Saquinavir-Piperine Eutectic Mixture: Preparation, Characterization, and Dissolution Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SQV Eutectic Mixture Screening
2.3. SQV-PIP Eutectic System
2.3.1. Determination of Mixtures Composition at the Eutectic Point
2.3.2. Preparation of Bulk Mixtures at the Eutectic Composition
2.4. Solid-State Characterization of the Eutectic Mixtures
2.4.1. Differential Scanning Calorimetry (DSC) Analysis
2.4.2. Powder X-ray Diffraction (PXRD)
2.4.3. Fourier Transform Infrared (FT-IR)
2.4.4. Scanning Electron Microscopy (SEM)
2.5. Powder Dissolution Test
Determination of Drug Content
3. Results and Discussion
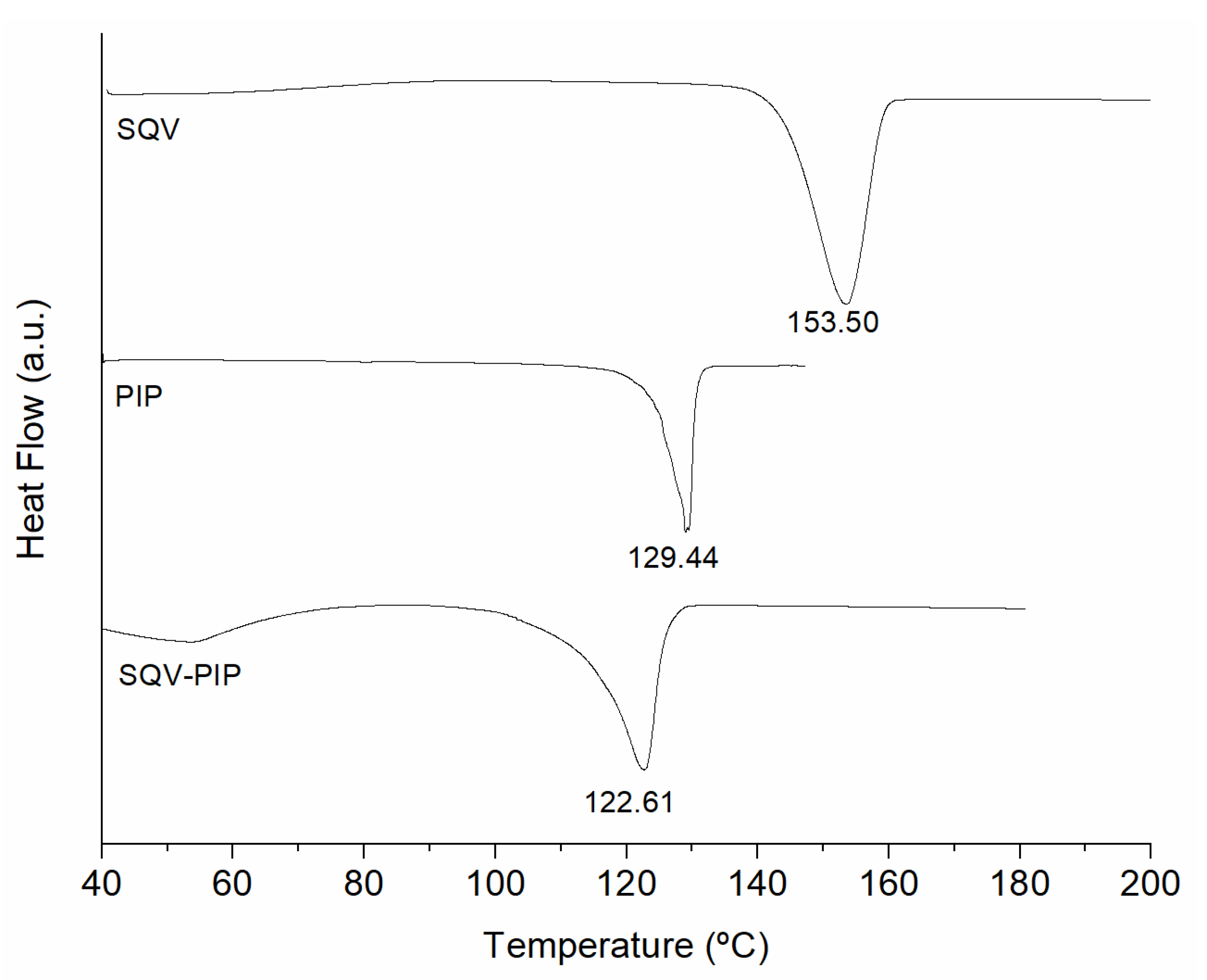
3.1. Eutectic Mixture Screening
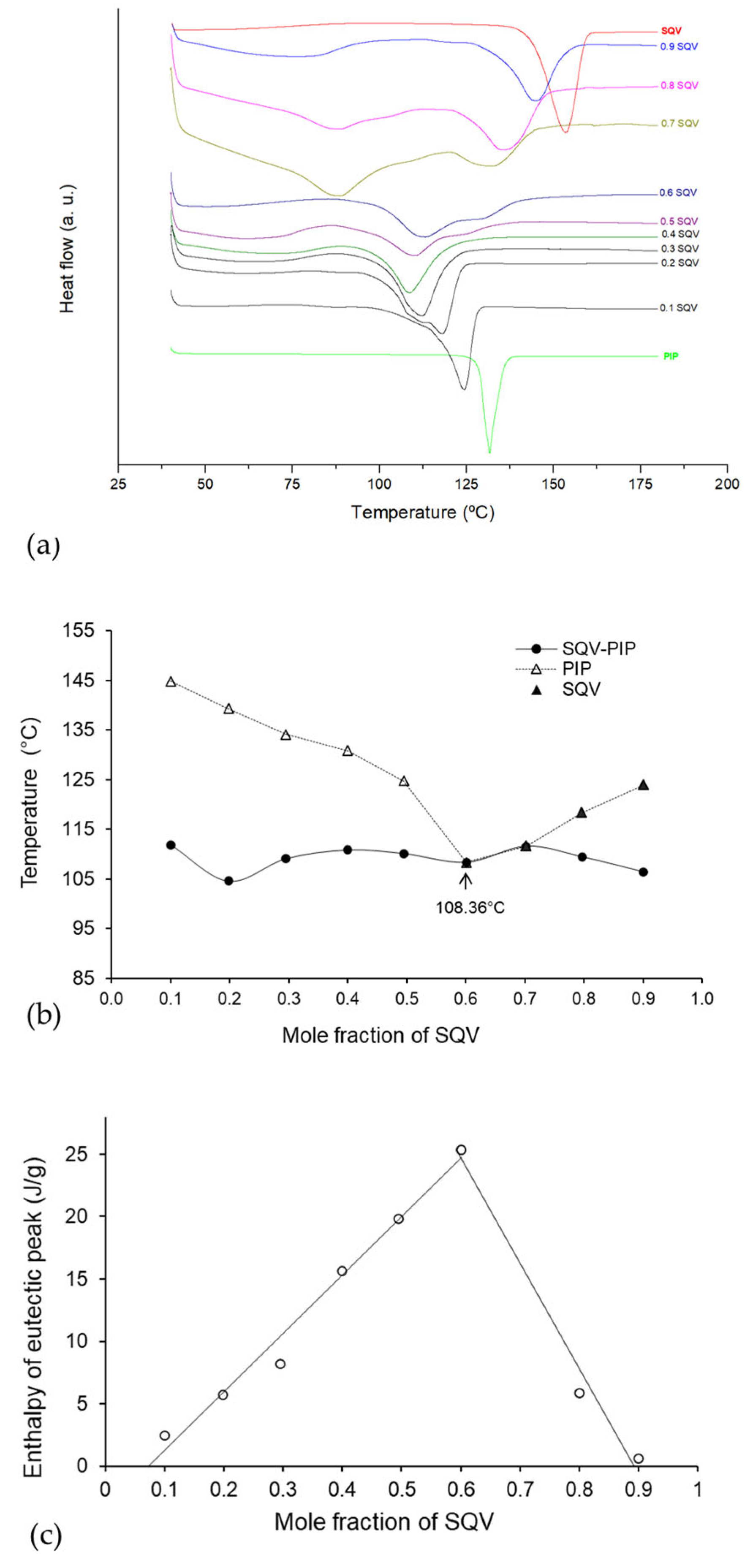
3.2. Binary Phase and Tammann Diagrams
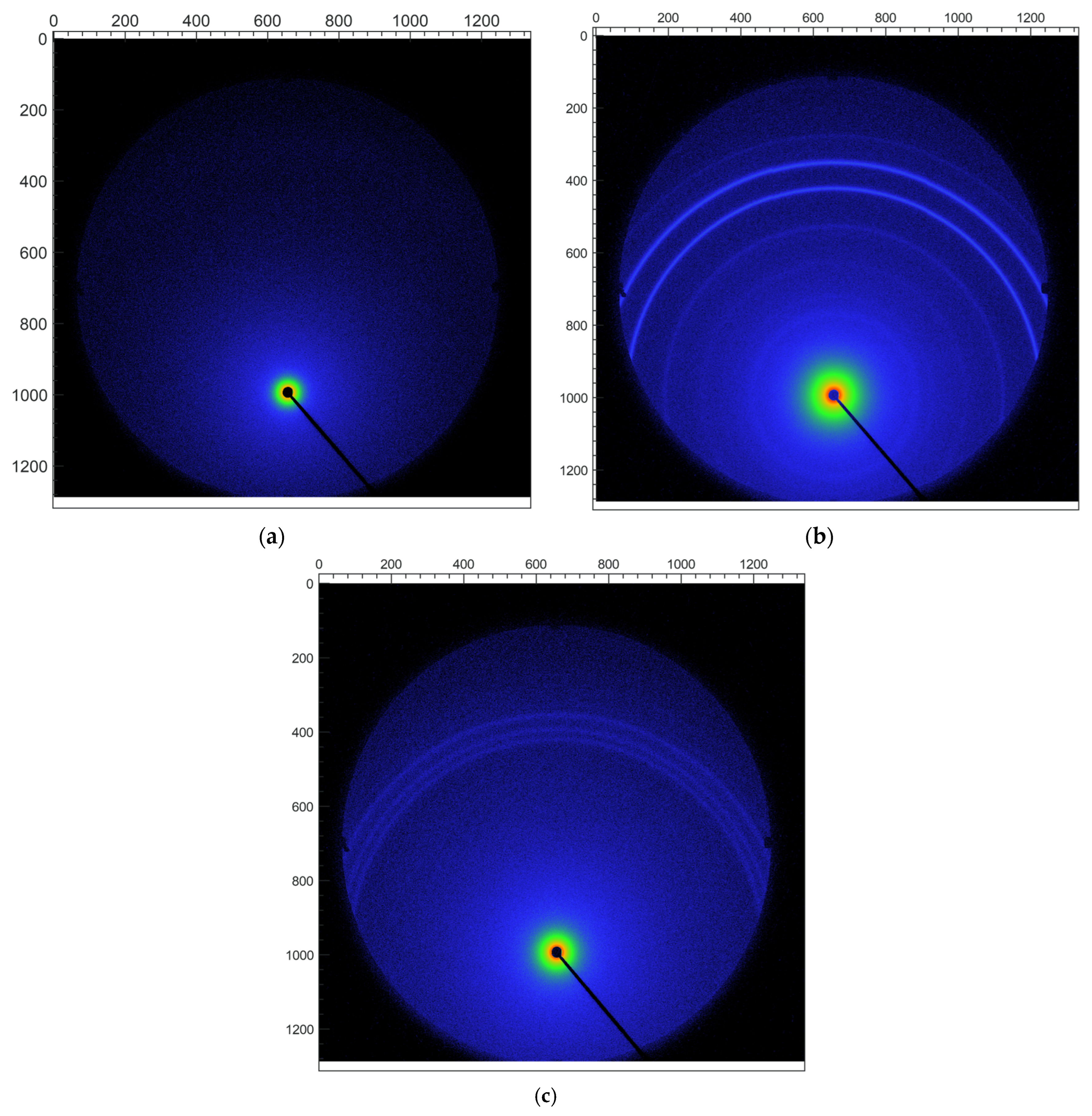
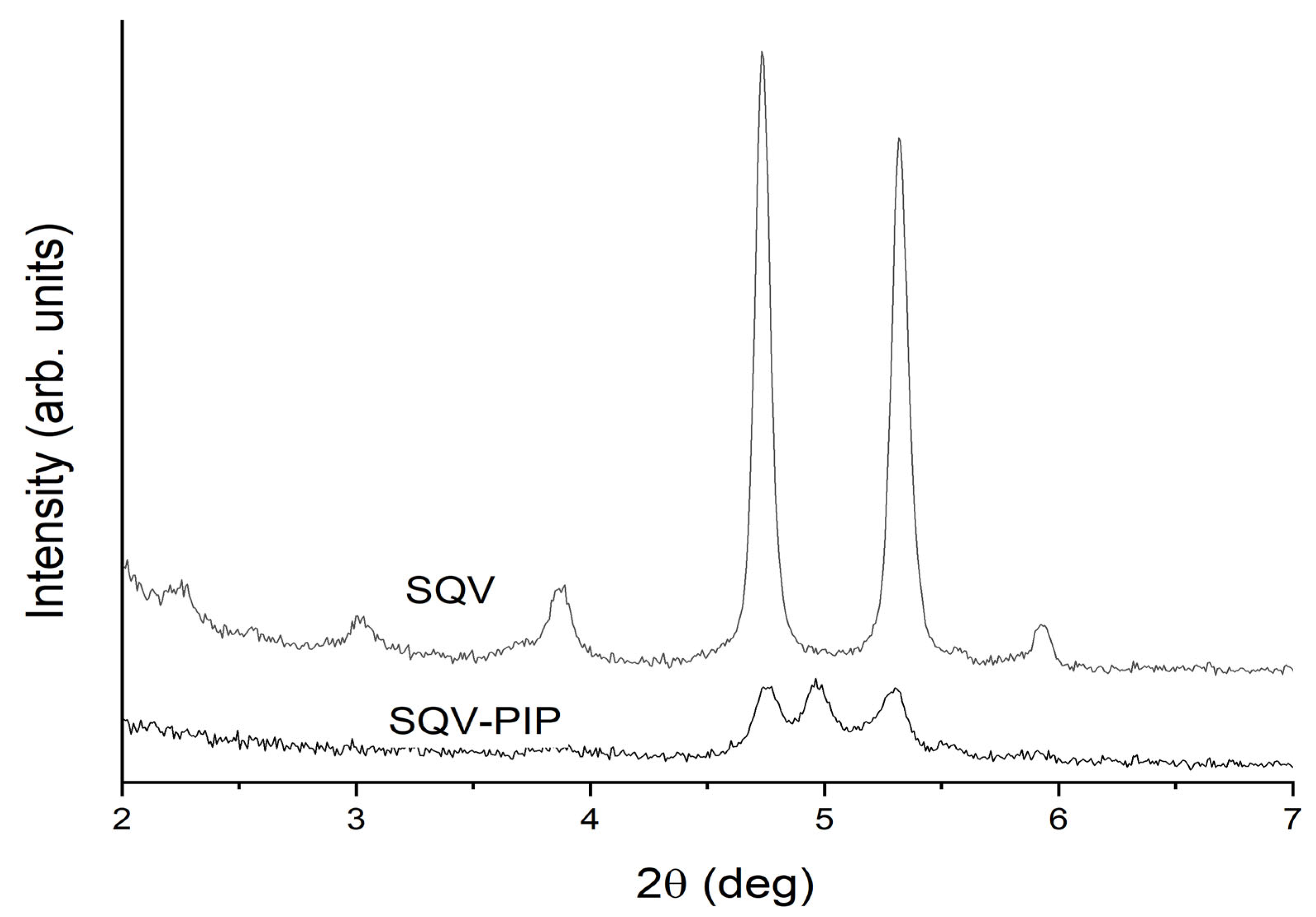
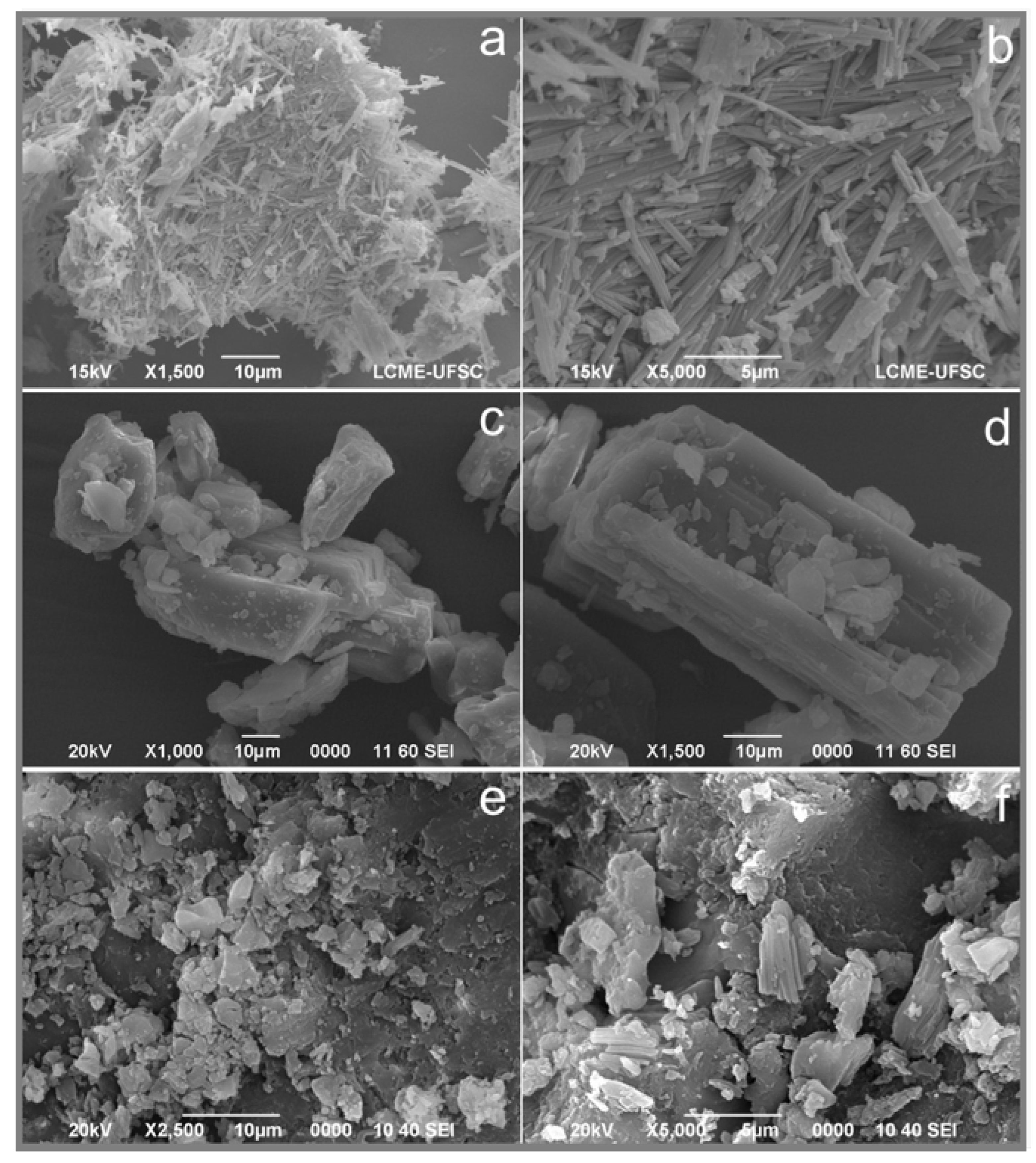
3.3. Solid-State Characterization of SQV-PIP Eutectic System
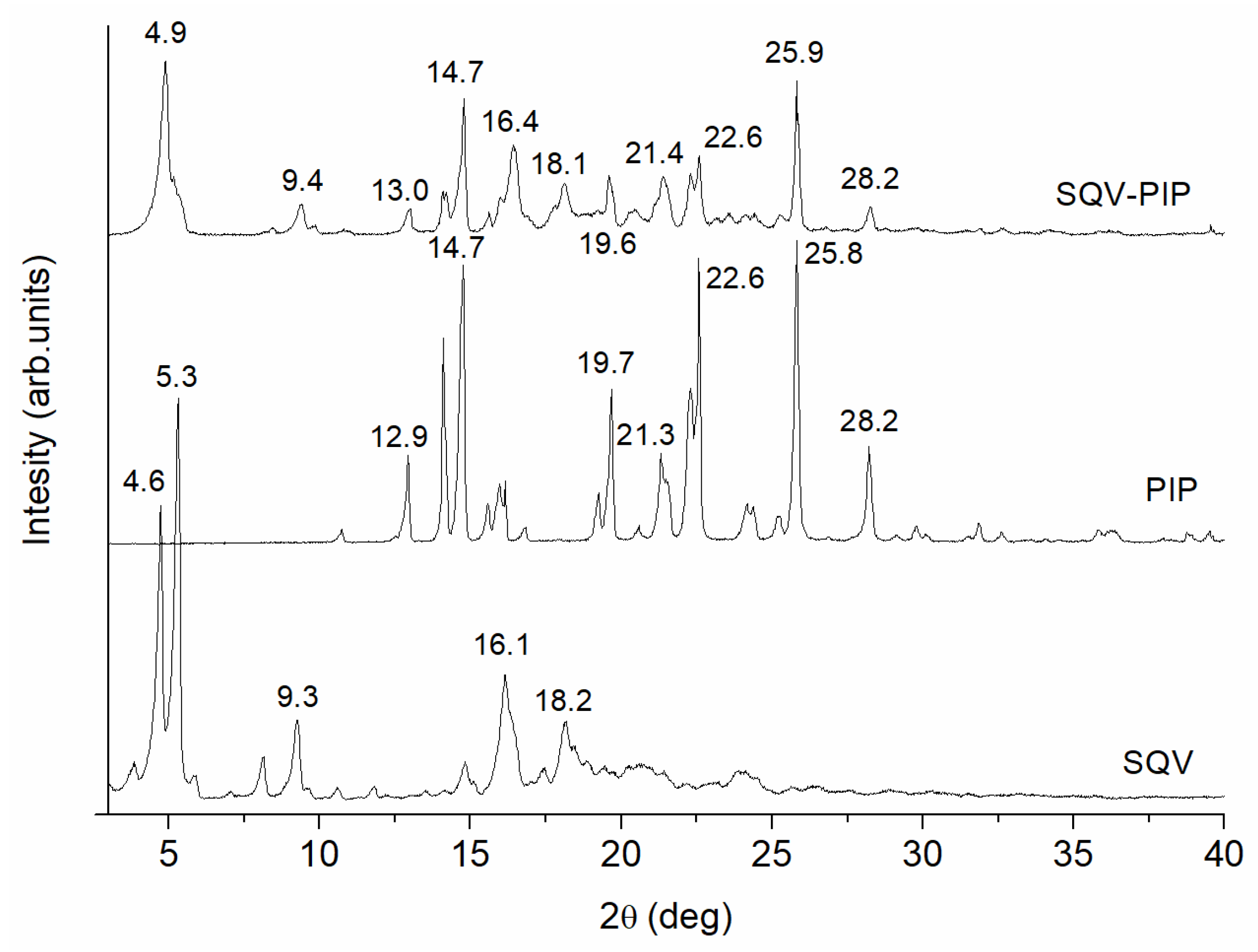
3.3.1. PXRD Analyses
3.3.2. FT-IR Analyses
3.3.3. Scanning Electron Microscopy
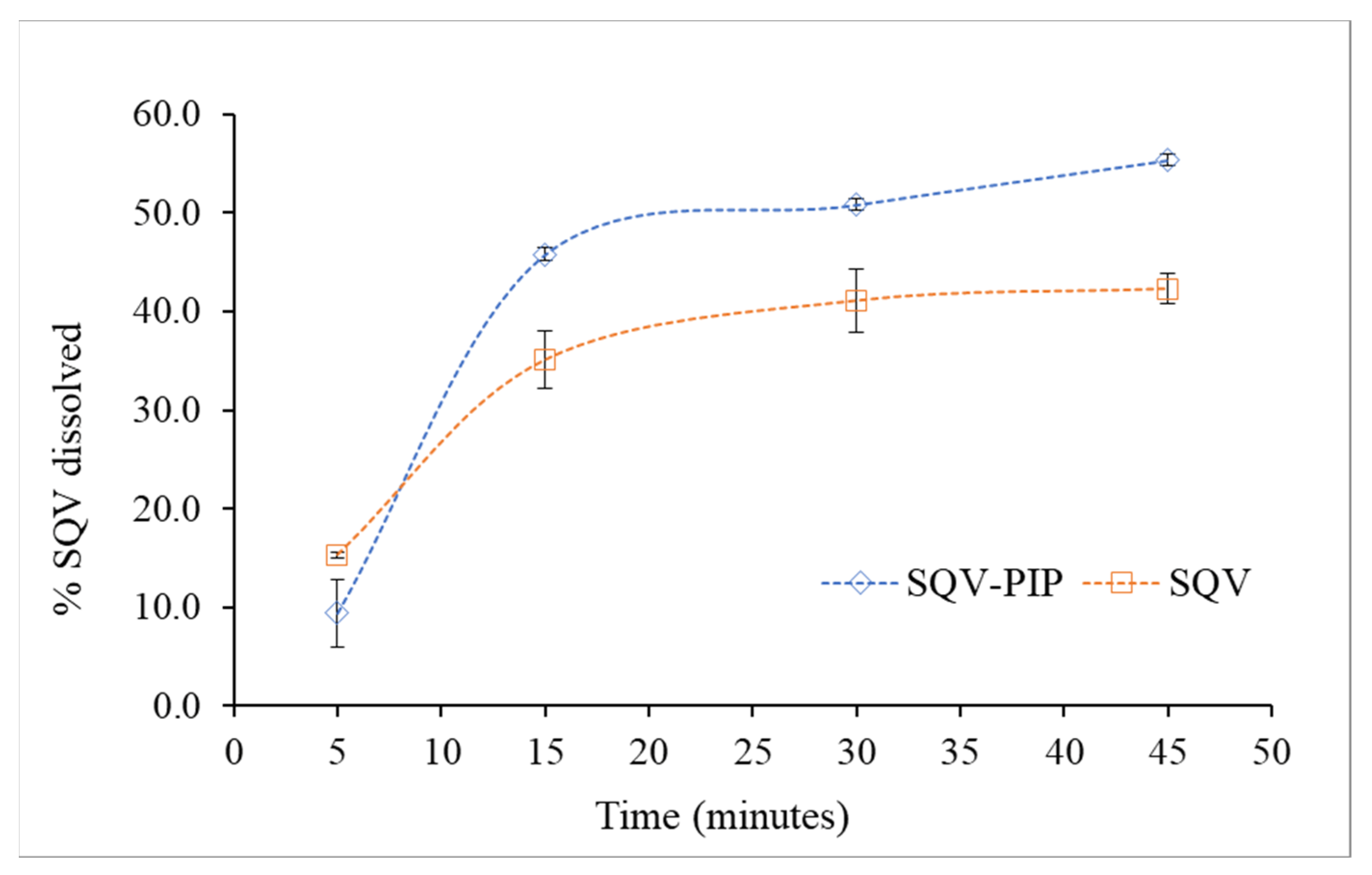
3.3.4. Powder Dissolution Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Persson, L.C.; Porter, C.J.H.; Charman, W.N.; Bergström, C.A.S. Computational prediction of drug solubility in lipid based formulation excipients. Pharm. Res. 2013, 30, 3225–3237. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Gong, W.; Mondal, P.K.; Ahmadi, S.; Wu, Y.; Rohani, S. Cocrystals, Salts, and Salt-Solvates of olanzapine; selection of coformers and improved solubility. Int. J. Pharm. 2021, 608, 121063. [Google Scholar] [CrossRef]
- Bansal, K.; Pant, P.; Rao, P.R.T.; Padhee, K.; Sathapathy, A.; Kochhar, P.S. Micronization and dissolution enhancement of norethindrone. Int. J. Res. Pharm. Chem. 2011, 1, 315–319. [Google Scholar]
- Chu, K.R.; Lee, E.; Jeong, S.H.; Park, E.S. Effect of particle size on the dissolution behaviors of poorly water-soluble drugs. Arch. Pharm. Res. 2012, 35, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Csicsák, D.; Szolláth, R.; Kádár, S.; Ambrus, R.; Bartos, C.; Balogh, E.; Antal, I.; Köteles, I.; Tőzsér, P.; Bárdos, V.; et al. The Effect of the Particle Size Reduction on the Biorelevant Solubility and Dissolution of Poorly Soluble Drugs with Different Acid-Base Character. Pharmaceutics 2023, 15, 278. [Google Scholar] [CrossRef]
- Bastin, R.J.; Bowker, M.J.; Slater, B.J. Salt selection and optimisation procedures for pharmaceutical new chemical entities. Org. Process Res. Dev. 2000, 4, 427–435. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanoparticle Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Newman, A. 2009 Re V iews. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Gala, U.; Pham, H.; Chauhan, H. Pharmaceutical Applications of Eutectic Mixtures. J. Dev. Drugs 2013, 2, 3. [Google Scholar] [CrossRef]
- Kim, D.; Jang, S.; Kim, I.W. Eutectic formation of naproxen with some dicarboxylic acids. Pharmaceutics 2021, 13, 2081. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, R.; Chen, Y.; Zhang, Y.; Liu, X.; Liu, B.F.; Zhang, G.; Hao, C. Drug-drug eutectic mixtures of celecoxib with tapentadol and milnacipran which could improve analgesic and antidepressant efficacy. J. Drug Deliv. Sci. Technol. 2022, 67, 102995. [Google Scholar] [CrossRef]
- Pathak, S.M.; Musmade, P.; Dengle, S.; Karthik, A.; Bhat, K.; Udupa, N. Enhanced oral absorption of saquinavir with Methyl-Beta-Cyclodextrin-Preparation and in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2010, 41, 440–451. [Google Scholar] [CrossRef]
- Surjyanarayan, M.; Snigdha, S.M.; Naazneen, S.; Vandana, B.P. Design and development of Saquinavir microemulsion for the oral bioavailability enhancement. Int. J. PharmTech Res. 2009, 1, 1442–1448. [Google Scholar]
- Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Vega-Baudrit, J.R.; Guillén-Girón, T.; Vargas-Huertas, F.; Navarro-Hoyos, M.; Araya-Sibaja, A.M. Evaluation of Piperine as Natural Coformer for Eutectics Preparation of Drugs Used in the Treatment of Cardiovascular Diseases. AAPS PharmSciTech 2022, 23, 127. [Google Scholar] [CrossRef]
- Phansalkar, P.S.; Zhang, Z.; Verenich, S.; Gerk, P.M. Pharmacokinetics and Bioavailability Enhancement of Natural Products. In Natural Products for Cancer Chemoprevention; Springer International Publishing: Cham, Switzerland, 2020; pp. 109–141. [Google Scholar]
- Vippagunta, S.R.; Wang, Z.; Hornung, S.; Krill, S. Molecular Nanomedicine towards Cancer. J. Pharm. Sci. 2007, 96, 294–304. [Google Scholar] [CrossRef]
- Park, H.; Seo, H.J.; Ha, E.-S.; Hong, S.; Kim, J.-S.; Kim, M.-S.; Hwang, S.-J. Preparation and characterization of glimepiride eutectic mixture with l-arginine for improvement of dissolution rate. Int. J. Pharm. 2020, 581, 119288. [Google Scholar] [CrossRef] [PubMed]
- Law, D.; Wang, W.; Schmitt, E.A.; Long, M.A. Prediction of Poly(Ethylene) Glycol-Drug Eutectic Compositions Using an Index Based on the van’t Hoff Equation. Pharm. Res. 2002, 19, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Cherukuvada, S.; Nangia, A. Eutectics as improved pharmaceutical materials: Design, properties and characterization. Chem. Commun. 2014, 50, 906–923. [Google Scholar] [CrossRef] [PubMed]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef] [PubMed]
- Broberg, B.F.J.; Evers, H.C.A. Local Anesthetic Mixture for Topical Application and Method for Obtaining Local Anesthesia. U.S. Patent US4529601A, 16 July 1985. [Google Scholar]
- Araya-Sibaja, A.; Vega-Baudrit, J.; Guillén-Girón, T.; Navarro-Hoyos, M.; Cuffini, S. Drug Solubility Enhancement through the Preparation of Multicomponent Organic Materials: Eutectics of Lovastatin with Carboxylic Acids. Pharmaceutics 2019, 11, 112. [Google Scholar] [CrossRef]
- Górniak, A.; Złocińska, A.; Trojan, M.; Pęcak, A.; Karolewicz, B. Preformulation Studies of Ezetimibe-Simvastatin Solid Dispersions in the Development of Fixed-Dose Combinations. Pharmaceutics 2022, 14, 912. [Google Scholar] [CrossRef]
- Chadha, R.; Sharma, M.; Haneef, J. Multicomponent solid forms of felodipine: Preparation, characterisation, physicochemical and in-vivo studies. J. Pharm. Pharmacol. 2017, 69, 254–264. [Google Scholar] [CrossRef]
- Rycerz, L. Practical remarks concerning phase diagrams determination on the basis of differential scanning calorimetry measurements. J. Therm. Anal. Calorim. 2013, 113, 231–238. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Shah, H.S.; Nahar, K.; Dave, R.; Morris, K.R. Contribution of Crystal Lattice Energy on the Dissolution Behavior of Eutectic Solid Dispersions. ACS Omega 2020, 5, 9690–9701. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chormale, J.H.; Bansal, A.K. Deep eutectic systems: An overview of fundamental aspects, current understanding and drug delivery applications. Int. J. Pharm. 2021, 610, 121203. [Google Scholar] [CrossRef]
- Gala, U.; Chuong, M.C.; Varanasi, R.; Chauhan, H. Characterization and Comparison of Lidocaine-Tetracaine and Lidocaine-Camphor Eutectic Mixtures Based on Their Crystallization and Hydrogen-Bonding Abilities. AAPS PharmSciTech 2015, 16, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Cherukuvada, S.; Guru Row, T.N. Comprehending the Formation of Eutectics and Cocrystals in Terms of Design and Their Structural Interrelationships. Cryst. Growth Des. 2014, 14, 4187–4198. [Google Scholar] [CrossRef]
- Balakrishnan, I.; Jawahar, N.; Senthil, V.; Debosmita, D. A brief review on eutectic mixture and its role in pharmaceutical field. Int. J. Res. Pharm. Sci. 2020, 11, 3017–3023. [Google Scholar] [CrossRef]
- Jesus, A.R.; Paiva, A.; Duarte, A.R.C. Current developments and future perspectives on biotechnology applications of natural deep eutectic systems. Curr. Opin. Green Sustain. Chem. 2023, 39, 100731. [Google Scholar] [CrossRef]
- Pfund, L.Y.; Chamberlin, B.L.; Matzger, A.J. The Bioenhancer Piperine is at Least Trimorphic. Cryst. Growth Des. 2015, 15, 2047–2051. [Google Scholar] [CrossRef]
- Bandi, P.; ReddyKura, R.; ReddyRapolu, R.; ReddyDasari, M.; ReddyKesireddy, S.; Chander, R.S.R. Novel Polymorphs of Saquinavir. European Patent EP2398773A2, 17 February 2009. [Google Scholar]
- Larkin, P.J. IR and Raman Spectra–Structure Correlations. In Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–134. [Google Scholar]
- Heinz, A.; Strachan, C.J.; Atassi, F.; Gordon, K.C.; Rades, T. Characterizing an amorphous system exhibiting trace crystallinity: A case study with saquinavir. Cryst. Growth Des. 2008, 8, 119–127. [Google Scholar] [CrossRef]
- Dressman, J.B.; Reppas, C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur. J. Pharm. Sci. 2000, 11, S73–S80. [Google Scholar] [CrossRef]
- Rossi, R.C.; Dias, C.L.; Bajerski, L.; Bergold, A.M.; Fröehlich, P.E. Development and validation of discriminating method of dissolution for fosamprenavir tablets based on in vivo data. J. Pharm. Biomed. Anal. 2011, 54, 439–444. [Google Scholar] [CrossRef]
- Varma, M.M.; Pandi, J.K. Dissolution, Solubility, XRD, and DSC Studies on Flurbiprofen-Nicotinamide Solid Dispersions. Drug Dev. Ind. Pharm. 2005, 31, 417–423. [Google Scholar] [CrossRef]
- Zaini, E.; Wahyuni, Y.S.; Halim, A.; Yuliandra, Y. Preparation of Eutectic Mixture of Ketoprofen and Nicotinamide for Enhanced Dissolution Rate. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 161–164. [Google Scholar]
- Alshaikh, R.A.; Essa, E.A.; El Maghraby, G.M. Eutexia for enhanced dissolution rate and anti-inflammatory activity of nonsteroidal anti-inflammatory agents: Caffeine as a melting point modulator. Int. J. Pharm. 2019, 563, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Sathisaran, I.; Dalvi, S.V. Crystal Engineering of Curcumin with Salicylic Acid and Hydroxyquinol as Coformers. Cryst. Growth Des. 2017, 17, 3974–3988. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.Y.; Back, S.Y.; Han, H.-K. Piperine-mediated drug interactions and formulation strategy for piperine: Recent advances and future perspectives. Expert Opin. Drug Metab. Toxicol. 2018, 14, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, A.; Thusu, N.; Zutshi, U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: Influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine 2002, 9, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Kajii, H.; Horie, T.; Hayashi, M.; Awazu, S. Fluorescence study on the interaction of salicylate with rat small intestinal epithelial cells: Possible mechanism for the promoting effects of salicylate on drug absorption. Life Sci. 1985, 37, 523–530. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fandaruff, C.; Quirós-Fallas, M.I.; Vega-Baudrit, J.R.; Navarro-Hoyos, M.; Lamas, D.G.; Araya-Sibaja, A.M. Saquinavir-Piperine Eutectic Mixture: Preparation, Characterization, and Dissolution Profile. Pharmaceutics 2023, 15, 2446. https://doi.org/10.3390/pharmaceutics15102446
Fandaruff C, Quirós-Fallas MI, Vega-Baudrit JR, Navarro-Hoyos M, Lamas DG, Araya-Sibaja AM. Saquinavir-Piperine Eutectic Mixture: Preparation, Characterization, and Dissolution Profile. Pharmaceutics. 2023; 15(10):2446. https://doi.org/10.3390/pharmaceutics15102446
Chicago/Turabian StyleFandaruff, Cinira, María Isabel Quirós-Fallas, José Roberto Vega-Baudrit, Mirtha Navarro-Hoyos, Diego German Lamas, and Andrea Mariela Araya-Sibaja. 2023. "Saquinavir-Piperine Eutectic Mixture: Preparation, Characterization, and Dissolution Profile" Pharmaceutics 15, no. 10: 2446. https://doi.org/10.3390/pharmaceutics15102446




