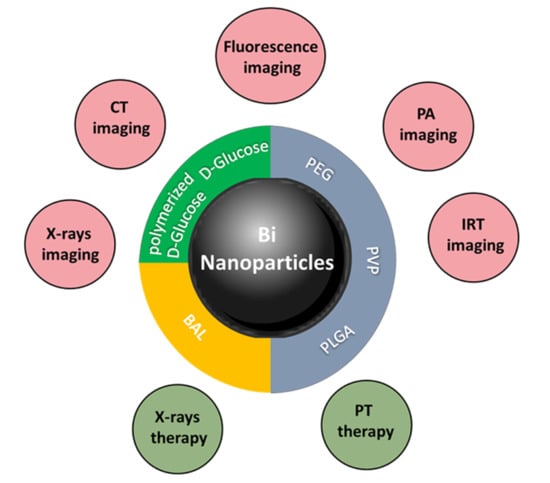
Medical Applications of Metallic Bismuth Nanoparticles
Abstract
:1. Introduction
2. Metallic Bismuth Nanoparticles as Imaging Contrast Agents
2.1. Metallic Bismuth Nanoparticles as Contrast Agents for X-ray Imaging
2.1.1. d-Glucose or Polymerized d-Glucose Coatings
2.1.2. PLGA Coating
2.1.3. BSA Coating
2.2. Metallic Bismuth Nanoparticles for Dual-Modal Imaging: X-ray and Fluorescence
2.3. Metallic Bismuth Nanoparticles for Photoacoustic Imaging
3. Metallic Bismuth Nanoparticles as X-ray Radiosensitizers
4. Metallic Bismuth Nanoparticles for Theranostic Applications
4.1. Metallic Bismuth Nanoparticles for Dual X-ray Contrast and NIR-Photothermal Therapy (Thermoradiotherapy)
4.2. Metallic Bismuth Nanoparticles for Multimodal Imaging and Multimodal Therapy
4.2.1. X-ray Imaging and PTT/RT
4.2.2. X-ray CT/PA Imaging and PTT/RT
4.2.3. CT/PA Imaging and PTT
4.2.4. CT/IRT Imaging and Chemo-Photothermal Therapy (CPTT)
4.2.5. CT/PA/IRT Imaging and PTT
4.2.6. CT/MRI Imaging and Chemo/Photothermal/Chemodynamic Therapy
5. Metallic Bismuth Nanoparticles as Bactericidal, Fungicidal, Antiparasitic and Antibiofilm Agents
5.1. Bi Citrate NPs
5.2. Bi Subnitrate NPs
5.3. Bi Subsalicylate NPs
5.4. Bi PVP NPs
5.5. BisBal NPs
5.6. Miscellaneous
6. Biocompatibility and Toxicity of Metallic Bismuth Nanoparticles
6.1. Coating of d-Glucose and Its Derivatives
6.2. Polymer Coatings
6.2.1. PEG
6.2.2. PLGA
6.2.3. PVP
6.3. BisBAL Coating
6.4. Other Coatings
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ALP | Alkaline Phosphatase |
| AST | Aspartate aminotransferase |
| Au NPs | Gold nanoparticles |
| Bi NPs | Metallic bismuth nanoparticles |
| BSA | Bovine serum albumin |
| BSS | Bismuth subsalicylate |
| BUN | Blood urea nitrogen |
| CCK-8 | Cell counting kit-8 |
| CPTT | Chemo-photothermal therapy |
| CREA | Creatinine |
| CT | Computed tomography |
| DLPC | 1,2-dilauroyl-sn-glycero-3 phosphocholine |
| DSPE | 2-distearoyl-sn-glycero-3-phosphoethanolamine |
| FBS | Fetal Bovine Serum |
| FITC | Fluorescein isothiocyanate |
| G | GANEX |
| GI | Gastrointestinal |
| HGFs | Human gingival fibroblasts |
| HU | Hounsfield units |
| IRT | Infrared thermal |
| LDH | Lactate dehydrogenase |
| MDC | Monodansylcadaverine |
| MIC | Minimal inhibitory concentration |
| MTA | Mineral Trioxide Aggregate |
| NAC | N-acetylcysteine |
| NIR | Near infrared |
| PA | Photoacoustic |
| PAI | Photoacoustic imaging |
| PEG | Polyethylene glycol |
| PLGA | Poly (DL-lactic-co-glycolic acid) |
| PPy | Polypyrrole |
| PTT | Photothermal therapy |
| PVP | Poly (vinylpyrrolidone |
| RBC | Red blood cell |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| SPR | Surface plasmon resonance |
| UA | Uric acid |
| XCAs | X-ray contrast agents |
References
- Gomez, C.; Hallot, G.; Port, M. Bismuth Metallic Nanoparticles. In Inorganic Framework as Smart Nanomedicines; William Andrew: Norwich, NY, USA, 2018; pp. 1–699. [Google Scholar]
- Shahbazi, M.A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Tang, R.; Yu, H.; Gibbons, P.C.; Buhro, W.E. Size- and Shape-Controlled Synthesis of Bismuth Nanoparticles. Chem. Mater. 2008, 20, 3656–3662. [Google Scholar] [CrossRef]
- Xia, F.; Xu, X.; Li, X.; Zhang, L.; Zhang, L.; Qiu, H.; Wang, W.; Liu, Y.; Gao, J. Preparation of Bismuth Nanoparticles in Aqueous Solution and Its Catalytic Performance for the Reduction of 4-Nitrophenol. Ind. Eng. Chem. Res. 2014, 53, 10576–10582. [Google Scholar] [CrossRef]
- Pothula, K.; Tang, L.; Zha, Z.; Wang, Z. Bismuth Nanoparticles: An Efficient Catalyst for Reductive Coupling of Nitroarenes to Azo-Compounds. RSC Adv. 2015, 5, 83144–83148. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, Y.; Li, S.; Ge, S. Preparation and Photocatalytic Performance of Bi Nanoparticles by Microwave-Assisted Method Using Ascorbic Acid as Reducing Agent. Catal. Commun. 2015, 72, 97–100. [Google Scholar] [CrossRef]
- Lusic, H.; Grinsta, M.W. X-ray-Computed Tomography Contrast Agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-Sized CT Contrast Agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Nanoparticule X-Ray Computed Tomography Contrast Agents: From Design Validation to in Vivo Applications. Acc. Chem. Res. 2012, 45, 1817–1827. [Google Scholar] [CrossRef]
- Shilo, M.; Reuveni, T.; Motiei, M.; Popovtezer, R. Nanoparticles as Computed Tomography Contrast Agents: Current Status and Future Perspectives R Eview. Nanomedecine 2012, 7, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y.; States, U. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Naha, P.C.; Benavides-Montes, V.; Litt, H.I.; Goforth, A.M.; Cormode, D.P. Synthesis, X-Ray Opacity, and Biological Compatibility of Ultra-High Payload Elemental Bismuth Nanoparticle X-Ray Contrast Agents. Chem. Mater. 2014, 26, 2266–2274. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, X.; Zhang, C.; Jiang, Y.; Fu, Y.Y.; Yu, C.; Sun, S.K.; Yan, X.P. Facile Synthesis of Uniform-Sized Bismuth Nanoparticles for CT Visualization of Gastrointestinal Tract in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 12720–12726. [Google Scholar] [CrossRef]
- Chakravarty, S.; Unold, J.; Shuboni-mulligan, D.D.; Blanco-fernandez, B.; Shapiro, E.M. Surface Engineering of Bismuth Nanocrystals to Counter Dissolution. Nanoscale 2016, 8, 13217–13222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swy, E.R.; Schwartz-Duval, A.S.; Shuboni, D.D.; Latourette, M.T.; Mallet, C.L.; Parys, M.; Cormode, D.P.; Shapiro, E.M. Dual-Modality, Fluorescent, PLGA Encapsulated Bismuth Nanoparticles for Molecular and Cellular Fluorescence Imaging and Computed Tomography. Nanoscale 2014, 6, 13104–13112. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhuang, J.; Zhang, X.; Yue, C.; Zhu, N.; Yang, L.; Wang, Y.; Chen, T.; Wang, Y.; Zhang, L.W. Autophagy associated cytotoxicity and cellular uptake mechanisms of bismuth nanoparticles in human kidney cells. Toxicol. Lett. 2017, 275, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; He, F.; Dong, Y.; Yang, D.; Dai, Y.; Xu, L.; Lv, R.; Gai, S.; Yang, P.; Lin, J. Bismuth Nanoparticles with “ Light ” Property Served as Multi-Functional Probe for X-ray Computed Tomography and Fluorescence Imaging. Chem. Mater. 2018, 30, 3301–3307. [Google Scholar] [CrossRef]
- Torrisia, L.; Silipignia, L.; Restucciaa, N.; Cuzzocreab, S.; Cutroneoc, M.; Barrecaa, F.; Faziod, B.; Di Marcod, G.; Guglielminoe, S. Laser-generated bismuth nanoparticles for applications in imaging and radiotherapy. J. Phys. Chem. Solids 2018, 119, 62–70. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Hu, Y.; Li, Z.; Fan, X.; Sun, Y.; Besenbacher, F.; Chen, C.; Yu, M. Biocompatible PEGylated Bismuth Nanocrystals: “All-in-One” Theranostic Agent with Triple-Modal Imaging and Efficient in Vivo Photothermal Ablation of Tumors. Biomaterials 2017, 141, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, A.; Zhao, C.; Yang, K.; Chen, X.; Li, W. Ultrasmall Semimetal Nanoparticles of Bismuth for Dual Modal Computed Tomography/Photoacoustic Imaging and Synergistic Thermoradiotherapy. ACS Nano 2017, 11, 3990–4001. [Google Scholar] [CrossRef]
- Yang, S.; Li, Z.; Wang, Y.; Fan, X.; Miao, Z.; Hu, Y.; Li, Z.; Sun, Y.; Besenbacher, F.; Yu, M. Multifunctional Bi @ PPy-PEG Core—Shell Nanohybrids for Dual-Modal Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1605–1615. [Google Scholar] [CrossRef]
- Yang, C.; Guo, C.; Guo, W.; Zhao, X.; Liu, S.; Han, X. Multifunctional Bismuth Nanoparticles as Theranostic Agent for PA/CT Imaging and NIR Laser-Driven Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 1, 820–830. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.; Chen, W.; Li, Q. Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Su, M. Nanoparticle Location and Material Dependent Dose Enhancement in X-Ray Radiation Therapy. J. Phys. Chem. C Nanomater. Interfaces 2012, 116, 23047–23052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.; Luo, Y.; Sun, Z.; Wang, C.; Zhang, M.; Fu, H.; Qiao, Y.; Su, M. Biosensors and Bioelectronics X-Ray Enabled Detection and Eradication of Circulating Tumor Cells with Nanoparticles. Biosens. Bioelectron. 2012, 38, 348–354. [Google Scholar] [CrossRef]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor Targeted, Stealthy and Degradable Bismuth Nanoparticles for Enhanced X-Ray Radiation Therapy of Breast Cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef]
- Jiao, L.; Li, Q.Q.; Deng, J.; Okosi, N.; Xia, J.; Su, M. Nanocellulose Templated Growth of Ultra-Small Bismuth Nanoparticles for Enhanced Radiation Therapy. Nanoscale 2018, 10, 6751–6757. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Wang, Z.; Zhang, J.; Liu, Z.; Zhu, B.; Yu, J.; Zhu, M.; Peng, C.; Chen, Z. Thiol-Capped Bi Nanoparticles as Stable and All-in-One Type Theranostic Nanoagents for Tumor Imaging and Thermoradiotherapy. Biomaterials 2018, 161, 279–291. [Google Scholar] [CrossRef]
- Luo, Y.; Hossain, M.; Wang, C.; Qiao, Y.; An, J.J.; Ma, L.; Su, M. Targeted Nanoparticles for Enhanced X-ray Radiation Killing of Multidrug-Resistant Bacteria. Nanoscale 2013, 5, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; An, R.; Zhang, P.; Yao, S.; Song, S.; Dong, L.; Xu, X.; Du, K.; Feng, J. Ultrafast Synthesis of Ultrasmall Poly (Vinylpyrrolidone)—Protected Bismuth Nanodots as a Multifunctional Theranostic Agent for In Vivo Dual-Modal CT/Photothermal-Imaging-Guided Photothermal Therapy. Adv. Funct. Mater. 2017, 27, 1–10. [Google Scholar] [CrossRef]
- Lu, S.; Xu, D.; Liao, R.; Luo, J.; Liu, Y.; Qi, Z.; Zhang, C.; Ye, N.; Wu, B.; Xu, H. Single-Component Bismuth Nanoparticles as a Theranostic Agent for Multimodal Imaging-Guided Glioma Therapy. Comput. Struct. Biotechnol. J. 2019, 17, 619–627. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Chen, X.; Li, S.; Han, Q.; Li, L.; Wang, C. Biomolecules-assisted synthesis of degradable bismuth nanoparticles for dual-modal imaging-guided chemo-photothermal therapy. Chem. Eng. J. 2020, 382, 122720. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Li, X.; Li, Y.; Li, C.; Wang, X.; Wang, J.; Guan, S.; Xu, Y.; Deng, G.; et al. A biocompatible theranostic agent based on stable bismuth nanoparticles for X-ray computed tomography/magnetic resonance imaging-guided enhanced chemo/photothermal/chemodynamic therapy for tumours. J. Colloid Interface Sci. 2021, 604, 80–90. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Diaz, D.; Arevalo-Niño, K.; Garza-Enriquez, M.; De la Garza-Ramos, M.A.; Cabral-Romero, C. Zerovalent Bismuth Nanoparticles Inhibit Streptococcus Mutans Growth and Formation of Biofilm. Int. J. Nanomed. 2012, 7, 2109–2113. [Google Scholar] [CrossRef] [Green Version]
- Nazari, P.; Dowlatabadi-Bazaz, R.; Mofid, M.R.; Pourmand, M.R.; Daryani, N.E.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shahverdi, A.R. The Antimicrobial Effects and Metabolomic Footprinting of Carboxyl-Capped Bismuth Nanoparticles against Helicobacter Pylori. Biotechnol. Appl. Biochem. 2014, 172, 570–579. [Google Scholar] [CrossRef]
- Flores-Castaneda, M.; Vega-Jimenez, A.L.; Berea, S.E.; Camps, E.; Pe, M.; Rodil, S.E. Antibacterial Effect of Bismuth Subsalicylate Nanoparticles Synthesized by Laser Ablation. J. Nanoparticle Res. 2015, 17, 431. [Google Scholar] [CrossRef]
- Vega-Jimenez, A.L.; Almaguer-Flores, A.; Flores-Castaneda, M.; Camps, E.; Uribe-Ramirez, M.; Aztatzi-Aguilar, O.G.; De Vizcaya-Ruiz, A. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology 2017, 28, 435101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasquez-Munoz, R.; Arellano-Jimenez, M.J.; Lopez-Ribot, J.L. Bismuth nanoparticles obtained by a facile synthesis method exhibit antimicrobial activity against Staphylococcus aureus and Candida albicans. BMC Biomed. Eng. 2020, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, A.R.; Rene, B.; Sa, R.I.; Cabral-Romero, C. Synthesis and Characterization of Lipophilic Bismuth Dimercaptopropanol Nanoparticles and Their Effects on Oral Microorganisms Growth and Biofilm Formation. J. Nanopart. Res. 2014, 16, 2456. [Google Scholar] [CrossRef]
- Badireddy, A.R.; Chellam, S.; Marinakos, S.M.; Wiesner, M.R. Lipophilic Nano-Bismuth Inhibits Bacterial Growth, Attachment, and Biofilm Formation. Surf. Innov. 2013, 1, 181–189. [Google Scholar] [CrossRef]
- Rodríguez-Luis, O.E.; Hernández-delgadillo, R.; Pineda-aguilar, N.; Vargas-villarreal, J.; González-salazar, F.; Garza-gonzález, J.N.; Hernández-garcía, M.E.; Chellam, S.; Cabral-romero, C.; De Odontología, F.; et al. Effect of Bismuth Lipophilic Nanoparticles (BisBAL NPs) on Trichomonas Vaginalis Growth. J. Nanosci. Nanotechnol. 2017, 17, 4618–4622. [Google Scholar] [CrossRef]
- Azad, A.; Rostamifar, S.; Modaresi, F.; Bazrafkan, A.; Rezaie, Z. Assessment of the Antibacterial Effects of Bismuth Nanoparticles against Enterococcus faecalis. Biomed. Res. Int. 2020, 2020, 5465439. [Google Scholar] [CrossRef]
- Rostamifar, S.; Azad, A.; Bazrafkan, A.; Modaresi, F.; Atashpour, S.; Jahromi, Z.K. New Strategy of Reducing Biofilm Forming Bacteria in Oral Cavity by Bismuth Nanoparticles. Biomed. Res. Int. 2021, 2021, 6695692. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; Del Angel-Mosqueda, C.; Solís-Soto, J.M.; Munguia-Moreno, S.; Pineda-Aguilar, N.; Sánchez-Nájera, R.I. Antimicrobial and Antibiofilm Activities of MTA Supplemented with Bismuth Lipophilic Nanoparticles. Dent. Mater. J. 2017, 36, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieznichenko, L.S.; Gruzina, T.G.; Dybkova, S.M.; Ushkalov, V.O.; Ulberg, Z.R. Investigation of Bismuth Nanoparticles Antimicrobial Activity against High Pathogen Microorganisms. Am. J. Bioterror. Biosecur. Biodefens. 2015, 2, 1004–1008. [Google Scholar]
- Luo, Y.; Wang, C.; Qiao, Y.; Hossain, M.; Ma, L.; Su, M. In vitro cytotoxicity of surface modified bismuth nanoparticles. J. Mater Sci. Mater. Med. 2012, 23, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Hamood, S.A.; Aldahan, Z.T. Bismuth (0) Nanoparticle as Anti-Breast Cancer Agent Synthesis and Investigation. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 809–816. [Google Scholar]
- Brown-Delgadillo, R.; Badireddy, A.R.; Zaragoza-Magaña, V.; Sánchez-nájera, R.I.; Chellam, S.; Cabral-romero, C. Effect of Lipophilic Bismuth Nanoparticles on Erythrocytes. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Delgadillo, R.; Appala Raju, B.; Martinez-Sanmiguel, J.J.; Contreras-Cordero, J.F.; Martinez-Gonzalez, G.I.; Sanchez-Najera, R.I.; Shankaraman, C.; Cabral-Romero, C. Cytotoxic Effect of Lipophilic Bismuth Dimercaptopropanol Nanoparticles on Epithelial Cells. J. Nanosci. Nanotechnol. 2016, 16, 203–209. [Google Scholar] [CrossRef]
- Cabral-Romero, C.; Solis-Soto, J.M.; Sanchez-Perrez, Y.; Pineda-Aguilar, N.; Meester, I.; Perez-Carillo, E.; Nakagoshi-Cepeda, S.E.; Sanchez-Najera, R.I.; Nakagoshi-Cepeda, M.A.A.; Hernandez-Delgadillo, R.; et al. Antitumor Activity of a Hydrogel loaded with Lipophilic Bismuth Nanoparticles on Cervical, Prostate, and Colon Human Cancer Cells. Anti-Cancer Drugs 2020, 31, 251–259. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; Garcia-Cuellar, C.M.; Sanchez-Perrez, Y.; Pineda-Aguilar, N.; Martinez-Martinez, M.A.; Rangel-Padilla, E.E.; Nakagoshi-Cepeda, S.E.; Solis-Soto, J.M.; Sanchez-Najera, R.I.; Nakagoshi-Cepeda, M.A.A.; et al. In vitro Evaluation of the Antitumor Effects of Bismuth Lipophilic Nanoparticles (BisBAL NPs) on Breast Cancer Cells. Inter. J. Nanomed. 2018, 13, 6089–6097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakibaie, M.; Forootanfar, H.; Ameri, A.; Adeli-Sardou, M.; Jafari, M.; Rahimi, H.R. Cytotoxicity of Biologically Synthesised Bismuth Nanoparticles against HT-29 Cell Line. Inst. Eng. Technol. 2018, 12, 653–657. [Google Scholar] [CrossRef]
- Shakibaie, M.; Amiri-Moghadam, P.; Ghazanfari, M.; Adeli-Sardou, M.; Jafari, M.; Forootanfar, H. Cytotoxic and antioxidant activity of the biogenic bismuth nanoparticles produced by Delftia sp. SFG. Mater. Res. Bull. 2018, 104, 155–163. [Google Scholar] [CrossRef]
- Da Luz, J.Z.; Machado, T.N.; Bezerra, A.G.; Oliveira Ribeiro, C.A.; Neto, F.F. Cytotoxicity of Bismuth Nanoparticles in the Murine Macrophage Cell Line RAW 264.7. J. Mater. Sci. Mater. Med. 2020, 31, 95. [Google Scholar] [CrossRef] [PubMed]
- Reus, T.L.; Machado, T.N.; Bezerra, A.G., Jr.; Hilzendeger Marcon, B.; Campos Paschoal, A.C.; Kuligovski, C.; de Aguiar, A.M.; Dallagiovanna, B. Dose-dependent cytotoxicity of bismuth nanoparticles produced by LASiS in a reference mammalian cell line BALB/c 3T3. Toxicol. Vitro 2018, 53, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, H.; Zhang, X.; Wang, Y.; Song, Z.; Zhao, J.; Shi, H.; Li, R.; Wang, Y.; Zhang, L.W. The protective role of autophagy in nephrotoxicity induced by bismuth nanoparticles through AMPK/mTOR pathway. Nanotoxicology 2018, 12, 586–601. [Google Scholar] [CrossRef]

| Entry | Capping Agent | Diameter TEM (nm) | Biological Applications | Proof of Concept | Reference |
|---|---|---|---|---|---|
| 1 | PVP, APTES and conjugation with folic acid | 30 | X-ray radiosensitizers to detect and kill circulating tumor cells | In vitro | Hossain et al. 2012 |
| 2 | PVP and conjugation with Pseudomonas aeruginosa polyclonal antibody | 30 | X-ray radiosensitizers to eliminate bacteria | In vitro | Luo et al. 2013 |
| 3 | Red blood cell membrane and conjugation with folic acid | 56 | X-ray radiosensitizers for breast cancer | In vitro & in vivo (mice) | Deng et al. 2018 |
| 4 | Cellulose nanofiber | 2–10 | X-ray radiosensitizers for breast cancer | In vitro & in vivo (mice) | Jiao et al. 2018 |
| 5 | 1-Dodecanethiol PEGylated phospholipid | 40 | CT tomography & photothermal and radiotherapy treatment of tumors. | In vitro & in vivo (mice) | Yu et al. 2018 |
| 6 | DSPE-PEG5000 and conjugation to peptide LyP-1 | 3.6 | CT tomography & photoacoustic imaging agent & NIR-photothermal and radiotherapy treatment of tumors. | In vitro & in vivo (mice) | Yu et al. 2017 |
| 7 | DLPC (,2-dilauroyl-sn-glycero-3-phosphocholine) | 47 | CT tomography & photoacoustic imaging agent & NIR-photothermal treatment of tumors. | In vitro & in vivo (mice) | Yang et al. 2018 |
| 8 | Poly (vinylpyrrolidone) | 2.7 | CT &photothermal-imaging-guided photothermal therapy | In vitro & in vivo (mice) | Lei et al. 2017 |
| 9 | Ppy PEG | 70 | CT tomography & photoacoustic imaging agent & NIR-photothermal treatment of tumors | In vitro & in vivo (mice) | Yang Sisi et al. 2017 |
| 10 | DSPE PEG | 100 **** | CT tomography & photoacoustic imaging agent & NIR-photothermal treatment of tumors | In vitro & in vivo (mice) | Lu et al. 2019 |
| 11 | GEL, BSA, HSA | 15–19 | CT tomography & infrared thermal & antitumor PTT | In vitro & in vivo (mice) | Liu et al. 2020 |
| 12 | PEG | 41 | Trimodal imaging (CT, photoacoustic and infrared thermal) & antitumor PTT | In vitro & in vivo (mice) | Li et al. 2017 |
| 13 | d-glucose & 1,2-propanediol | 74 | CT tomography | In vitro | Brown et al. 2014 |
| 14 | PLGA and SiO2 | 12 | CT tomography | In vitro | Chakravarty et al. 2016 |
| 15 | PLGA | 120 * | CT tomography | In vitro & ex vivo (chicken wing forearm) | Swy et al. 2014 |
| 16 | PEG NH2 | 4 to 100 | CT tomography and fluorescence imaging | In vitro & in vivo (mice) | Bi et al. 2018 |
| 17 | Polymerized d-glucose | 22 | CT tomography (GI tract) | In vitro & in vivo (mice) | Wei et al. 2016 |
| 18 | BSA | 6–7 | CT tomography, fluorescence imaging and cytotoxicity | In vitro & in vivo (mice) | Liu et al. 2017 |
| 19 | Surfactant (not described) | 10–100 * | CT tomography and radiotherapy | In vivo (mice) | Torisi et al. 2018 |
| 20 | Mesoporous silica | 115 nm **** | CT tomography/magnetic resonance imaging chemo/photothermal/chemodynamic therapy | In vitro & in vivo (mice) | Zhao et al. 2021 |
| Entry | Capping Agent | Diameter TEM (nm) | Biological Applications | Proof of Concept | Reference |
|---|---|---|---|---|---|
| 1 | Citrate | 3.3 | Antimicrobial activity against Streptococcus mutans and inhibition of biofilm formation by Streptococcus mutans | In vitro | Hernandez et al. 2012 |
| 2 | Carboxylic groups | 100 | Antibacterial activity against Helicobacter pylori | In vitro | Nazari et al. 2013 |
| 3 | Subsalycilate | 22, 31, 45 and 58 ** | Antibacterial activity against E. coli, P. aeruginosa, S. aureus and S. epidermidis | In vitro | Mariela Flores-Castaneda et al. 2015 |
| 4 | Subsalycilate | 4-22 | Antibacterial activity against Actinomyces israelii, Aggregatibacter actinomycetemcomitans serotype b, Capnocytophaga gingivalis, Eikenella corrodens, Fusobacterium nucleatum subsp. nucleatum, Parvimonas micra, Porphyromonas gingivalis, Prevotella intermedia, Streptococcus mutans and Streptococcus sanguinis | In vitro | Vega-Jimenez et al. 2017 |
| 5 | PVP | 8 | Antimicrobial activity and antibiofilm activity on Staphylococcus aureus and Candida albicans | In vitro | Vazquez-Munoz et al. 2020 |
| 6 | 2,3-dimercapto-1-propanol | 28 | Antibacterial activity against Streptococcus mutans and Streptococcus gordonii Antimycotical activity against Candida albicans Inhibition of biofilm formation by a multispecies population of S. mutans, L. casei, S. gordonii and C. albicans | In vitro | Badireddy et al. 2014 |
| 7 | 2,3-dimercapto-1-propanol | 3–15 | Antibacterial activity Inhibition of biofilm formation by Pseudomonas aeruginosa | In vitro | Badireddy et al. 2014 |
| 8 | 2,3-dimercapto-1-propanol | 25 * | Antiparasitic activity Inhibition of Trichomonas vaginalis growth | In vitro | Rodríguez-Luis et al. 2017 |
| 9 | 2,3-dimercapto-1-propanol | 40 | Antibacterial activity of Enterococcus faecalis and Streptococcus salivarius | In vitro | Azad et al. 2020 Rostamifar et al. 2021 |
| 10 | Mineral trioxide aggregate supplemented with Bi NPs coated with 2,3-dimercapto-1-propanol | ND | Antibacterial, antifongical activity of Enterococcus faecalis, Escherichia coli, and Candida albicans Inhibition of biofilm formation by Enterococcus faecalis and mechanical properties of mineral trioxide aggregate (MTA) supplemented with Bi NPs | In vitro | Hernandez-Delgadillo et al. 2017 |
| 11 | ND | 40 | Antimicrobial activity of Campylobacter jejuni Pl—09.c, Listeria monocytogenes ATCC 19112, Yersinia enterocolitica 12/15- 08, Salmonella typhimurium N°16, Escherichia coli N°4; Mycoplasma arginini G 230, Acholeplasma laidlawii ATCC 23206, Bacillus anthracis M-71, Leptospira Pomona microorganisms | In vitro | Rieznichenko et al. 2015 |
| Entry | Capping Agent | Diameter TEM (nm) | Proof of Concept | Cytotoxicity | Toxicity In Vivo | Reference |
|---|---|---|---|---|---|---|
| 1 | Cellulose nanofiber | 2–10 | In vitro & in vivo (mice) | MTT assay: 4T1 breast cancer cells | ND | Jiao et al. 2018 |
| 2 | 1-Dodecanethiol PEGylated phospholipid | 40 | In vitro & in vivo (mice) | kit-8 (CCK-8) assay: 4T1 cancer cells | Toxicity on mice (IV injection): hematology & junctional liver and kidney markers | Yu et al. 2018 |
| 3 | DSPE-PEG5000 and conjugation to peptide LyP-1 | 3.6 | In vitro & in vivo (mice) | kit-8 (CCK-8) assay: 4T1 cancer cells and hepatic L02 cells | ND | Yu et al. 2017 |
| 4 | DLPC (,2-dilauroyl-sn-glycero-3-phosphocholine) | 47 | In vitro & in vivo (mice) | MTT assay: MDA-MB-231 and MCF-10A cells | Body weight changes, histology of main organ and blood analysis | Yang et al. 2018 |
| 5 | Poly (vinylpyrrolidone) | 2,7 | In vitro & in vivo (mice) | MTT assay: U14 cells | Histology of main organ and blood analysis | Lei et al. 2017 |
| 6 | Ppy PEG | 70 | In vitro & in vivo (mice) | kit-8 (CCK-8) assay: HUVEC & 4T1 cancer cells | Histology of main organ and serum biochemistry | Yang Sisi et al. 2017 |
| 7 | DSPE PEG | 100 **** | In vitro & in vivo (mice) | MTT assay: C6 & Cos-7 cells | Histology of main organ, liver function and blood analysis | Lu et al. 2019 |
| 8 | GEL, BSA, HSA | 15–19 | In vitro & in vivo (mice) | MTT assay: HepG-2 & HeLa cells | Toxicity on nude mice (IV injection): hematology & major organs | Liu et al. 2020 |
| 9 | PEG | 41 | In vitro & in vivo (mice) | kit-8 (CCK-8) assay: HUVEC & HeLa cancer cells hemolytic behavior on Red Blood Cell | Histology of main organ and serum biochemistry | Li et al. 2017 |
| 10 | d-glucose & 1,2-propanediol | 74 | In vitro | MTS assays: HeLa cell & J774.A macrophage cell | ND | Brown et al. 2014 |
| 11 | PLGA and SiO2 | 12 | In vitro | MTT assay: Raw264.7 macrophage | ND | Chakravarty et al. 2016 |
| 12 | PLGA | 120 * | In vitro & ex vivo (chicken wing forearm) | MTT assay STO mouse fibroblast | Toxicity on rats (IV & IP injection): hematology & Blood chemistry, abnormalities & pathological & histopathological analysis | Swy et al. 2014 |
| 13 | PEG NH2 | 4 to 100 | In vitro & in vivo (mice) | MTT assay: L929 cells | ND | Bi et al. 2018 |
| 14 | Polymerized d-glucose | 22 | In vitro & in vivo (mice) | MTT assay: HeLa cells | Toxicity on mice (oral administration) blood chemistry & histopathological analysis | Wei et al. 2016 |
| 15 | Subsalycilate | 4-22 | In vitro | LDH and MTS assays in Human gingival fibroblast (HGF-1) cells | ND | Vega-Jimenez et al. 2017 |
| 16 | Mineral trioxide aggregate supplemented with Bi NPs coated with 2,3-dimercapto-1-propanol | ND | In vitro | Human gingival fibroblasts (HGF) | ND | Hernandez-Delgadillo et al. 2017 |
| 17 | PEG-SiO2, NH2-SiO2, SiO2 or PVP | 20 | In vitro | MTT, G6PD and calcein AM/EthD-1 assays: HeLa, MG-63 | ND | Luo et al. 2012 |
| 18 | PVP | 78 *** | In vitro | Breast cancer cell line MCF-7 | ND | Hammood et al. 2016 |
| 19 | 2,3-dimercapto-1-propanol | 18 | In vitro | Erythrocytes, leukocytes, & neutrophils | ND | Hernandez-Delgadillo et al. 2015 |
| 20 | 2,3-dimercapto-1-propanol | 18 | In vitro | Monkey kidney cells | ND | Hernandez-Delgadillo et al. 2016 |
| 21 | 2,3-dimercapto-1-propanol | 24 * | In vitro & in vivo (mice) | HeLa, DU145, HCT-116 | Histology of main organ | Cabral-Romero et al. 2020 |
| 22 | 2,3-dimercapto-1-propanol | 28 * | In vitro | Human breast cancer cell line MCF-7 | ND | Hernandez-Delgadillo et al. 2018 |
| 23 | ND | 40–120 | In vitro | MTT assay: HT29 cells | ND | Shakibaie et al. 2018 |
| 24 | ND | 20-120 | In vitro | MTT assay: lung (A549), breast (MCF-7) cancer cells, 3T3 fibroblast cells | ND | Shakibaie et al. 2018 |
| 25 | BSA | 4–87 **** | In vitro | Murine macrophage cell line RAW 264.7 | ND | Zablocki da Luz et al. 2020 |
| 26 | BSA | 20 | In vitro | BALB/c 3T3 cells | ND | Reus et al. 2018 |
| 27 | BSA | 6-7 | In vitro & in vivo (mice) | MTT assay: HEK293, HepG2, HUVEC, A549 cells | Mouse mammary carcinoma cell line 4T1 (IV injection) | Liu et al. 2017 |
| 28 | BSA | 6–7 | In vitro & in vivo (mice) | HEK223 cells | Histology of kidney and nephrotoxicity on Balb/c mice (IV injection) | Liu et al. 2018 |
| 29 | Mesoporous silica | 115 nm **** | In vitro & in vivo (mice) | CCK-8 assay HUVECs and HeLa cells | Body weight changes, histology of main organ | Zhao et al. 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, C.; Hallot, G.; Laurent, S.; Port, M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics 2021, 13, 1793. https://doi.org/10.3390/pharmaceutics13111793
Gomez C, Hallot G, Laurent S, Port M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics. 2021; 13(11):1793. https://doi.org/10.3390/pharmaceutics13111793
Chicago/Turabian StyleGomez, Catherine, Gauthier Hallot, Sophie Laurent, and Marc Port. 2021. "Medical Applications of Metallic Bismuth Nanoparticles" Pharmaceutics 13, no. 11: 1793. https://doi.org/10.3390/pharmaceutics13111793