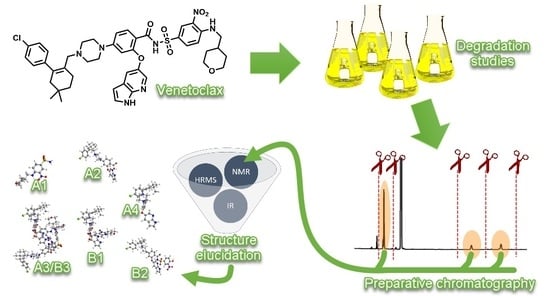
On the Stability and Degradation Pathways of Venetoclax under Stress Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Equipment and Software
2.3. Ultra High Performance Liquid Chromatography (UHPLC) Method Conditions
2.4. MS and HRMS Method Conditions
2.5. Preparative Chromatography Conditions
2.6. NMR Method Conditions
2.7. DSC Measurements Conditions
2.8. Fourier-Transform Infrared (FTIR) Measurements Conditions
2.9. Preparation of Sample Solutions
2.9.1. Degradation Studies Samples
2.9.2. Samples for Isolation of Degradation Products
2.9.3. Preparation of Isolated Degradation Products for Analyses
3. Results and Discussion
3.1. Forced Degradation Studies
3.1.1. Degradation of Venetoclax in Acidic Conditions
3.1.2. Degradation of Venetoclax in Basic Conditions
3.1.3. Degradation of Venetoclax in Oxidative Conditions
3.2. Characterization of Observed Key Degradation Products of Venetoclax
3.3. Degradation Pathways of Venetoclax
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Identification of Degradation Products
Appendix A.1. Degradation Product A1
Appendix A.2. Degradation Product A2
Appendix A.3. Degradation Product A3/B3
Appendix A.4. Degradation Product A4
Appendix A.5. Degradation Product B1
Appendix A.6. Degradation Product B2
References
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Birkinshaw, R.W.; Gong, J.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 2019, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Lutz, R.J. Role of the BH3 (Bcl-2 homology 3) domain in the regulation of apoptosis and Bcl-2-related proteins. Biochem. Soc. Trans. 2000, 28, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, J.; Letai, A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018, 25, 56–64. [Google Scholar] [CrossRef]
- Dai, H.; Meng, X.W.; Kaufmann, S.H. Mitochondrial apoptosis and BH3 mimetics. F1000Research 2016, 5, 2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Finger, L.R.; Yunis, J.; Nowell, P.C.; Croce, C.M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 1984, 226, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.D.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 2016, 16, 99–109. [Google Scholar] [CrossRef]
- Petch, A.; Al-rubeai, M. The Bcl-2 family. In Cell Engineering: Apoptosis; Al-Rubeai, M., Fussenegger, M., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2004; Volume 4, pp. 25–47. ISBN 978-1-4020-2217-3. [Google Scholar] [CrossRef]
- Ku, B.; Liang, C.; Jung, J.U.; Oh, B.H. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011, 21, 627–641. [Google Scholar] [CrossRef]
- Mandal, T.; Shin, S.; Aluvila, S.; Chen, H.C.; Grieve, C.; Choe, J.Y.; Cheng, E.H.; Hustedt, E.J.; Oh, K.J. Assembly of Bak homodimers into higher order homooligomers in the mitochondrial apoptotic pore. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Delbridge, A.R.D.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullard, A. Pioneering apoptosis-targeted cancer drug poised for FDA approval. Nat. Rev. Drug Discov. 2016, 15, 147–149. [Google Scholar] [CrossRef]
- Drugbank Venetoclax. Available online: https://www.drugbank.ca/drugs/DB11581 (accessed on 6 January 2020).
- U.S. Food and Drug Administration. Search Orphan Drug Designations and Approvals. Available online: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=600117 (accessed on 27 February 2020).
- European Medicines Agency Venclyxto (venetoclax) EPAR: An Overview of Venclyxto and Why it is Authorised in the EU; European Medicines Agency: London, UK, 2018.
- U.S. National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 27 February 2020).
- Žigart, N.; Časar, Z. A literature review of the patent publications on venetoclax–a selective Bcl-2 inhibitor: Discovering the therapeutic potential of a novel chemotherapeutic agent. Expert Opin. Ther. Pat. 2019, 29, 487–496. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency In Assessment Report of Venclycto (International Designation: Venetoclax); European Medicines Agency: London, UK, 2016.
- ICH harmonised tripartite guideline, Stability testing of new drug substances and products Q1A(R2). In Proceedings of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 6 February 2003.
- ICH harmonised tripartite guideline, Stability testing: Photostability testing of new drug substances and products Q1B. In Proceedings of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 6 November 1996.
- Baertschi, S.W. Pharmaceutical Stress Testing: Predicting Drug Degradation; Taylor & Francis Group, LLC: Indianapolis, IN, USA, 2005; ISBN 0824740211. [Google Scholar]
- Alsante, K.M.; Friedmann, R.C.; Hatajik, T.D.; Lohr, L.L.; Sharp, T.R.; Snyder, K.D.; Szczesny, E.J. Degradation and impurity analysis for pharmaceutical drug candidates. In Handbook of Modern Pharmaceutical Analysis; Ahuja, S., Scypinski, S., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 85–172. ISBN 0-12-045555-2. [Google Scholar]
- Alsante, K.M.; Ando, A.; Brown, R.; Ensing, J.; Hatajik, T.D.; Kong, W.; Tsuda, Y. The role of degradant profiling in active pharmaceutical ingredients and drug products. Adv. Drug Deliv. Rev. 2007, 59, 29–37. [Google Scholar] [CrossRef]
- Deokate, U.; Gorde, A.M. Forced degradation and stability testing: Strategies and analytical perspectives. Int. J. Pharm. Sci. Rev. Res. 2014, 26, 242–250. [Google Scholar]
- Aubry, A.-F.; Tattersall, P.; Ruan, J. Development of stability indicating methods. In Handbook of Stability Testing in Pharmaceutical Development; Huynh-Ba, K., Ed.; Springer Science: New York, NY, USA, 2009; pp. 139–161. ISBN 978-0-387-85627-8. [Google Scholar] [CrossRef]
- Liu, H.; Michmerhuizen, M.J.; Lao, Y.; Wan, K.; Salem, A.H.; Sawicki, J.; Serby, M.; Vaidyanathan, S.; Wong, S.L.; Agarwal, S.; et al. Metabolism and disposition of a novel B-cell lymphoma-2 inhibitor venetoclax in humans and characterization of its unususal metabolites. Drug Metab. Dispos. 2017, 45, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Bansal, G.; Singh, M.; Jindal, K.C.; Singh, S. Ultraviolet-photodiode array and high-performance liquid chromatographic/mass spectrometric studies on forced degradation behavior of glibenclamide and development of a validated stability-indicating method. J. AOAC Int. 2008, 91, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.W.; Abdelwahab, N.S.; El-Zeiny, B.A.; Tohamy, S.I. Stability indicating tlc-densitometric method for determination of chlorpropamide. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1575–1585. [Google Scholar] [CrossRef]
- Peron, F.; Fossey, C.; Cailly, T.; Fabis, F. N-Tosylcarboxamide as a transformable directing group for Pd-Catalyzed C-H ortho-arylation. Org. Lett. 2012, 14, 1827–1829. [Google Scholar] [CrossRef] [PubMed]
- Ammazzalorso, A.; De Filippis, B.; Giampietro, L.; Amoroso, R. N-acylsulfonamides: Synthetic routes and biological potential in medicinal chemistry. Chem. Biol. Drug Des. 2017, 90, 1094–1105. [Google Scholar] [CrossRef]
- Kluger, R.; Howe, G.W.; Mundle, S.O.C. Avoiding CO2 in catalysis of decarboxylation. In Advances in Physical Organic Chemistry; Elsevier Ltd.: Oxford, UK, 2013; Volume 47, pp. 85–128. ISBN 9780124077546. [Google Scholar] [CrossRef]
- Jivani, S.G.; Stella, V.J. Mechanism of decarboxylation of p-aminosalicylic acid. J. Pharm. Sci. 1985, 74, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Ruelle, P. Theoretical study on the mechanism of the thermal decarboxylation of salicylic and p-aminobenzoic acids; Models for aqueous solution. J. Chem. Soc. Perkin Trans. 2 1986, 1953–1959. [Google Scholar] [CrossRef]
- Jones-Mensah, E.; Karki, M.; Magolan, J. Dimethyl Sulfoxide as a synthon in organic chemistry. Synthesis 2016, 48, A–P. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Köse, M.; Sylvester, K.; Weighardt, H.; Thimm, D.; Borges, G.; Förster, I.; Von Kügelgen, I.; Müller, C.E. Diindolylmethane derivatives: Potent agonists of the immunostimulatory Orphan G Protein-Coupled receptor GPR84. J. Med. Chem. 2017, 60, 3636–3655. [Google Scholar] [CrossRef]
- Johansson, H.; Jørgensen, T.B.; Gloriam, D.E.; Bräuner-Osborne, H.; Pedersen, D.S. 3-Substituted 2-phenyl-indoles: Privileged structures for medicinal chemistry. RCS Adv. 2019, 3, 945–960. [Google Scholar] [CrossRef]
- Tocco, G.; Zedda, G.; Casu, M.; Simbula, G.; Begala, M. Solvent-free addition of indole to aldehydes: Unexpected synthesis of novel 1-[1-(1H-indol-3-yl)alkyl]-1h-indoles and preliminary evaluation of their cytotoxicity in hepatocarcinoma cells. Molecules 2017, 22, 1747. [Google Scholar] [CrossRef]
- Sun, C.; Zou, X.; Li, F. Direct use of methanol as an alternative to formaldehyde for the synthesis of 3,3’-Bisindolylmethanes (3,3’-BIMs). Chemistry 2013, 19, 14030–14033. [Google Scholar] [CrossRef]
- Hughes, D.L. Patent review of manufacturing routes to oncology drugs: Carfilzomib, osimertinib, and venetoclax. Org. Process Res. Dev. 2016, 20, 2028–2042. [Google Scholar] [CrossRef]
- Robnik, B.; Naumoska, K.; Časar, Z. A novel testing approach for oxidative degradation dependent incompatibility of amine moiety containing drugs with pegs in solid-state. Pharmaceutics 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modhave, D.; Barrios, B.; Paudel, A. PVP-H2O2 complex as a new stressor for the accelerated oxidation study of pharmaceutical solids. Pharmaceutics 2019, 11, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robnik, B.; Likozar, B.; Wang, B.; Stanić Ljubin, T.; Časar, Z. Understanding and kinetic modeling of complex degradation pathways in the solid dosage form: The case of saxagliptin. Pharmaceutics 2019, 11, 452. [Google Scholar] [CrossRef] [Green Version]
- Nikitina, G.V.; Pevzner, M.S. Imidazole and benzimidazole N-oxides (review). Chem. Heterocycl. Compd. 1993, 29, 127–151. [Google Scholar] [CrossRef]
- Buján, E.I.; Salum, M.L. A simple synthesis of benzimidazole N-oxides from 2-nitroaniline derivatives—scope and limitations. Can. J. Chem. 2004, 82, 1322–1327. [Google Scholar] [CrossRef]
- Buján, E.I.; Salum, M.L. From N-(dinitrophenyl) amino acids to benzimidazole N-oxides. Synthesis, kinetics and mechanism. J. Phys. Org. Chem. 2006, 19, 187–195. [Google Scholar] [CrossRef]
- Salum, M.L.; de Rossi, R.H.; Buján, E.I. Medium effect on the reaction of N-Butyl-2,4,6-trinitroaniline with NaOH. Eur. J. Org. Chem. 2007, 2164–2174. [Google Scholar] [CrossRef]
- Hanusek, J.; Macháček, V. Intramolecular base-catalyzed reactions involving interaction between benzene nitro groups and ortho carbon chains. Collect. Czechoslov. Chem. Commun. 2009, 74, 811–833. [Google Scholar] [CrossRef]
- Nikitina, P.A.; Perevalov, V.P. Methods of synthesis and physicochemical properties of 1-hydroxyimidazoles, imidazole 3-oxides, and their benzoannulated analogs. Chem. Heterocycl. Compd. 2017, 53, 123–149. [Google Scholar] [CrossRef]
- Sarmiento Tagle, M.G.; Salum, M.L.; Buján, E.I.; Argüello, G.A. Time evolution and competing pathways in photodegradation of trifluralin and three of its major degradation products. Photochem. Photobiol. Sci. 2005, 4, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Buján, E.I.; Cañas, A.I.; Rossi, R.H. De Amines as leaving groups in nucleophilic aromatic substitution reactions. Part 5. Substitution vs. N-oxide formation in the reaction of N-n-butyl-2,6-dinitroaniline with hydroxide ions. J. Chem. Soc. Perkin Trans. 2 2001, 1973–1977. [Google Scholar] [CrossRef]
- Cafiero, P.A.C.; French, C.S.; Mcfarlane, M.D.; Mackie, R.K.; Smith, D.M. o-Nitroaniline derivatives. Part 14. Cyclisation leading to benzimidazole N-oxides, N-hydroxybenzimidazolones and N-hydroxyquinoxaline-2,3-diones: A mechanistic borderline. J. Chem. Soc. Perkin Trans. 1 1997, 1375–1384. [Google Scholar] [CrossRef]
- Machin, J.; Mackie, R.K.; Mcnab, H.; Reed, G.A.; Sagar, A.J.G.; Smith, D.M. o-Nitroaniline Derivatives. Part V. Cyclisation of N-Acylated Derivatives of N-Benzyl- and N-p-Nitrobenzyl-o-nitroaniline: A Comparison of Carboxamides and Sulphonamides. J. Chem. Soc. Perkin 1 1976, 394–399. [Google Scholar] [CrossRef]
- Machin, B.J.; Smith, D.M. o-Nitroaniline Derivatives. Part 7. The synthesis of 2-Alkoxybenzimidazole N-Oxides (2-Alkoxy-N-hydroxybenzimidazoles) from o-Nitro anilines. J. Chem. Soc. Perkin 1 1979, 1371–1378. [Google Scholar] [CrossRef]
- Forlani, L.; Boga, C.; Mazza, M.; Cavrini, V.; Andrisano, V. Unusual reaction of 1,4-diamino-2-nitrobenzene derivatives toward nucleophiles: Catalysis by sodium sulphite. Tetrahedron 1998, 54, 4647–4654. [Google Scholar] [CrossRef]
- De Vargas, E.B.; De Rossi, R.H. Amines as leaving groups in nucleophilic aromatic substitution reactions. II. Hydrolysis of N-(2,4,6-trinitrophenyl)amines. J. Phys. Org. Chem. 1989, 2, 507–518. [Google Scholar] [CrossRef]
- Buján, E.I.; Remedi, M.V.; De Rossi, R.H. Amines as leaving groups in nucleophilic aromatic substitution reactions. Part 4. σ-adduct formation in the hydrolysis of 1-amino-2,4,6-trinitrobenzenes. J. Chem. Soc. Perkin Trans. 2 2000, 969–975. [Google Scholar] [CrossRef]










| Stress Condition | 5% Degradation | 10% Degradation | 20% Degradation |
|---|---|---|---|
| 1 M HCl, 50 °C | 2 days | 3 days | 7 days |
| 1 M NaOH, 50 °C | 1 day | 1 day | 2 days |
| 3% H2O2, 50 °C | 7 days | not achieved | not achieved |
| ACVA, 50 °C | 2 days | 14 days | not achieved |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žigart, N.; Črnugelj, M.; Ilaš, J.; Časar, Z. On the Stability and Degradation Pathways of Venetoclax under Stress Conditions. Pharmaceutics 2020, 12, 639. https://doi.org/10.3390/pharmaceutics12070639
Žigart N, Črnugelj M, Ilaš J, Časar Z. On the Stability and Degradation Pathways of Venetoclax under Stress Conditions. Pharmaceutics. 2020; 12(7):639. https://doi.org/10.3390/pharmaceutics12070639
Chicago/Turabian StyleŽigart, Nina, Martin Črnugelj, Janez Ilaš, and Zdenko Časar. 2020. "On the Stability and Degradation Pathways of Venetoclax under Stress Conditions" Pharmaceutics 12, no. 7: 639. https://doi.org/10.3390/pharmaceutics12070639