid in cationic liposomes on siRNA delivery into the lung by intravenous injection of cationic lipoplex. J. Drug Target. 2019, 27, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Moghimipour, E.; Kouchak, M.; Ramezani, Z.; Amini, M.; Angali, K.A.; Saremy, S.; Dorkoosh, F.A.; Rezaei, M. New folate receptor targeted nano liposomes for delivery of 5-fluorouracil to cancer cells: Strong implication for enhanced potency and safety. Life Sci. 2019, 227, 39–50. [Google Scholar] [CrossRef]
- Hattori, Y.; Shimizu, S.; Ozaki, K.-I.; Onishi, H. Effect of Cationic Lipid Type in Folate-PEG-Modified Cationic Liposomes on Folate Receptor-Mediated siRNA Transfection in Tumor Cells. Pharmaceutics 2019, 11, 181. [Google Scholar] [CrossRef]
- Gao, W. Preparation and Evaluation of Folate Receptor Mediated Targeting Liposomes. Liposome-Based Drug Deliv. Syst. 2018, 1–12. [Google Scholar]
- Yashwant, G.; Anekant, J.; Priyanka, J.; Jain, S.K. Design and development of folate appended liposomes for enhanced delivery of 5-FU to tumor cells. J. Drug Target. 2007, 15, 231–240. [Google Scholar]
- Li, M.; Shi, K.; Tang, X.; Wei, J.; Cun, X.; Chen, X.; Yu, Q.; Zhang, Z.; He, Q. pH-sensitive folic acid and dNP2 peptide dual-modified liposome for enhanced targeted chemotherapy of glioma. Eur. J. Pharm. Sci. 2018, 124, 240–248. [Google Scholar] [CrossRef]
- Gabizon, A.; Horowitz, A.T.; Goren, D.; Tzemach, D.; Mandelbaum-Shavit, F.; Qazen, M.M.; Zalipsky, S. Targeting folate receptor with folate linked to extremities of poly (ethylene glycol)-grafted liposomes: In vitro studies. Bioconjugate Chem. 1999, 10, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Park, S.H.; Kang, M.H.; Park, M.J.; Choi, Y.W. Folic acid-tethered Pep-1 peptide-conjugated liposomal nanocarrier for enhanced intracellular drug delivery to cancer cells: Conformational characterization and in vitro cellular uptake evaluation. Int. J. Nanomed. 2013, 8, 1155. [Google Scholar]
- Qiu, L.; Dong, C.; Kan, X. Lymphoma-targeted treatment using a folic acid-decorated vincristine-loaded drug delivery system. Drug Des. Dev. Ther. 2018, 12, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Na, K.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005, 5, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.B.; Kao, G.Y.; Moase, E.H.; Zalipsky, S.; Allen, T.M. Attachment of antibodies to sterically stabilized liposomes: Evaluation, comparison and optimization of coupling procedures. Biochim. Et Biophys. Acta Biomembr. 1995, 1239, 133–144. [Google Scholar] [CrossRef]
- Abdus, S.; Sultana, Y.; Aqil, M. Liposomal drug delivery systems: An update review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar]
- Briuglia, M.L.; Rotella, C.; Mcfarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Huang, L. Role of cholesterol in the stability of pH-sensitive, large unilamellar liposomes prepared by the detergent-dialysis method. Biochim. Et Biophys. Acta (BBA) Biomembr. 1989, 981, 254–260. [Google Scholar] [CrossRef]
- Haloupek, N.; Rafferty, J.; Haloupek, K.; Tejada, E.; Mamistvalova, T.; Chong, P. Investigation of the Role of Cholesterol Superlattice in Release Kinetics of Drugs from Stealth Liposomes. Biophys. J. 2012, 102, 83a. [Google Scholar] [CrossRef] [Green Version]
- Kirpotin, D.; Hong, K.; Mullah, N.; Papahadjopoulos, D.; Zalipsky, S. Liposomes with detachable polymer coating: Destabilization and fusion of dioleoylphosphatidylethanolamine vesicles triggered by cleavage of surface-grafted poly(ethylene glycol). FEBS Lett. 1996, 388, 115–118. [Google Scholar] [CrossRef]
- Ellens, H.; Bentz, J.; Szoka, F.C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: Role of bilayer contact. Biochemistry 1984, 23, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kirchmeier, M.J.; Moase, E.H.; Zalipsky, S.; Allen, T.M. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim. Et Biophys. Acta (BBA) Biomembr. 2001, 1515, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Duzgunes, N.; Straubinger, R.M.; Baldwin, P.A.; Friend, D.S.; Papahadjopoulos, D. Proton-induced fusion of oleic acid-phosphatidylethanolamine liposomes. Biochemistry 1985, 24, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.; Litzinger, D.C.; Huang, L. Structural and functional comparisons of pH-sensitive liposomes composed of phosphatidylethanolamine and three different diacylsuccinylglycerols. Biochim. Et Biophys. Acta (BBA) Biomembr. 1990, 1025, 234–242. [Google Scholar] [CrossRef]
- Xiong, S.; Yu, B.; Wu, J.; Li, H.; Lee, R.J. Preparation, therapeutic efficacy and intratumoral localization of targeted daunorubicin liposomes conjugating folate-PEG-CHEMS. Biomed. Pharmacother. 2011, 65, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, Y.; Zhao, P.; Zhang, S.; Li, M.; He, C.; Zhang, X.; Yang, T.; Yan, R.; Ye, P. Co-delivery of doxorubicin and imatinib by pH sensitive cleavable PEGylated nanoliposomes with folate-mediated targeting to overcome multidrug resistance. Int. J. Pharm. 2018, 542, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Kingma, P.S.; Whitsett, J.A. In defense of the lung: Surfactant protein A and surfactant protein D. Curr. Opin. Pharmacol. 2006, 6, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kishore, U.; Greenhough, T.J.; Waters, P.; Shrive, A.K.; Ghai, R.; Kamran, M.F.; Bernal, A.L.; Reid, K.B.; Madan, T.; Chakraborty, T. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol. Immunol. 2006, 43, 1293–1315. [Google Scholar] [CrossRef] [PubMed]
- Eugénia, N.; Mangialavori, I.C.; Ana, L.; Azoia, N.G.; Sárria, M.P.; Patrícia, N.; Jaime, F.; Johan, H.R.; Ulyana, S.; Alexandra, R. Peptide Anchor for Folate-Targeted Liposomal Delivery. Biomacromolecules 2015, 16, 2904. [Google Scholar]
- Jaradat, D. Thirteen decades of peptide synthesis: Key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation. Amino Acids 2018, 50, 39–68. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, N.; Chhikara, A.; Chopra, M. Recent Progress in Combinatorial Solid Phase Synthesis: Techniques, Characterization and its Application in Drug Development. Curr. Biochem. Eng. 2017, 4, 9–33. [Google Scholar] [CrossRef]
- Hoshi, T.; Saiki, H.; Anzai, J.I. Preparation of spatially ordered multilayer thin films of antibody and their binding properties. Biosens. Bioelectron. 2000, 15, 623–628. [Google Scholar] [CrossRef]
- Rusinova, E.; Tretyachenko-Ladokhina, V.; Vele, O.E.; Senear, D.F.; Ross, J.A. Alexa and Oregon Green dyes as fluorescence anisotropy probes for measuring protein–protein and protein–nucleic acid interactions. Anal. Biochem. 2002, 308, 18–25. [Google Scholar] [CrossRef]
- Ingle, S.G.; Pai, R.V.; Monpara, J.D.; Vavia, P.R. Liposils: An effective strategy for stabilizing Paclitaxel loaded liposomes by surface coating with silica. Eur. J. Pharm. Sci. 2018, 122, S0928098718302811. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.F.; Malachias, Â.; Poundlana, G.; Magalhãespaniago, R.; Mosqueira, V.C.F.; Oliveira, M.C.; Barros, A.L.D.; Leite, E.A. Paclitaxel-Loaded pH-Sensitive Liposome: New Insights on Structural and Physicochemical Characterization. Langmuir 2018, 34, 5728–5737. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dong, A.; Guo, R.; Yang, M.; Deng, L.; Zhang, J. DOX/ICG Coencapsulated Liposome-Coated Thermosensitive Nanogels for NIR-Triggered Simultaneous Drug Release and Photothermal Effect. ACS Biomater. Sci. Eng. 2018, 4, 2424–2434. [Google Scholar] [CrossRef]
- Xia, Y.; Mei, F.; Dong, J.; Xu, C.; Zhen, L.; Ning, P.; Qi, Z. pH sensitive liposomes delivering tariquidar and doxorubicin to overcome multidrug resistance of resistant ovarian cancer cells. Colloids Surf. B Biointerfaces 2018, 170, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mukherjee, B.; Chaudhuri, S.; Roy, T.; Mukherjee, A.; Sengupta, S. Methotrexate Aspasomes Against Rheumatoid Arthritis: Optimized Hydrogel Loaded Liposomal Formulation with In Vivo Evaluation in Wistar Rats. AAPS PharmSciTech 2018, 19, 1320–1336. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Rip, J.; Gaillard, P.J.; Ecm, D.L.; Hammarlund-Udenaes, M. The impact of liposomal formulations on the release and brain delivery of methotrexate: An in vivo microdialysis study. J. Pharm. Sci. 2018, 106, 2606–2613. [Google Scholar] [CrossRef]
- Gregory, M.E.; Kurzman, I.; Rosenthal, R.; Fox, L.; Madewell, B.R. Liposome-Encapsulated MTP-PE with cis- platin in the canine osteosarcoma model-a randomized study. J. Immunother. 1992, 11, 133. [Google Scholar]
- Bandak, S.; Goren, D.; Horowitz, A.; Tzemach, D.; Gabizon, A. Pharmacological studies of cisplatin encapsulated in long-circulating liposomes in mouse tumor models. Anticancer Drugs 1999, 10, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Y.; Peng, S.; Chen, X.; Zou, L.; Liu, W.; Liu, C. Liposomes consisting of pluronic F127 and phospholipid: Effect of matrix on morphology, stability and curcumin delivery. J. Dispers. Sci. Technol. 2019, 1–7. [Google Scholar] [CrossRef]
- Behl, G.; Kumar, P.; Sikka, M.; Fitzhenry, L.; Chhikara, A. PEG-coumarin nanoaggregates as π-π stacking derived small molecule lipophile containing self-assemblies for anti-tumour drug delivery. J. Biomater. Sci. Polym. Ed. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, G.M.M.E.; Williams, A.C.; Barry, B.W. Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in-vitro. J. Pharm. Pharmacol. 2010, 53, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Hitzman, C.J.; Elmquist, W.F.; Wattenberg, L.W.; Wiedmann, T.S. Development of a respirable, sustained release microcarrier for 5-fluorouracil I: In vitro assessment of liposomes, microspheres, and lipid coated nanoparticles. J. Pharm. Sci. 2006, 95, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Shi, L.; Ding, W.; Koga, K.; Kawano, K.; Hakoshima, M.; Maitani, Y. Novel irinotecan-loaded liposome using phytic acid with high therapeutic efficacy for colon tumors. J. Control. Release 2009, 136, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Sadzuka, Y.; Hirotsu, S.; Hirota, S. Effective Irinotecan (CPT-11)-containing Liposomes: Intraliposomal Conversion to the Active Metabolite SN-38. Cancer Sci. 2010, 90, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Valero, L.; Alhareth, K.; Espinoza, J.R.; Viricel, W.; Leblond, J.; Chissey, A.; Dhotel, H.; Roques, C.; Campiol, D.A.; Escriou, V. Liposomes as Gene Delivery Vectors for Human Placental Cells. Molecules 2018, 23, 1085. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Wang, B.; Zhang, J.; Liu, Y.H.; Zhang, W.; Xiao, Y.P.; Zhao, R.M. Reduction-responsive liposomal nanocarriar with self-reporting ability for efficient gene delivery. J. Mater. Chem. B 2018, 6, 2860–2868. [Google Scholar]
- Kumar, P.; Liu, B.; Behl, G. A Comprehensive Outlook of Synthetic Strategies and Applications of Redox-Responsive Nanogels in Drug Delivery. Macromol. Biosci. 2019, 1900071. [Google Scholar] [CrossRef]
- Soe, Z.C.; Thapa, R.K.; Ou, W.; Gautam, M.; Nguyen, H.T.; Jin, S.G.; Ku, S.K.; Oh, K.T.; Choi, H.G.; Yong, C.S. Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy. Colloids Surf. B Biointerfaces 2018, 170, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.H.; Zhi, D.F.; Zhao, Y.N.; Chen, H.Y.; Meng, Y.; Zhang, C.M.; Zhang, S.B. Cationic lioposomes with folic acid as targeting ligand for gene delivery. Bioorganic Med. Chem. Lett. 2016, 26, 4025–4029. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Horowitz, A.T.; Zalipsky, S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv. Drug Deliv. Rev. 2004, 56, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Stevenson, B.R.; Rogers, J.A. Folic acid–PEO-labeled liposomes to improve gastrointestinal absorption of encapsulated agents. J. Control. Release 1999, 60, 189–198. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, H.; Yu, M.; Cao, S.; Zhang, F.; Chang, J.; Niu, R. Paclitaxel-Loaded, Folic-Acid-Targeted and TAT-Peptide-Conjugated Polymeric Liposomes: In Vitro and In Vivo Evaluation. Pharm. Res. 2010, 27, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chen, W.; Wu, J.; Li, H. Folic acid-coupled nano-paclitaxel liposome reverses drug resistance in SKOV3/TAX ovarian cancer cells. Anticancer Drugs 2014, 25, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Hilgenbrink, A.R.; Low, P.S. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Peres-Filho, M.J.; Santos, A.P.D.; Nascimento, T.L.; Ávila, R.I.D.; Ferreira, F.S.; Valadares, M.C.; Lima, E.M. Antiproliferative Activity and VEGF Expression Reduction in MCF7 and PC-3 Cancer Cells by Paclitaxel and Imatinib Co-encapsulation in Folate-Targeted Liposomes. AAPS PharmSciTech 2017, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Saremy, S.; Dorkoosh, F.A. A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes. Biomed. Pharmacother. 2018, 108, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Dorkoosh, F.A.; Handali, S. Folic acid-modified liposomal drug delivery strategy for tumor targeting of 5-fluorouracil. Eur. J. Pharm. Sci. 2017, 114, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, R.; Babu, A.; Amreddy, N.; Basalingappa, K.; Mehta, M.; Chen, A.; Yan, D.Z.; Kompella, U.B.; Munshi, A.; Ramesh, R. Folate receptor-targeted nanoparticle delivery of HuR-RNAi suppresses lung cancer cell proliferation and migration. J. Nanobiotechnol. 2016, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Zhang, Y.G.; Yang, Y.H.; Ma, C.C.; Wang, P.; Du, W.; Li, L.; Xiang, R.; Song, X.R.; Zhao, X. In vivo ovarian cancer gene therapy using CRISPR-Cas9 system. Hum. Gene Ther. 2018, 29, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, T.; Wu, B.; Zhang, X. Insights into the therapeutic potential of hypoxia-inducible factor-1α small interfering RNA in malignant melanoma delivered via folate-decorated cationic liposomes. Int. J. Nanomed. 2016, 11, 991–1002. [Google Scholar]
- Nogueira, E.; Lager, F.; Le, R.D.; Nogueira, P.; Freitas, J.; Charvet, C.; Renault, G.; Loureiro, A.; Almeida, C.R.; Ohradanova-Repic, A. Enhancing Methotrexate Tolerance with Folate Tagged Liposomes in Arthritic Mice. J. Biomed. Nanotechnol. 2015, 11, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Li, H. Combination of NF-kB targeted siRNA and methotrexate in a hybrid nanocarrier towards the effective treatment in rheumatoid arthritis. J. Nanobiotechnol. 2018, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Poh, S.; Chelvam, V.; Ayala-López, W.; Putt, K.S.; Low, P.S. Selective liposome targeting of folate receptor positive immune cells in inflammatory diseases. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Miraglia, E.; Viarisio, D.; Costamagna, C.; Pescarmona, G.; Ghigo, D.; Bosia, A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [Google Scholar] [PubMed]
- Chegaev, K.; Riganti, C.; Lazzarato, L.; Rolando, B.; Guglielmo, S.; Campia, I.; Fruttero, R.; Bosia, A.; Gasco, A. Nitric oxide donor doxorubicins accumulate into doxorubicin-resistant human colon cancer cells inducing cytotoxicity. ACS Med. Chem. Lett. 2011, 2, 494–497. [Google Scholar] [CrossRef]
- Chegaev, K.; Fraix, A.; Gazzano, E.; Abd-Ellatef, G.E.F.; Blangetti, M.; Rolando, B.; Conoci, S.; Riganti, C.; Fruttero, R.; Gasco, A. Light-regulated NO release as a novel strategy to overcome doxorubicin multidrug resistance. ACS Med. Chem. Lett. 2017, 8, 361–365. [Google Scholar] [CrossRef]
- Riganti, C.; Rolando, B.; Kopecka, J.; Campia, I.; Chegaev, K.; Lazzarato, L.; Federico, A.; Fruttero, R.; Ghigo, D. Mitochondrial-targeting nitrooxy-doxorubicin: A new approach to overcome drug resistance. Mol. Pharm. 2012, 10, 161–174. [Google Scholar] [CrossRef]
- Li, H.; Guo, K.; Wu, C.; Shu, L.; Guo, S.; Hou, J.; Zhao, N.; Wei, L.; Man, X.; Zhang, L. Controlled and Targeted Drug Delivery by a UV-responsive Liposome for Overcoming Chemo-resistance in Non-Hodgkin Lymphoma. Chem. Biol. Drug Des. 2015, 86, 783–794. [Google Scholar] [CrossRef]
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-hodgkin lymphoma. Lancet 2012, 380, 848–857. [Google Scholar] [CrossRef]
- Mahmoud, E.; Adil, N.; Marianna, F. Non-viral nucleic acid delivery: Key challenges and future directions. Curr Drug Deliv 2011, 8, 235–244. [Google Scholar]
- Gao, Y.; Liu, X.L.; Li, X.R. Research progress on siRNA delivery with nonviral carriers. Int. J. Nanomed. 2011, 2011, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fisher, K.A.; Darcy, R.; Cryan, J.F.; O’Driscoll, C. Therapeutic targeting in the silent era: Advances in non-viral siRNA delivery. Mol. Biosyst. 2010, 6, 1143–1161. [Google Scholar] [CrossRef] [PubMed]
- Kabilova, T.O.; Shmendel, E.V.; Gladkikh, D.V.; Chernolovskaya, E.L.; Markov, O.V.; Morozova, N.G.; Maslov, M.A.; Zenkova, M.A. Targeted delivery of nucleic acids into xenograft tumors mediated by novel folate-equipped liposomes. Eur. J. Pharm. Biopharm. 2017, 123, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tsunekawa, Y.; Hernandezbenitez, R.; Wu, J.; Jie, Z.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Zhe, L. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally engineered Cas9 nucleases with improved specificity. Science 2016, 351, 84. [Google Scholar] [CrossRef]
- Modell, J.W.; Jiang, W.; Marraffini, L.A. CRISPR–Cas systems exploit viral DNA injection to establish and maintain adaptive immunity. Nature 2017, 544, 101. [Google Scholar] [CrossRef]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 2016, 34, 933–941. [Google Scholar] [CrossRef]
- Chandrupatla, D.M.S.H.; Molthoff, C.F.M.; Lammertsma, A.A.; Laken, C.J.V.D.; Jansen, G. The folate receptor β as a macrophage-mediated imaging and therapeutic target in rheumatoid arthritis. Drug Deliv. Transl. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mcduffie, F.C. Morbidity impact of rheumatoid arthritis on society. Am. J. Med. 1985, 78, 1–5. [Google Scholar] [CrossRef]
- Janssens, H.J.E.M.; Lagro, H.A.H.M.; Peet, P.G.V.; Gorter, K.J.; Pas, P.V.D.; Paardt, M.V.D.; Woutersen-Koch, H. NHG-Standaard Artritis. Huisarts En Wet. 2009, 52, 439–453. [Google Scholar]
- Olsen, N.J.; Stein, C.M. New Drugs for Rheumatoid Arthritis. New Engl. J. Med. 2004, 350, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Tzemach, D.; Gorin, J.; Mak, L.; Amitay, Y.; Shmeeda, H.; Zalipsky, S. Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor models. Cancer Chemother. Pharmacol. 2009, 66, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Q.; Lee, R.J. A folate receptor-targeted liposomal formulation for paclitaxel. Int. J. Pharm. 2006, 316, 148–153. [Google Scholar] [CrossRef]
- Mary Jo, T.; Waters, D.J.; Low, P.S. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 2004, 213, 165–172. [Google Scholar]
- Szebeni, J.; Muggia, F.; Gabizon, A.; Barenholz, Y. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: Prediction and prevention. Adv. Drug Deliv. Rev. 2011, 63, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Fujimoto, K. A reduction-triggered delivery by a liposomal carrier possessing membrane-permeable ligands and a detachable coating. Colloids Surf. B Biointerfaces 2006, 49, 15–21. [Google Scholar] [CrossRef]
- Shin, J.; Shum, P.; Thompson, D.H. Acid-triggered release via dePEGylation of DOPE liposomes containing acid-labile vinyl ether PEG–lipids. J. Control. Release 2003, 91, 187–200. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Kogure, K.; Oishi, M.; Nagasaki, Y.; Kihira, Y.; Ueno, M.; Kobayashi, H.; Kikuchi, H.; Harashima, H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Ito, E.; Akita, H.; Oishi, M.; Nagasaki, Y.; Futaki, S.; Harashima, H. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J. Control. Release 2009, 139, 127–132. [Google Scholar] [CrossRef] [PubMed]






| Pharmaceutical Agent | Lipids Composition | Liposomes Formulation Method | Application | Ref. |
|---|---|---|---|---|
| Paclitaxel and 99mTc | DOPE, CHEMS, DSPE-PEG, FA-PEG-DSPE, and DSPE-PEG-DTPA | Film hydration method | Anticancer and imaging (In vitro MDA-MB-231 cells) | [27] |
| 99mTc-EC20 (FA-conjugated 99mTc) | Egg PC and cholesterol | Film hydration method | Rheumatoid arthritis and imaging (In vivo female Lewis rats) | [28] |
| Dil (carbocyanine dye) | DMPC, cholesterol, dihexadecyl phosphate, DSPE-PEG, and FA-PEG-DSPE | Microfluidic hydrodynamic flow focusing method | Imaging (In vitro RAW 264.7, SKOV3, and MCF-7 cells) | [53] |
| No drug used | DMPC, cholesterol, dihexadecyl phosphate, DMPE-PEG, and FA-PEG-DSPE | Microfluidic hydrodynamic flow focusing method | Anticancer | [54] |
| DiI/DiR (carbocyanine dye) and TAT peptide (CYGRKKRRQRRR) | DMPC, cholesterol, DEPE-PEG, FA-PEG-DSPE, and DSPE-PEG-TAT | Microfluidic hydrodynamic flow focusing method | Anticancer and imaging (In vitro RAW 264.7, SKOV3, and MCF-7 cells and In vivo nude Balb/C mice) | [57] |
| Paclitaxel | SPC, cholesterol, DSPE-PEG-OMe, DSPE-PEG-Hz-FA, and DSPE-PEG-dNP2 | Film hydration method | Anticancer (In vitro C6 cells) | [81] |
| Vincristine | PLGA, FA-PEG-SA | Ultrasonication method | Anticancer (In vitro Raji, HUVEC, and A20 cells) | [84] |
| DOX and imatinib | DOPE, HSPC, CHEMS, cholesterol, mPEG2000-Hz-VES, and FA-PEG-CHEMS | Film hydration method | Anticancer (In vitro MCF-7 and MCF-7/ADR cells) | [97] |
| Celastrol and irinotecan | DPPC, cholesterol, and FA-PEG-DSPE | Film hydration method | Anticancer (In vitro MCF-7, MDA-MB-231, and A549 cells) | [122] |
| 5-FU | DPPC, cholesterol, and FA-PEG-DSPE | Film hydration method | Anticancer (In vitro HT-29, Caco-2, CT26, HeLa, and MCF-7 cells) | [130] |
| Paclitaxel and imatinib | DSPC, cholesterol, and FA-PEG-DSPE | Film hydration method | Anticancer (In vitro MCF7 and PC-3 cells) | [129] |
| 5-FU | PC, cholesterol, and FA-PEG-DSPE | Film hydration method | Anticancer (In vitro CT26 cells) | [131] |
| DNA or siRNA | DOTAP, cholesterol, and FA-PEG-DSPE | Film hydration method | Anticancer (In vitro H1299 and CCD16 cells) | [132] |
| Single-guide RNA and CRISPR plasmid DNA co-expressing-Cas9 | DOTAP, cholesterol, FA-PEG-CHEMS, and methoxy-PEG-succinyl cholesterol | Film hydration method | Anticancer (In vitro gDNMT1 and in vivo Cas9 endonuclease) | [133] |
| HIF-1α siRNA | Soybean lecithin S100, cholesterol, and folate/oleic acid-diacylated oligochitosan | Film hydration method | Anticancer (In vitro Hypoxia-induced a375 cells) | [134] |
| Methotrexate | DOPE, cholesterol, DSPE-MPEG, and FA-peptide (SP-DS3) | Film hydration method | Rheumatoid arthritis (In vitro THP-1 macrophages and Jurkat T cells and in vivo mice) | [135] |
| Methotrexate and siRNA | DSPC, cholesterol, PEG-DSPE, and FA-PEG-DSPE | Film hydration method | Rheumatoid arthritis (In vitro Raw 264.7 cells and in vivo mice) | [136] |
| Betamethasone and DiD (carbocyanine dyes) | DSPC, cholesterol, PEG-DSPE, and FA-PEG-DSPE | Film hydration method | Imaging, rheumatoid arthritis, atherosclerosis, and psoriasis (In vitro RAW 264.7 cells and in vivo mice) | [137] |
| Folate-Conjugated Derivatives | Commonly Used Analogues | Advantages | Disadvantages |
|---|---|---|---|
| Uncleavable folate-conjugated phospholipids | FA-PEG-DSPE, FA-PEG-DPPE, and FA-PEG-SA |
|
|
| Acid-cleavable folate-conjugated phospholipids | FA-Hz-PEG-DSPE |
|
|
| Folate-conjugated cholesterol | FA-PEG-CHEMS |
|
|
| Folate-conjugated Proteins | SP-DS1, SP-DS2, and SP-DS3 |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Huo, P.; Liu, B. Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications. Pharmaceutics 2019, 11, 381. https://doi.org/10.3390/pharmaceutics11080381
Kumar P, Huo P, Liu B. Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications. Pharmaceutics. 2019; 11(8):381. https://doi.org/10.3390/pharmaceutics11080381
Chicago/Turabian StyleKumar, Parveen, Peipei Huo, and Bo Liu. 2019. "Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications" Pharmaceutics 11, no. 8: 381. https://doi.org/10.3390/pharmaceutics11080381
APA StyleKumar, P., Huo, P., & Liu, B. (2019). Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications. Pharmaceutics, 11(8), 381. https://doi.org/10.3390/pharmaceutics11080381





