Design of Targeted Flurbiprofen Biomimetic Nanoparticles for Management of Arthritis: In Vitro and In Vivo Appraisal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bovine Serum Albumin Nanoparticles (BSA NPs)
2.3. Preparation of Hyaluronic Acid-Coated BSA NPs (HA-BSA NPs)
2.4. Determination of Hyaluronic Acid Content
2.5. Physicochemical Characterization of Prepared BSA NPs
2.5.1. Measurement of Particle Size, Zeta Potential and Polydispersity Index
2.5.2. Measurement of Percentage Entrapment Efficiency (%EE) and Loading Content (%LC)
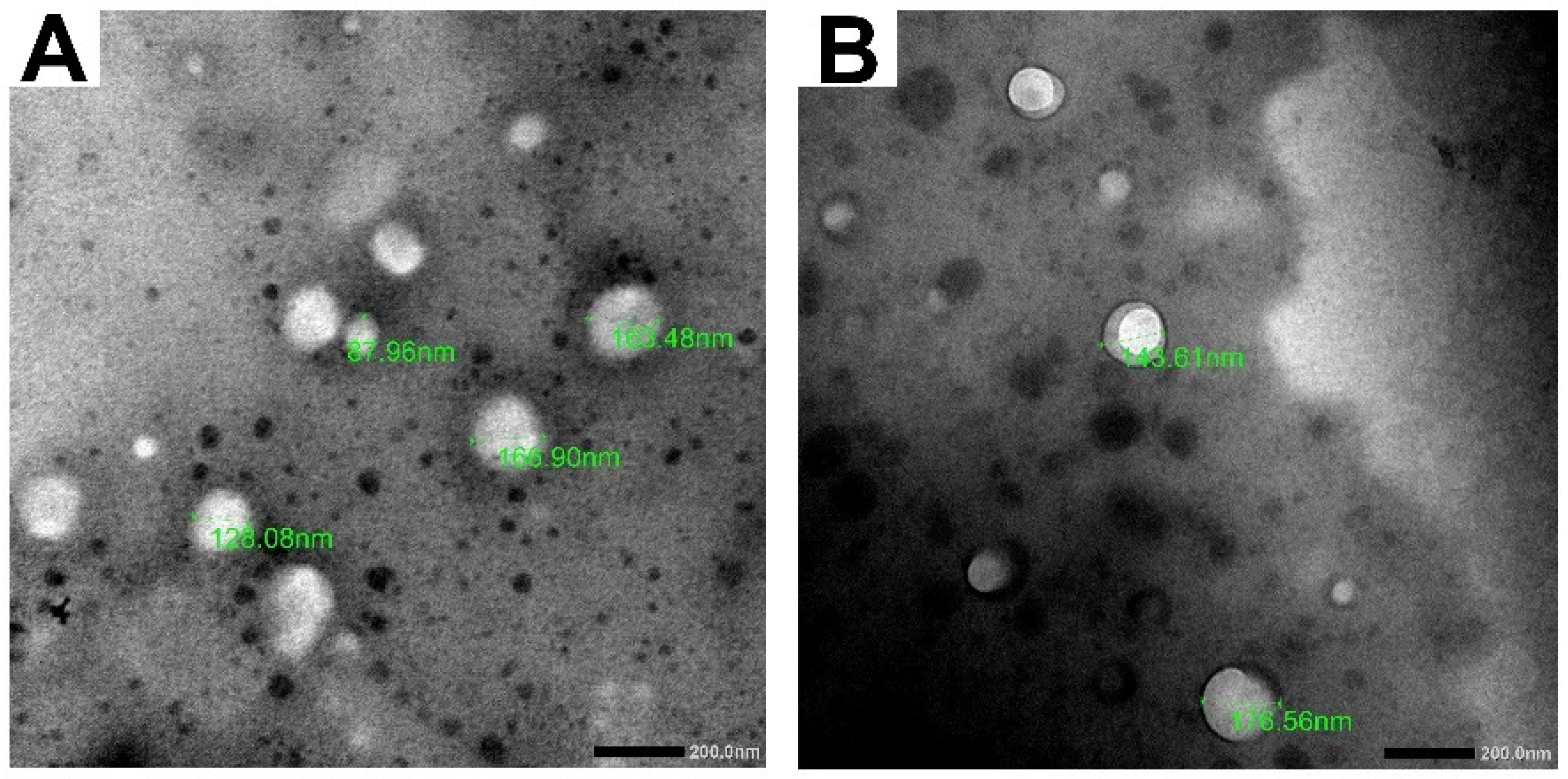
2.5.3. Transmission Electron Microscopy (TEM)
2.5.4. Differential Scanning Calorimetry (DSC)
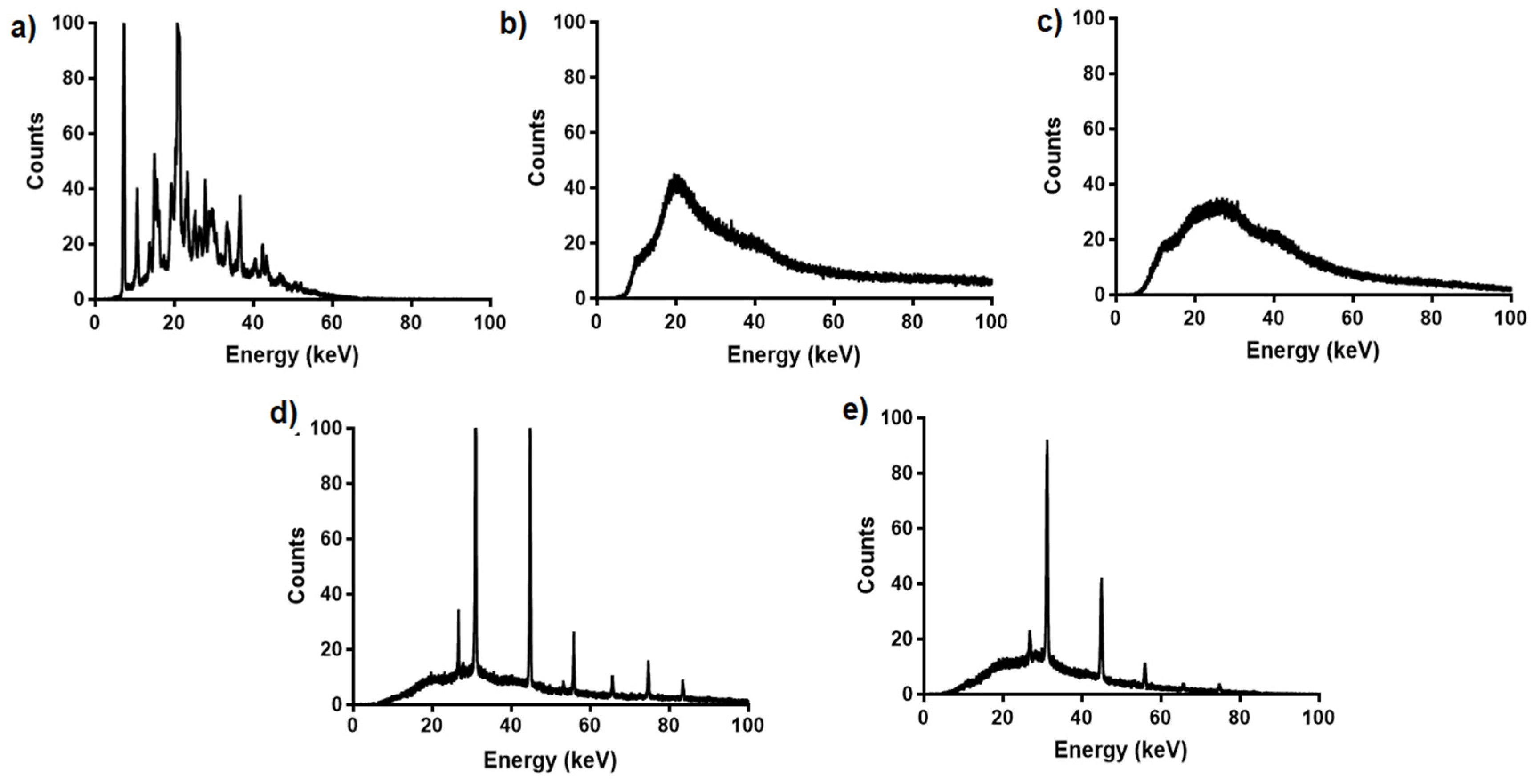
2.5.5. X-ray Diffraction (XRD) Analysis
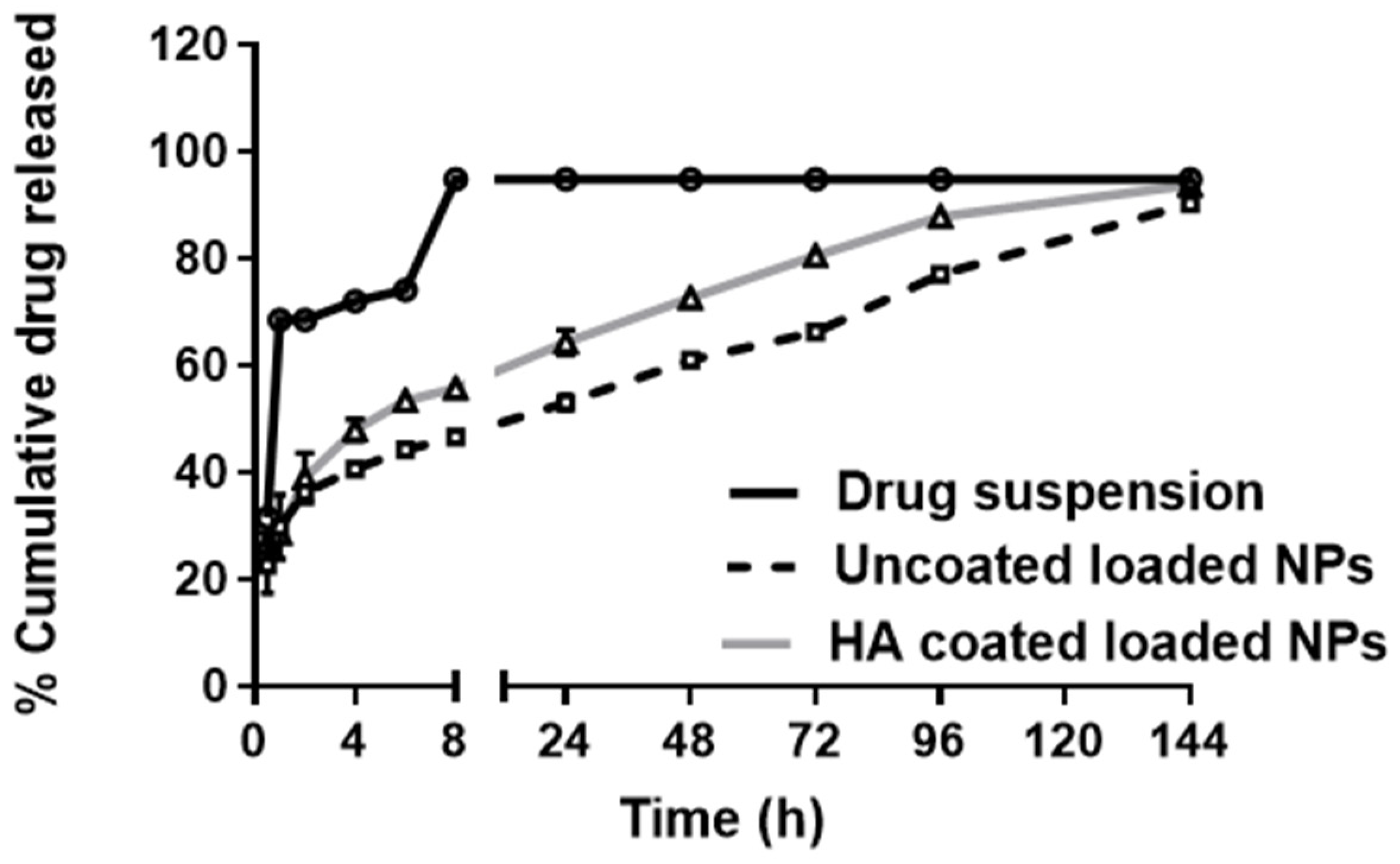
2.5.6. In Vitro Release of FLUR from BSA NPs
2.5.7. Stability Study
2.6. In Vivo Characterization of the Prepared NPs
2.6.1. Animals
2.6.2. Induction of Arthritis
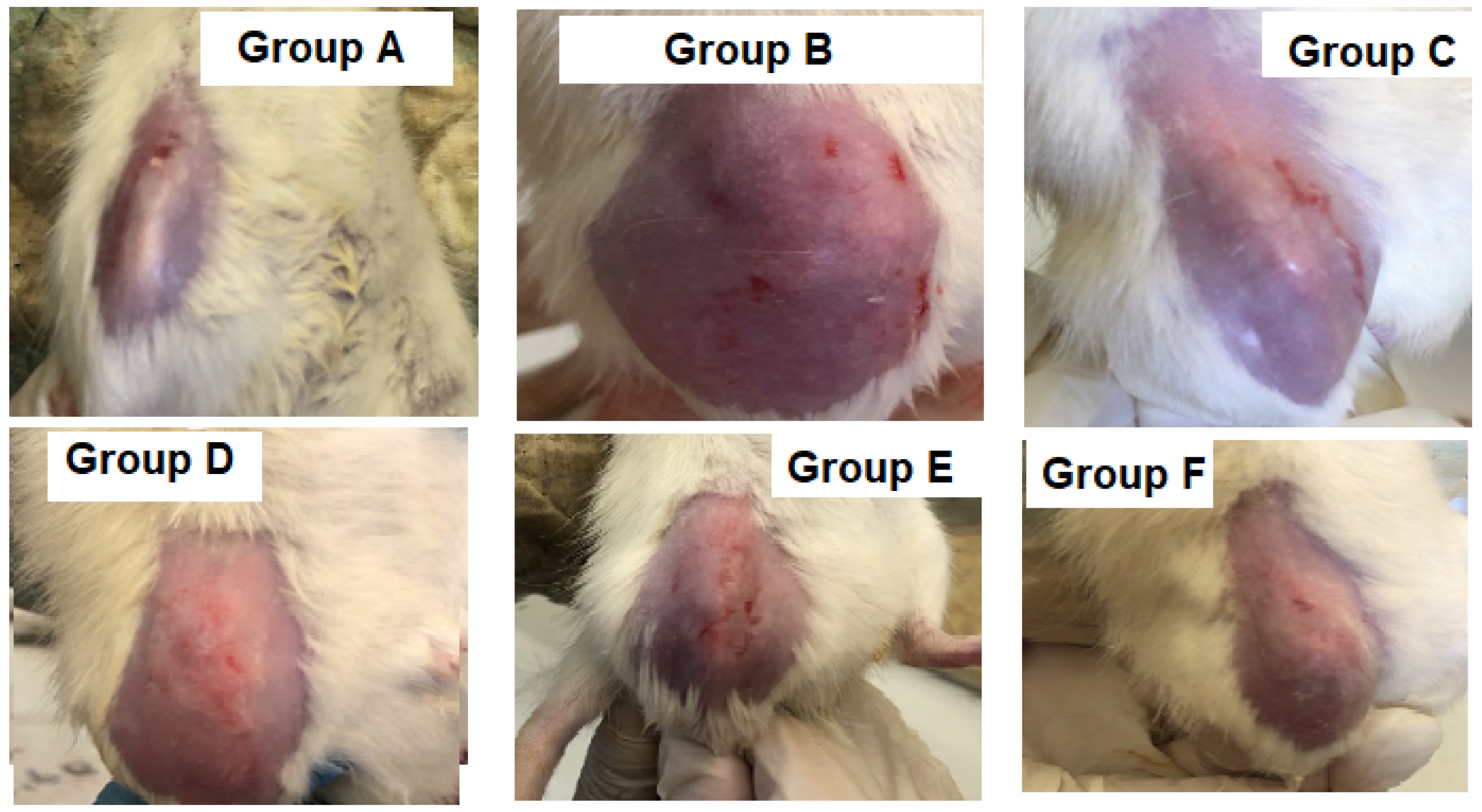
2.6.3. Knee Joint Swelling
2.6.4. Behavioral Testing (Knee Bend Test)
2.6.5. Termination and Tissue Collection
2.6.6. Evaluation of C-Reactive Protein (CRP) Level
2.6.7. Measurement of Inflammatory Mediators in Serum
2.6.8. Evaluation of Inflammatory Mediators’ Expression by Western Blotting
2.6.9. Histopathological Examination
2.6.10. Immunohistochemistry
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization and Physicochemical Characterization of the Prepared BSA NPs
3.1.1. Transmission Electron Microscopy (TEM)
3.1.2. Differential Scanning Calorimetry (DSC) and X-ray Diffraction (XRD) Analysis
3.1.3. In Vitro Release of FLUR from BSA NPs
3.1.4. Stability Study
3.2. In Vivo Characterization of the Prepared NPs
3.2.1. Knee Joint Swelling
3.2.2. Knee Bend Test
3.2.3. Measurement of C-Reactive Protein (CRP) Level
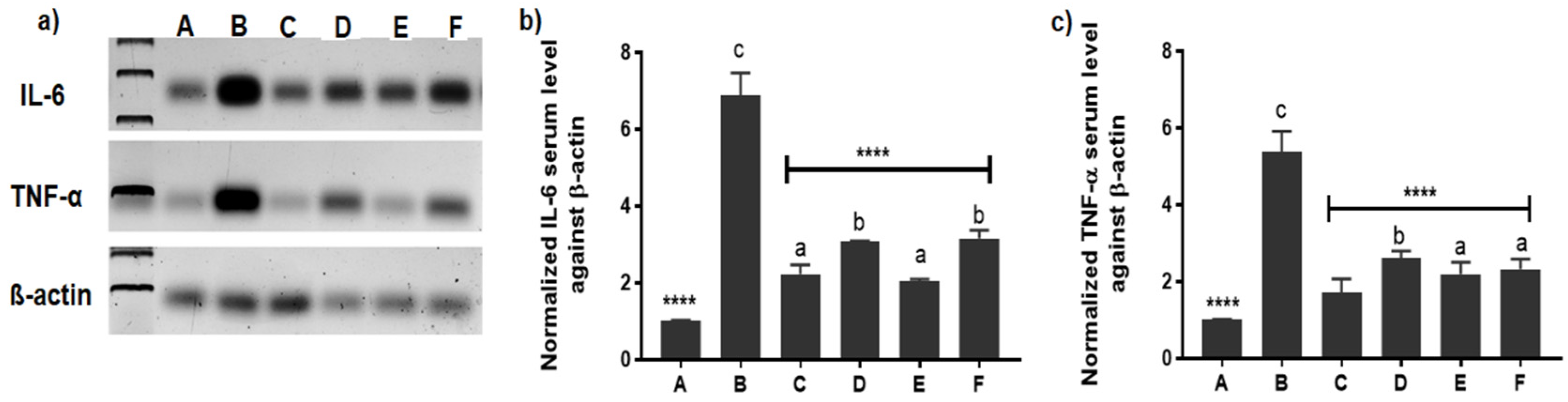
3.2.4. Measurement of Inflammatory Mediators in Serum
3.2.5. Evaluation of Inflammatory Mediators’ Expression by Western Blotting
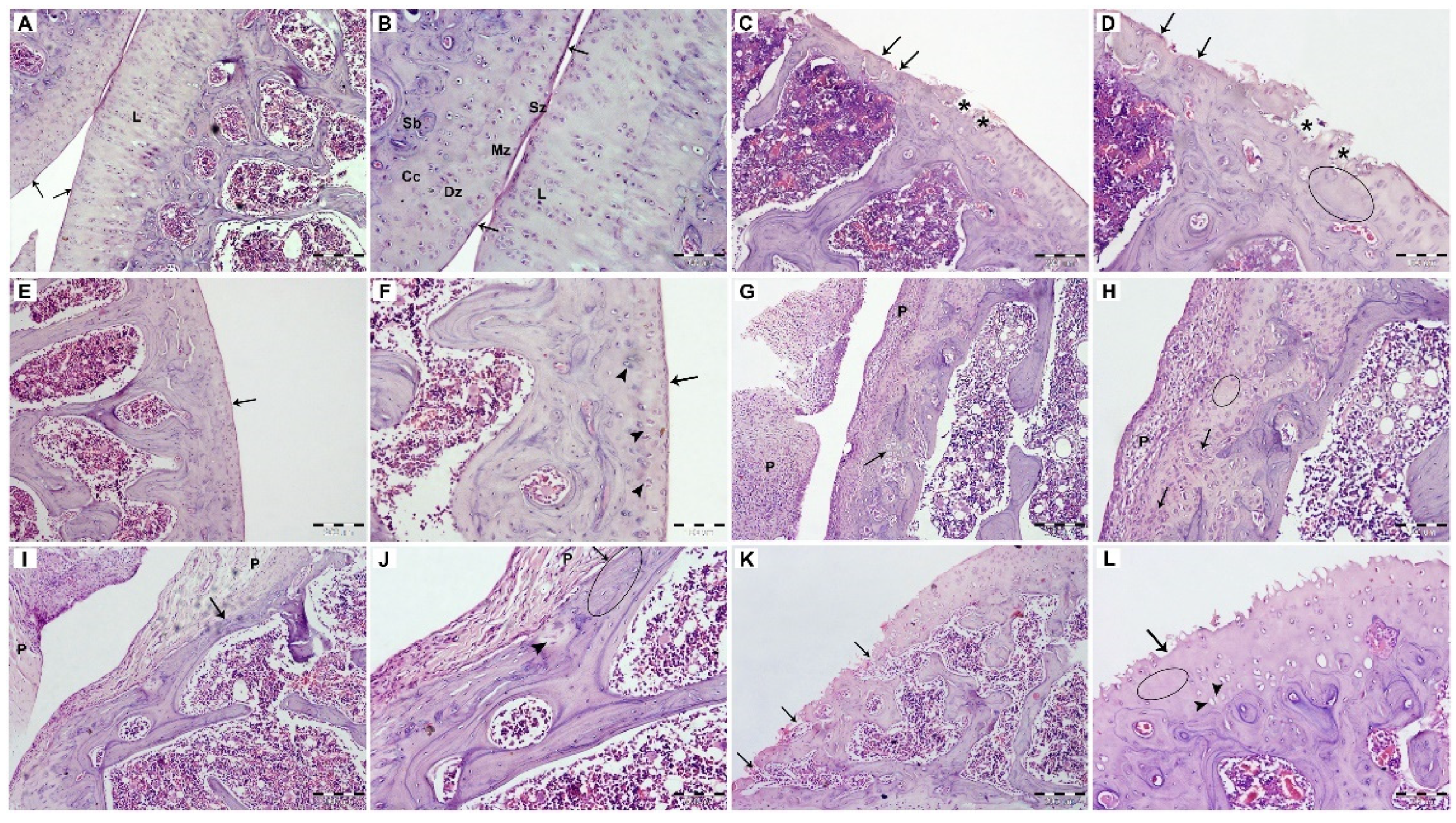
3.2.6. Histopathological Examination
3.2.7. Immunohistochemistry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chegini, S.P.; Varshosaz, J.; Taymouri, S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Qin, X.; Fang, J.; Sun, X. Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies. Acta Pharm. Sin. B 2021, 11, 1158–1174. [Google Scholar] [CrossRef]
- Zheng, M.; Jia, H.; Wang, H.; Liu, L.; He, Z.; Zhang, Z.; Yang, W.; Gao, L.; Gao, X.; Gao, F. Application of nanomaterials in the treatment of rheumatoid arthritis. R. Soc. Chem. 2021, 11, 7129–7137. [Google Scholar] [CrossRef]
- Sato, J.; Aoyama, M.; Yamazaki, M.; Okumura, S.; Takahashi, K.; Funakubo, M.; Mizumura, K. Artificially produced meteorological changes aggravate pain in adjuvant-induced arthritic rats. Neurosci. Lett. 2004, 354, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlenberg, J.M.; Fox, D.A. Advances in the medical treatment of rheumatoid arthritis. Hand Clin. 2011, 27, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Koenders, M.I.; van den Berg, W.B. Novel therapeutic targets in rheumatoid arthritis. Trends Pharmacol. Sci. 2015, 36, 189–195. [Google Scholar] [CrossRef]
- Dolati, S.; Sadreddini, S.; Rostamzadeh, D.; Ahmadi, M.; Jadidi-Niaragh, F.; Yousefi, M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed. Pharmacother. 2016, 80, 30–41. [Google Scholar] [CrossRef]
- Smolen, J.S.; van der Heijde, D.; Machold, K.P.; Aletaha, D.; Landewé, R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 2014, 73, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Timlin, H.; Bingham, C.O. Efficacy and safety implications of molecular constructs of biological agents for rheumatoid arthritis. Expert Opin. Biol. Ther. 2014, 14, 893–904. [Google Scholar] [CrossRef]
- Thakur, S.; Riyaz, B.; Patil, A.; Kaur, A.; Kapoor, B.; Mishra, V. Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: An overview. Biomed. Pharmacother. 2018, 106, 1011–1023. [Google Scholar] [CrossRef]
- Yang, M.; Feng, X.; Ding, J.; Chang, F.; Chen, X. Nanotherapeutics relieve rheumatoid arthritis. J. Control. Release 2017, 252, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.; Tilak, A.; Sharma, J.; Dave, V.; Sharma, S.; Yadav, R.; Patel, S.; Verma, K.; Tak, K. Flurbiprofen loaded ethosomes—Transdermal delivery of anti-inflammatory effect in rat model. Lipids Health Dis. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhilegaonkar, S.; Gaonkar, R. Formulation and evaluation of colon targeted drug delivery system of flurbiprofen for treatment of rheumatoid arthritis. Int. J. Pharm. Sci. Res. 2018, 9, 1865–1877. [Google Scholar] [CrossRef]
- Kawadkar, J.; Jain, R.; Kishore, R.; Pathak, A.; Chauhan, M.K. Formulation and evaluation of flurbiprofen-loaded genipin cross-linked gelatin microspheres for intra-articular delivery. J. Drug Target. 2013, 21, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Maudens, P.; Jordan, O.; Allemann, E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov. Today 2018, 23, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Pham, C.T. Intra-articular drug delivery systems for joint diseases. Curr. Opin. Pharmacol. 2018, 40, 67–73. [Google Scholar] [CrossRef]
- Jüni, P.; Hari, R.; Rutjes, A.W.; Fischer, R.; Silletta, M.G.; Reichenbach, S.; da Costa, B.R. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015, Cd005328. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Lefèvre-Colau, M.M.; Poiraudeau, S.; Rannou, F. Evidence and recommendations for use of intra-articular injections for knee osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 184–189. [Google Scholar] [CrossRef]
- Newberry, S.J.; Fitzgerald, J.D.; Maglione, M.A.; O’Hanlon, C.E.; Booth, M.; Motala, A.; Timmer, M.; Shanman, R.; Shekelle, P.G. AHRQ Technology Assessments. In Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2015. [Google Scholar]
- Mitragotri, S.; Yoo, J.-W. Designing micro- and nano-particles for treating rheumatoid arthritis. Arch. Pharm. Res. 2011, 34, 1887–1897. [Google Scholar] [CrossRef]
- O’Mary, H.; Del Rincόn, I.; Cui, Z. Nanomedicine for Intra-Articular Drug Delivery in Rheumatoid Arthritis. Curr. Med. Chem. 2016, 23, 2490–2506. [Google Scholar] [CrossRef]
- Pham, C.T.N. Nanotherapeutic approaches for the treatment of rheumatoid arthritis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Gorantla, S.; Singhvi, G.; Rapalli, V.K.; Waghule, T.; Dubey, S.K.; Saha, R.N. Targeted drug-delivery systems in the treatment of rheumatoid arthritis: Recent advancement and clinical status. Ther. Deliv. 2020, 11, 269–284. [Google Scholar] [CrossRef]
- Bader, R.A. The Development of Targeted Drug Delivery Systems for Rheumatoid Arthritis Treatment. In Rheumatoid Arthritis—Treatment, 2nd ed.; Lemmey, A., Ed.; IntechOpen: London, UK, 2012; pp. 111–132. [Google Scholar]
- Syed, A.; Devi, V.K. Potential of targeted drug delivery systems in treatment of rheumatoid arthritis. J. Drug Deliv. Sci. Technol. 2019, 53, 101217. [Google Scholar] [CrossRef]
- Jain, K.K. Drug delivery systems—An overview. Methods Mol. Biol. 2008, 437, 1–50. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, X. Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater. Sci. 2017, 5, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2018, 43, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholijani, N.; Abolmaali, S.S.; Kalantar, K.; Ravanrooy, M.H. Therapeutic Effect of Carvacrol-loaded Albumin Nanoparticles on Arthritic Rats. Iran J. Pharm. Res. 2020, 19, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.H.; Babu, J.R. Nano-Sized Drug Delivery. Mol. Pharm. Org. Process Res. 2013, 1, e108. [Google Scholar] [CrossRef] [Green Version]
- Naor, D.; Nedvetzki, S. CD44 in rheumatoid arthritis. Arthritis Res. Ther. 2003, 5, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kim, S.H.; Thambi, T.; You, D.G.; Jeon, J.; Lee, J.O.; Chung, B.Y.; Jo, D.G.; Park, J.H. A hyaluronic acid-methotrexate conjugate for targeted therapy of rheumatoid arthritis. Chem. Commun. 2014, 50, 7632–7635. [Google Scholar] [CrossRef]
- Heo, R.; Park, J.S.; Jang, H.J.; Kim, S.H.; Shin, J.M.; Suh, Y.D.; Jeong, J.H.; Jo, D.G.; Park, J.H. Hyaluronan nanoparticles bearing γ-secretase inhibitor: In vivo therapeutic effects on rheumatoid arthritis. J. Control. Release 2014, 192, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Liu, X.R.; Chen, Q.B.; Li, Y.; Zhou, J.L.; Zhou, L.Y.; Zou, T. Hyaluronic acid and albumin-based nanoparticles for drug delivery. J. Control. Release 2021, 331, 416–433. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Wu, L.; Li, W.; Chen, J.; Cheng, H.; Pan, J.; Cai, B. Hyaluronic acid-coated bovine serum albumin nanoparticles loaded with brucine as selective nanovectors for intra-articular injection. Int. J. Nanomed. 2013, 8, 3843–3853. [Google Scholar] [CrossRef] [Green Version]
- Wong, L.R.; Ho, P.C. Role of serum albumin as a nanoparticulate carrier for nose-to-brain delivery of R-flurbiprofen: Implications for the treatment of Alzheimer’s disease. J. Pharm. Pharmacol. 2018, 70, 59–69. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, Q. Establishment of CTAB Turbidimetric method to determine hyaluronic acid content in fermentation broth. Carbohydr. Polym. 2009, 78, 178–181. [Google Scholar] [CrossRef]
- Liu, Y.L.; Lin, H.M.; Zou, R.; Wu, J.C.; Han, R.; Raymond, L.N.; Reid, P.F.; Qin, Z.H. Suppression of complete Freund’s adjuvant-induced adjuvant arthritis by cobratoxin. Acta Pharmacol. Sin. 2009, 30, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Adães, S.; Mendonça, M.; Santos, T.N.; Castro-Lopes, J.M.; Ferreira-Gomes, J.; Neto, F.L. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Arthritis Res. Ther. 2014, 16, R10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, N.P.; Tao, C.; Yang, A.; Louie, L.; Zheng, T.; Yao, Z.; Soon-Shiong, P.; Magdassi, S. Protein Stabilized Pharmacologically Active Agents, Methods for the Preparation Therof and Methods for the Use Therof. U.S. Patent Patent Number 5,916,596, 29 June 1999. [Google Scholar]
- Rahimnejad, M.; Najafpour, G.; Bakeri, G. Investigation and modeling effective parameters influencing the size of BSA protein nanoparticles as colloidal carrier. Colloids Surf. A Physicochem. Eng. Asp. 2012, 412, 96–100. [Google Scholar] [CrossRef]
- Radwan, S.E.; El-Kamel, A. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [Google Scholar] [CrossRef]
- Galisteo-González, F.; Molina-Bolívar, J.A. Systematic study on the preparation of BSA nanoparticles. Colloids Surf. B Biointerfaces 2014, 123, 286–292. [Google Scholar] [CrossRef]
- Lomis, N.; Westfall, S.; Farahdel, L.; Malhotra, M.; Shum-Tim, D.; Prakash, S. Human Serum Albumin Nanoparticles for Use in Cancer Drug Delivery: Process Optimization and In Vitro Characterization. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norde, W.; Giacomelli, C.E. BSA structural changes during homomolecular exchange between the adsorbed and the dissolved states. J. Biotechnol. 2000, 79, 259–268. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Comparison of the Effectiveness of Four Different Crosslinking Agents with Hyaluronic Acid Hydrogel Films for Tissue-Culture Applications. Appl. Polym. Sci. 2007, 104, 3183–3191. [Google Scholar] [CrossRef]
- Chandran, S.; Roy, A.; Saha, R.N. Effect of pH and formulation variables on in vitro transcorneal permeability of flurbiprofen: A technical note. AAPS PharmSciTech 2008, 9, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Hou, J.; Zhong, Z.; Hao, N.; Lin, Y.; Li, C. Targeted delivery of hyaluronic acid-coated solid lipid nanoparticles for rheumatoid arthritis therapy. Drug Deliv. 2018, 25, 716–722. [Google Scholar] [CrossRef]
- Khan, D.; Qindeel, M.; Ahmed, N.; Khan, A.U.; Khan, S.; Rehman, A.U. Development of novel pH-sensitive nanoparticle-based transdermal patch for management of rheumatoid arthritis. Nanomedicine 2020, 15, 603–624. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, H.; Zhong, Z.; Zhou, M.; Lin, Y.; Tang, C.; Li, C. Co-Delivery of Prednisolone and Curcumin in Human Serum Albumin Nanoparticles for Effective Treatment of Rheumatoid Arthritis. Int. J. Nanomed. 2019, 14, 9113–9125. [Google Scholar] [CrossRef] [Green Version]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H.; Shu, X.; Wu, M.; Liu, J.; Hao, T.; Cui, H.; Zheng, L. Intra-articular delivery of flurbiprofen sustained release thermogel: Improved therapeutic outcome of collagenase II-induced rat knee osteoarthritis. Drug Deliv. 2020, 27, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Choy, E.H. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin. Arthritis Rheum. 2021, 51, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Esme, H.; Kesli, R.; Apiliogullari, B.; Duran, F.M.; Yoldas, B. Effects of flurbiprofen on CRP, TNF-α, IL-6, and postoperative pain of thoracotomy. Int. J. Med. Sci. 2011, 8, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Foster, W.; Carruthers, D.; Lip, G.Y.; Blann, A.D. Inflammatory cytokines, endothelial markers and adhesion molecules in rheumatoid arthritis: Effect of intensive anti-inflammatory treatment. J. Thromb. Thrombolysis 2010, 29, 437–442. [Google Scholar] [CrossRef]
- Wei, S.T.; Sun, Y.H.; Zong, S.H.; Xiang, Y.B. Serum Levels of IL-6 and TNF-alpha May Correlate with Activity and Severity of Rheumatoid Arthritis. Med. Sci. Monit. 2015, 21, 4030–4038. [Google Scholar] [CrossRef] [Green Version]
- Favalli, E.G. Understanding the Role of Interleukin-6 (IL-6) in the Joint and Beyond: A Comprehensive Review of IL-6 Inhibition for the Management of Rheumatoid Arthritis. Rheumatol. Ther. 2020, 7, 473–516. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Hou, Y.; Meng, X.; Wang, D.; Liu, J.; Sun, F.; Li, Y. Hyaluronic Acid Coated Acid-Sensitive Nanoparticles for Targeted Therapy of Adjuvant-Induced Arthritis in Rats. Molecules 2019, 24, 16. [Google Scholar] [CrossRef] [Green Version]
- Yokota, K.; Sato, K.; Miyazaki, T.; Aizaki, Y.; Tanaka, S.; Sekikawa, M.; Kozu, N.; Kadono, Y.; Oda, H.; Mimura, T. Characterization and Function of Tumor Necrosis Factor and Interleukin-6–Induced Osteoclasts in Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1145–1154. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, P.; Ding, J.; Zhou, W. Hyaluronic Acid-Coated MTX-PEI Nanoparticles for Targeted Rheumatoid Arthritis Therapy. Crystals 2021, 11, 321. [Google Scholar] [CrossRef]
- Lee, H.; Lee, M.Y.; Bhang, S.H.; Kim, B.S.; Kim, Y.S.; Ju, J.H.; Kim, K.S.; Hahn, S.K. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano 2014, 8, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ansari, M.Y. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef]
- Gul, A.; Kunwar, B.; Mazhar, M.; Faizi, S.; Ahmed, D.; Shah, M.R.; Simjee, S.U. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-κB and iNOS activation. Int. Immunopharmacol. 2018, 59, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Brune, K.; Rau, T.; Pahl, A. Flurbiprofen enantiomers inhibit inducible nitric oxide synthase expression in RAW 264.7 macrophages. Pharm. Res. 2001, 18, 151–156. [Google Scholar] [CrossRef] [PubMed]




| Formula | Amount (mg) | PS (nm) ± SD | PDI ± SD | ZP (mv) ± SD | ||
|---|---|---|---|---|---|---|
| BSA | HA | FLUR | ||||
| F1 | 30 | – | – | 349.61 ± 117.92 | 0.69 ± 0.09 | −6 ± 1.01 |
| F2 | 50 | – | – | 506.31 ± 52.12 | 0.58 ± 0.04 | −6.32 ± 1.15 |
| * F3 | 60 | – | – | 163.83 ± 24.39 | 0.35 ± 0.12 | −8 ± 1.53 |
| F4 | 60 | 1 | – | 440.42 ± 40.12 | 0.59 ± 0.19 | −20 ± 0.02 |
| * F5 | 60 | 2 | – | 339.41 ± 19.61 | 0.23 ± 0.17 | −24 ± 0 |
| F6 | 60 | 4 | – | 3046 ± 579.83 | 0.80 ± 0.28 | −24 ± 0.09 |
| * F7 | 60 | – | 15 | 172.21 ± 12.08 | 0.35 ± 0.02 | −6 ± 1.15 |
| F8 | 60 | – | 25 | 601.63 ± 61.58 | 0.59 ± 0.06 | −4.25 ± 2.13 |
| F9 | 60 | – | 40 | 2756 ± 257.92 | 0.47 ± 0.19 | −5.62 ± 3.22 |
| F10 | 60 | 1 | 15 | 531.91 ± 6.58 | 0.44 ± 0.08 | −27.72 ± 0.35 |
| * F11 | 60 | 2 | 15 | 257.12 ± 2.54 | 0.25 ± 0.01 | −48 ± 3 |
| F12 | 60 | 4 | 15 | 869.81 ± 128.52 | 0.98 ± 0.03 | −53 ± 0.23 |
| Storage Temperature 4 °C | ||||
|---|---|---|---|---|
| Formula | Loaded BSA NPs (F7) | Loaded HA-BSA NPs (F11) | ||
| Time (months) | 0 | 6 | 0 | 6 |
| PS (nm) ± SD | 172 ± 12.08 | 737.91 ± 43.01 | 257.12 ± 2.54 | 320.52 ± 59.61 |
| ZP ± SD | −6 ± 1.15 | −7 ± 0 | −48 ± 3 | −36 ± 0 |
| PDI ± SD | 0.35 ± 0.02 | 0.54 ± 0.03 | 0.25 ± 0.01 | 0.68 ± 0.06 |
| EE% ± SD | 90.35 ± 0.99 | 80.17 ± 0.97 | 90.12 ± 1.06 | 79.16 ± 0.76 |
| Group | Zero Time | Seven-Days Post-Induction | One-Week Post-Treatment | Two-Weeks Post-Treatment |
|---|---|---|---|---|
| Group A | 0 | 0 | 0 | 0 |
| Group B | 0 | 8 ± 2.74 *** | 8 ± 2.73 | 10 ± 0 |
| Group C | 0 | 8 ± 2.73 *** | 1.51 ± 1.36 a | 1 ± 1.37 a |
| Group D | 0 | 8.75 ± 2.5 *** | 3.125 ± 1.25 c | 2.52 ± 2.04 a |
| Group E | 0 | 9 ± 2.23 *** | 2.52 ± 1.76 b | 1.88 ± 1.25 a |
| Group F | 0 | 8 ± 2.73 *** | 4.51 ± 1.25 d | 4 ± 1.37 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, H.I.; El-Kamel, A.H.; Hammad, G.O.; Heikal, L.A. Design of Targeted Flurbiprofen Biomimetic Nanoparticles for Management of Arthritis: In Vitro and In Vivo Appraisal. Pharmaceutics 2022, 14, 140. https://doi.org/10.3390/pharmaceutics14010140
Mohamed HI, El-Kamel AH, Hammad GO, Heikal LA. Design of Targeted Flurbiprofen Biomimetic Nanoparticles for Management of Arthritis: In Vitro and In Vivo Appraisal. Pharmaceutics. 2022; 14(1):140. https://doi.org/10.3390/pharmaceutics14010140
Chicago/Turabian StyleMohamed, Hagar I., Amal H. El-Kamel, Ghada O. Hammad, and Lamia A. Heikal. 2022. "Design of Targeted Flurbiprofen Biomimetic Nanoparticles for Management of Arthritis: In Vitro and In Vivo Appraisal" Pharmaceutics 14, no. 1: 140. https://doi.org/10.3390/pharmaceutics14010140





