Tri-Layer Core–Shell Fibers from Coaxial Electrospinning for a Modified Release of Metronidazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
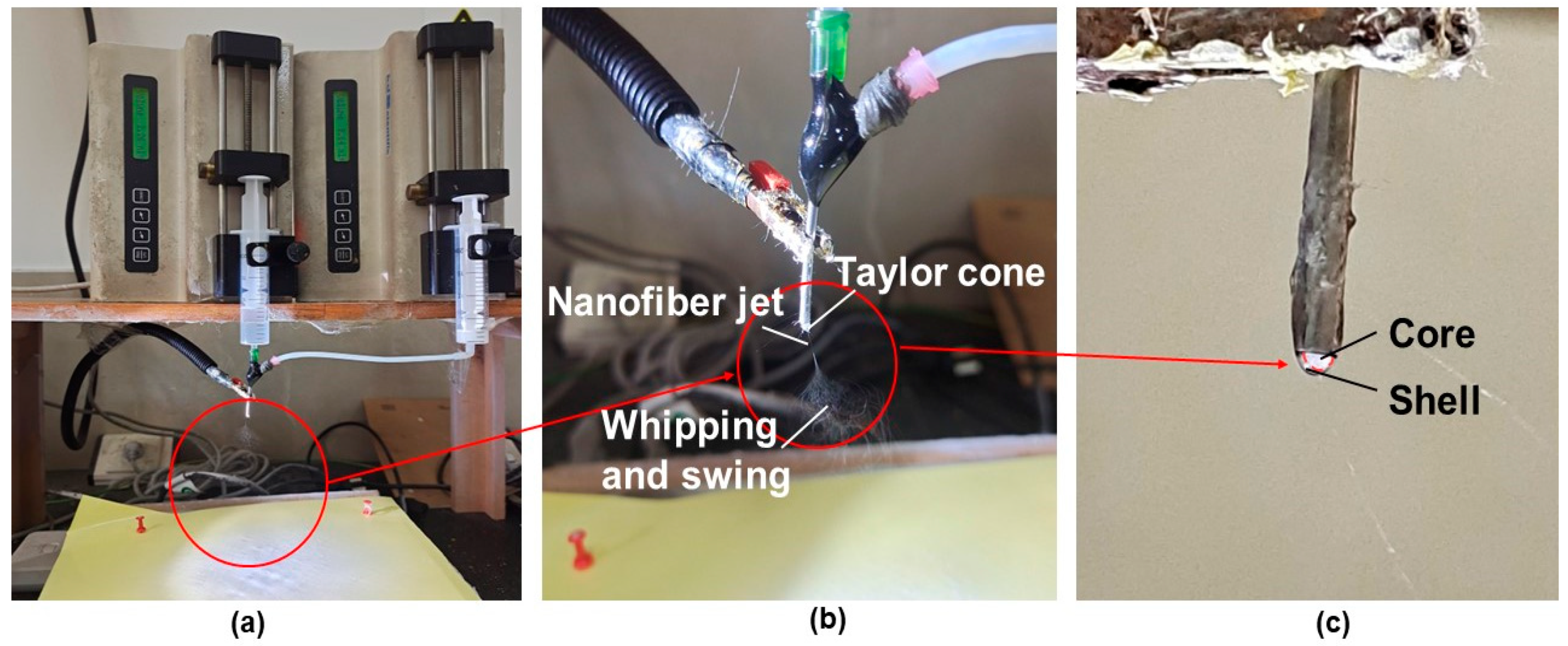
2.2. Electrospinning Process
2.2.1. Preparation of Core Emulsions
2.2.2. Preparation of Drug-Loaded Fibers by Coaxial Electrospinning
2.3. Characterization of Fiber
2.3.1. Emulsion Stability Observation
2.3.2. Morphology and Structure of Fibers
2.3.3. Physical States and Compatibility between the Fibrous Components
2.3.4. Water Contact Angle Test (WCA)
2.3.5. Mechanical Properties Test (Stretch Test)
2.4. In Vitro Dissolution Test
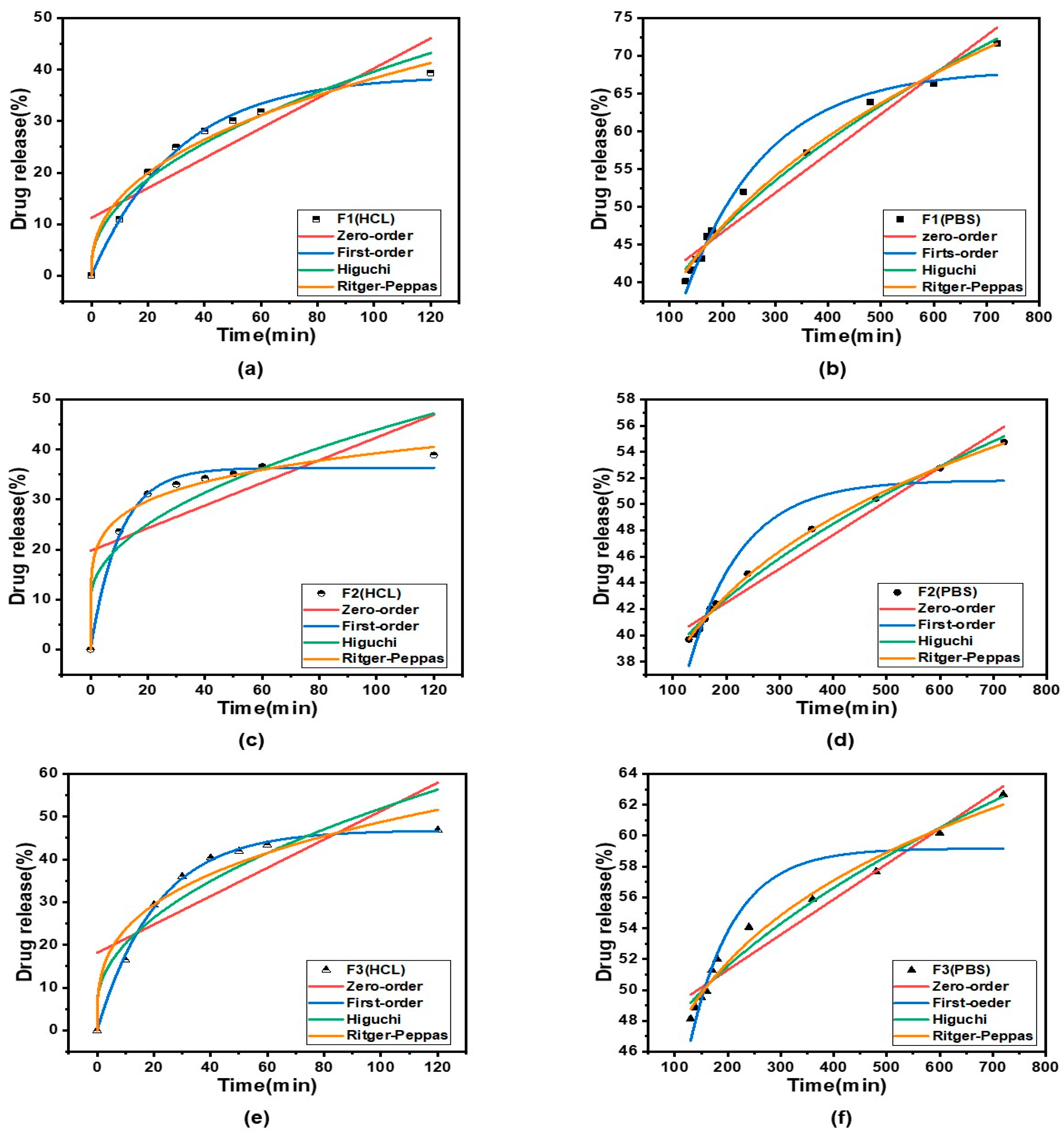
2.5. Drug-Release Kinetic Studies
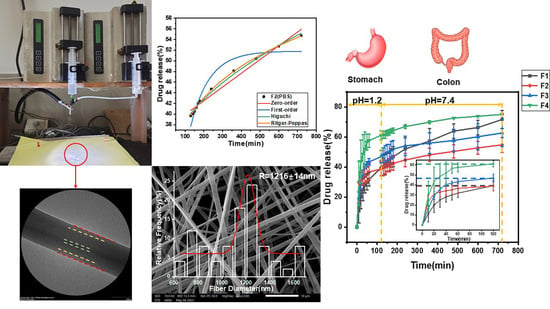
3. Results and Discussion
3.1. Emulsion Microstructure and Size
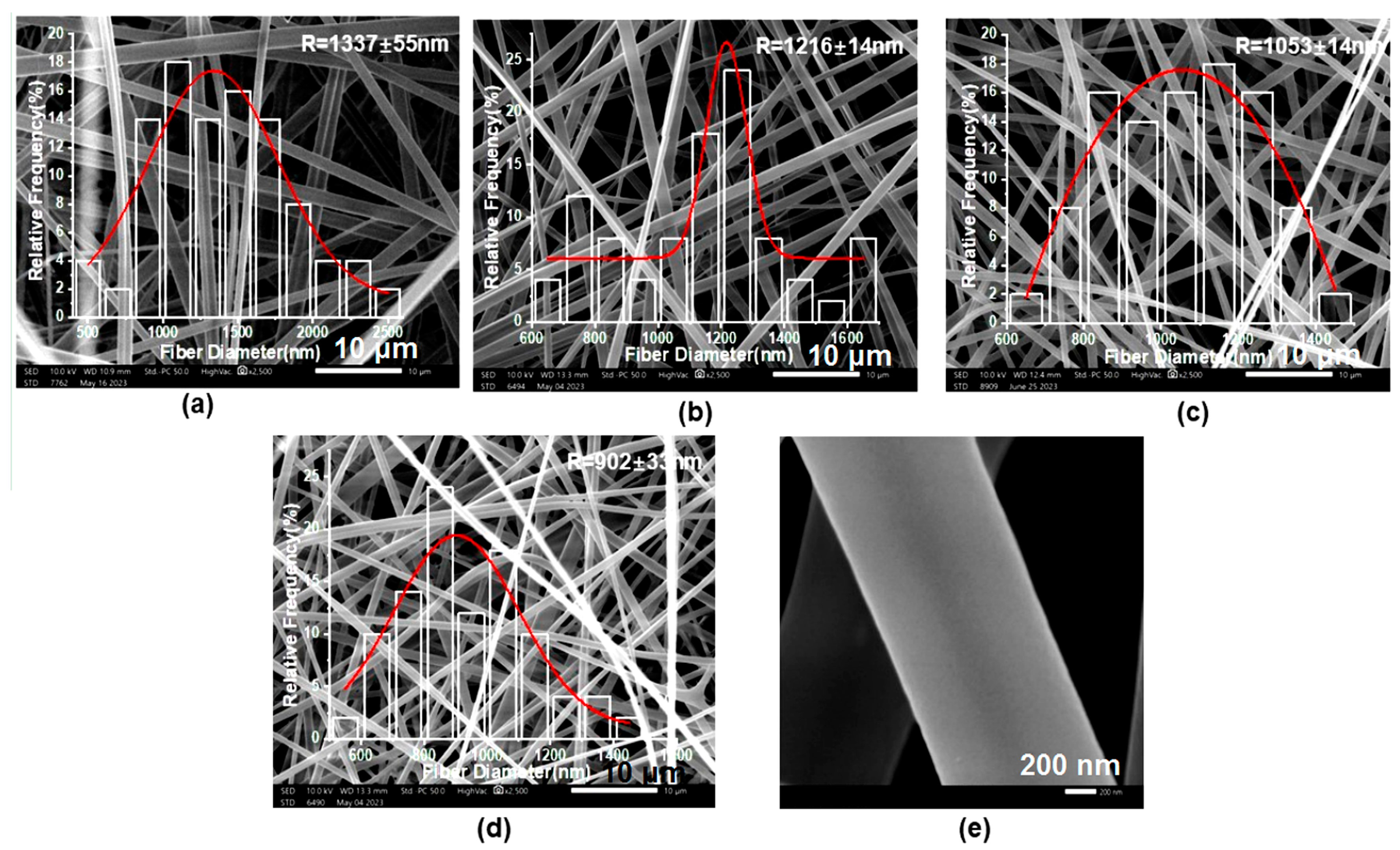
3.2. Morphology, Structure and Size Analysis of Electrospun Fibers and Their Formation Mechanism
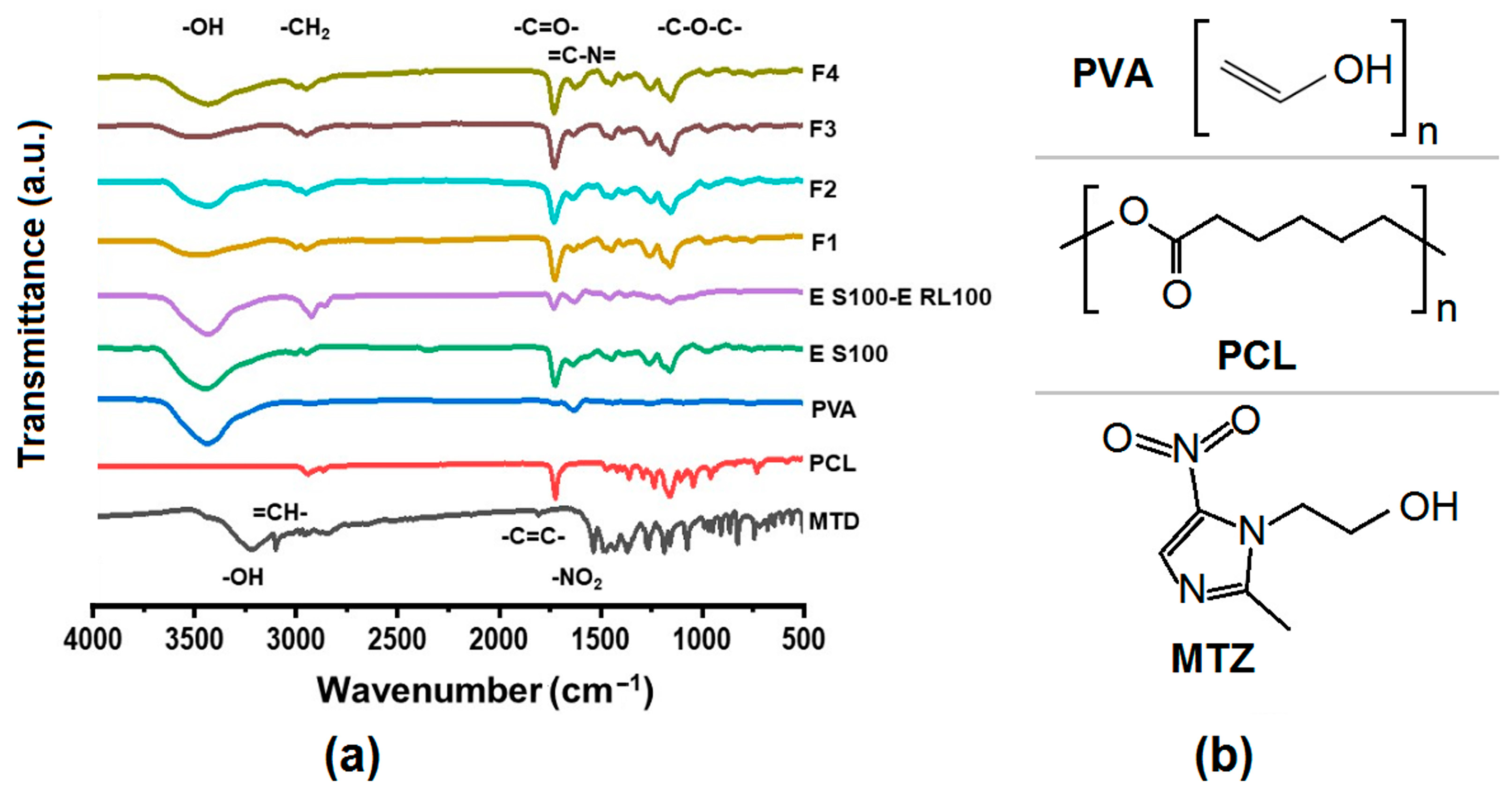
3.3. Compatibility and Physical State Analysis of Electrospinning Fibers
3.3.1. Compatibility between Fiber Components
3.3.2. Physical States of Components in Drug-Carrying Fibers
3.4. Analysis of Water Absorption and Wettability of Fiber Surface
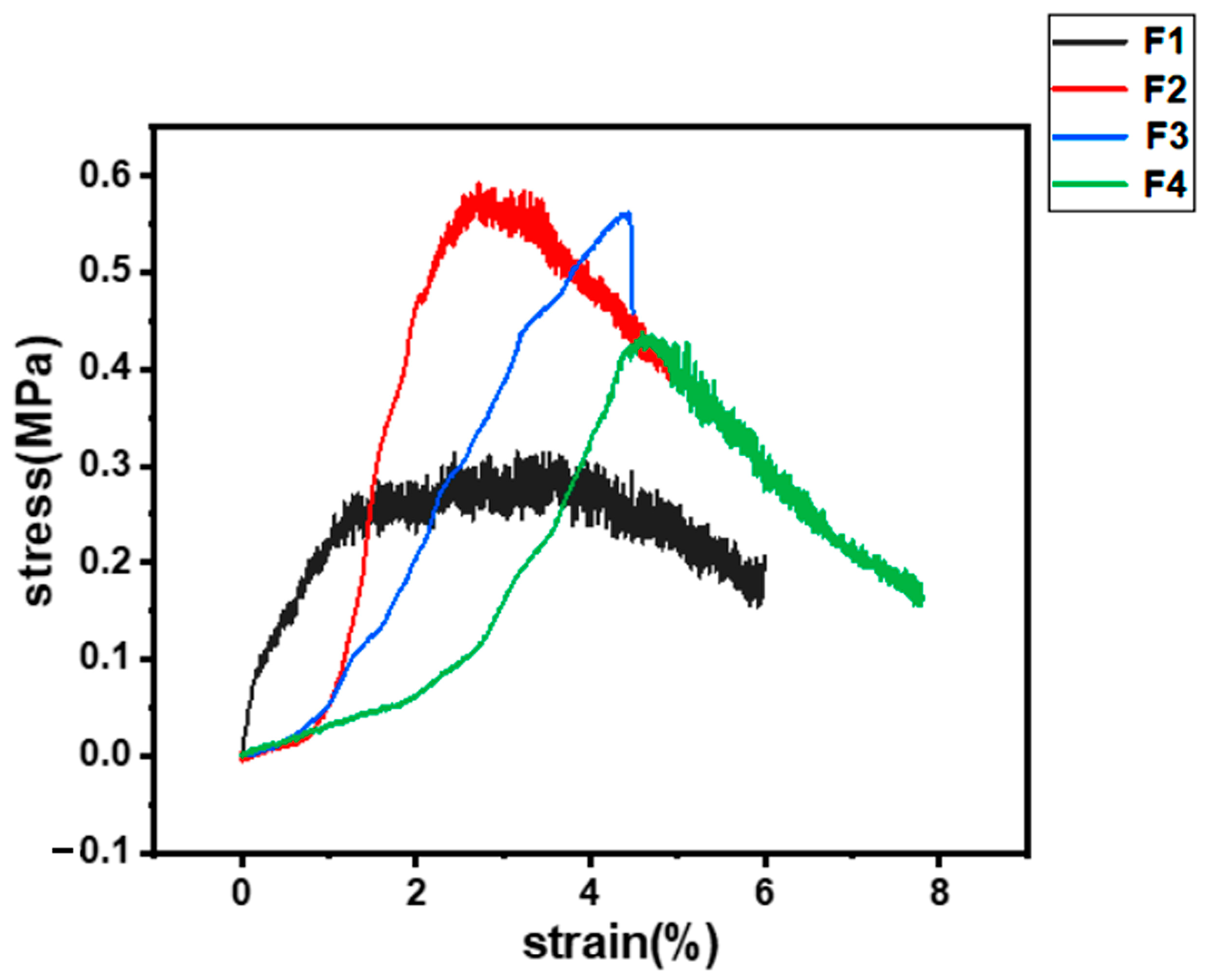
3.5. Mechanical Properties Test
3.6. Fiber Drug-Release Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.P.; Lee, J.; Im, E.; Jung, Y.; Yoo, J.-W. Advances in Colon-Targeted Nano-Drug Delivery Systems: Challenges and Solutions. Arch. Pharm. Res. 2020, 43, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, P.; Yu, D.-G.; Zhu, Y. Biphasic Drug Release from Electrospun Structures. Expert Opin. Drug Deliv. 2023, 20, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.-W.; Park, B.J.; Han, H.-K. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Wang, Q.; Li, Y.; Silva, D.Z.; Ruiz, M.E.L.; Ouyang, R.; Liu, B.; Miao, Y. Recent Development of Rhenium-Based Materials in the Application of Diagnosis and Tumor Therapy. Molecules 2023, 28, 2733. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering Exosomes for Targeted Drug Delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459.e29. [Google Scholar] [CrossRef]
- Almoshari, Y. Osmotic Pump Drug Delivery Systems—A Comprehensive Review. Pharmaceuticals 2022, 15, 1430. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, L.; Xie, M.; Li, H.; Ma, D.; Xue, W. A Colon-Targeted Oral Probiotics Delivery System Using an Enzyme-Triggered Fuse-Like Microcapsule. Adv. Healthc. Mater. 2021, 10, 2001953. [Google Scholar] [CrossRef]
- Balogh, A.; Farkas, B.; Domokos, A.; Farkas, A.; Démuth, B.; Borbás, E.; Nagy, B.; Marosi, G.; Nagy, Z.K. Controlled-Release Solid Dispersions of Eudragit® FS 100 and Poorly Soluble Spironolactone Prepared by Electrospinning and Melt Extrusion. Eur. Polym. J. 2017, 95, 406–417. [Google Scholar] [CrossRef]
- Dong, N.; Liu, Z.; He, H.; Lu, Y.; Qi, J.; Wu, W. “Hook & Loop” Multivalent Interactions Based on Disk-Shaped Nanoparticles Strengthen Active Targeting. J. Control. Release 2023, 354, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Z.; He, Y.; Xian, J.; Luo, R.; Zheng, C.; Zhang, J. Oral Colon-Targeting Core–Shell Microparticles Loading Curcumin for Enhanced Ulcerative Colitis Alleviating Efficacy. Chin. Med. 2021, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhou, X.; Xiao, B.; Duan, L.; Zhu, Z. Mucus-Penetrating Silk Fibroin-Based Nanotherapeutics for Efficient Treatment of Ulcerative Colitis. Biomolecules 2022, 12, 1263. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Vass, P.; Démuth, B.; Hirsch, E.; Nagy, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; Csontos, I.; Nagy, Z.K.; Marosi, G. Drying Technology Strategies for Colon-Targeted Oral Delivery of Biopharmaceuticals. J. Control. Release 2019, 296, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Wu, T.; Li, Z.; Teng, M.; Ma, L.; Qin, M.; Zhao, P.; Fu, Q. Preparation and Characterization of Novel Composite Nanoparticles Using Zein and Hyaluronic Acid for Efficient Delivery of Naringenin. Food Chem. 2023, 417, 135890. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Tran, T.T.D. Current Film Coating Designs for Colon-Targeted Oral Delivery. Curr. Med. Chem. 2021, 28, 1957–1969. [Google Scholar] [CrossRef]
- Zhang, G.; Han, W.; Zhao, P.; Wang, Z.; Li, M.; Sui, X.; Liu, Y.; Tian, B.; He, Z.; Fu, Q. Programmed PH-Responsive Core–Shell Nanoparticles for Precisely Targeted Therapy of Ulcerative Colitis. Nanoscale 2023, 15, 1937–1946. [Google Scholar] [CrossRef]
- Coban, O.; Aytac, Z.; Yildiz, Z.I.; Uyar, T. Colon Targeted Delivery of Niclosamide from β-Cyclodextrin Inclusion Complex Incorporated Electrospun Eudragit® L100 Nanofibers. Colloids Surf. B Biointerfaces 2021, 197, 111391. [Google Scholar] [CrossRef]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.-G.; Liu, Y.; Shao, J. Core–Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef]
- Turanlı, Y.; Acartürk, F. Fabrication and Characterization of Budesonide Loaded Colon-Specific Nanofiber Drug Delivery Systems Using Anionic and Cationic Polymethacrylate Polymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102511. [Google Scholar] [CrossRef]
- Akhgari, A.; Heshmati, Z.; Afrasiabi Garekani, H.; Sadeghi, F.; Sabbagh, A.; Sharif Makhmalzadeh, B.; Nokhodchi, A. Indomethacin Electrospun Nanofibers for Colonic Drug Delivery: In Vitro Dissolution Studies. Colloids Surf. B Biointerfaces 2017, 152, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Patriota, Y.B.G.; Arruda, I.E.S.; de Jesus Oliveira, A.C.; de Oliveira, T.C.; de Lemos Vasconcelos Silva, E.; Chaves, L.L.; de Oliveira Silva Ribeiro, F.; da Silva, D.A.; de La Roca Soares, M.F.; Soares-Sobrinho, J.L. Synthesis of Eudragit® L100-Coated Chitosan-Based Nanoparticles for Oral Enoxaparin Delivery. Int. J. Biol. Macromol. 2021, 193, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, C. Chiral Fiber Supramolecular Hydrogels for Tissue Engineering. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1847. [Google Scholar] [CrossRef]
- Song, N.; Ren, S.; Zhang, Y.; Wang, C.; Lu, X. Confinement of Prussian Blue Analogues Boxes Inside Conducting Polymer Nanotubes Enables Significantly Enhanced Catalytic Performance for Water Treatment. Adv. Funct. Mater. 2022, 32, 2204751. [Google Scholar] [CrossRef]
- Kou, L.; Yao, Q.; Zhang, H.; Chu, M.; Bhutia, Y.D.; Chen, R.; Ganapathy, V. Transporter-Targeted Nano-Sized Vehicles for Enhanced and Site-Specific Drug Delivery. Cancers 2020, 12, 2837. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- Zheng, S.; Li, D.; Li, W.; Chen, J.; Rao, X.; Wang, N.; Wang, B.; Luo, S.; Zhao, Y. MnO2 Nanosheets on a Carbon Nanofiber Freestanding Film by Electrospinning and In Situ Spraying for Lithium and Sodium Storage. ACS Appl. Energy Mater. 2022, 5, 3587–3594. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-Decorated BixTiyOz/TiO2 Flexible Carbon Nanofibers and Their Applications on Degradating of Organic Pollutants under Solar Radiation. J. Mater. Sci. Technol. 2023, 150, 114–123. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, V.; Patel, R. A Brief Review of the Essential Role of Nanovehicles for Improving the Therapeutic Efficacy of Pharmacological Agents against Tumours. Curr. Drug Deliv. 2022, 19, 301–316. [Google Scholar] [CrossRef]
- Li, D.; Cheng, Y.; Luo, Y.; Teng, Y.; Liu, Y.; Feng, L.; Wang, N.; Zhao, Y. Electrospun Nanofiber Materials for Photothermal Interfacial Evaporation. Materials 2023, 16, 5676. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Y.; Bai, Y.; Shi, H.; Zhou, W.; Chen, Y.; Liu, Y.; Yu, D.-G. Recent Combinations of Electrospinning with Photocatalytic Technology for Treating Polluted Water. Catalysts 2023, 13, 758. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-Layer Nanodepots for Sustained Release of Acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Li, D.; Yue, G.; Li, S.; Liu, J.; Li, H.; Gao, Y.; Liu, J.; Hou, L.; Liu, X.; Cui, Z.; et al. Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers. Engineering 2022, 13, 116–127. [Google Scholar] [CrossRef]
- Morais, D.C.; Fontes, M.L.; Oliveira, A.B.; Gabbai-Armelin, P.R.; Ferrisse, T.M.; De Oliveira, L.F.C.; Brighenti, F.L.; Barud, H.S.; De Sousa, F.B. Combining Polymer and Cyclodextrin Strategy for Drug Release of Sulfadiazine from Electrospun Fibers. Pharmaceutics 2023, 15, 1890. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhou, J. How Can Electrospinning Further Service Well for Pharmaceutical Researches? J. Pharm. Sci. 2023, 112, 2719–2723. [Google Scholar] [CrossRef]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [Google Scholar] [CrossRef]
- Chen, X.; Yan, S.; Wen, S.; Chen, J.; Xu, J.; Wang, C.; Lu, X. Chelating Adsorption-Engaged Synthesis of Ultrafine Iridium Nanoparticles Anchored on N-Doped Carbon Nanofibers toward Highly Efficient Hydrogen Evolution in Both Alkaline and Acidic Media. J. Colloid Interface Sci. 2023, 641, 782–790. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Zhang, C.; Xing, Z.; Ahmad, Z.; Ding, Q.; Chang, M.-W. Controlled engineering of multifunctional porous structures using tri-needle co-axial electrohydrodynamic flow and sacrificial media. Chem. Eng. J. 2022, 429, 132221. [Google Scholar] [CrossRef]
- Milano, F.; Masi, A.; Madaghiele, M.; Sannino, A.; Salvatore, L.; Gallo, N. Current Trends in Gelatin-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1499. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Chunta, S.; Wu, W.; Chen, Z.; Lu, Y. Enhanced Oral Bioavailability from Food Protein Nanoparticles: A Mini Review. J. Control. Release 2023, 354, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.K.; Kuppusamy, U.R.; Chua, K.H.; Arumugam, B. Emerging Strategies to Improve the Stability and Bioavailability of Insulin: An Update on Formulations and Delivery Approaches. Curr. Drug Deliv. 2022, 20, 1141–1162. [Google Scholar] [CrossRef]
- Wu, W.; Li, T. Deepening the Understanding of the In Vivo and Cellular Fate of Nanocarriers. Adv. Drug Deliv. Rev. 2022, 189, 114529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Gong, W.; Wang, B.; Yu, D.-G.; Zhu, Y. Integrating Chinese Herbs and Western Medicine for New Wound Dressings through Handheld Electrospinning. Biomedicines 2023, 11, 2146. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, T.; Zhang, Z.; Yu, D.G.; Liu, P.; Wang, L.; Zhu, Y. Electrospun Janus Core (Ethyl Cellulose//Polyethylene Oxide) @ Shell (Hydroxypropyl Methyl Cellulose Acetate Succinate) Hybrids for an Enhanced Colon-Targeted Prolonged Drug Absorbance. Adv. Compos. Hybrid Mater. 2023, 6, 189. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhao, P. The Key Elements for Biomolecules to Biomaterials and to Bioapplications. Biomolecules 2022, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Wang, B.; Chang, M.-W.; Sun, R.; Zhang, L. Sandwich-structured electrospun pH-responsive dental pastes for anti-caries. Colloids Surf. Physicochem. Eng. Asp. 2023, 668, 131399. [Google Scholar] [CrossRef]
- Râpă, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.-D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.-M.; Predescu, C. Bioactive Collagen Hydrolysate-Chitosan/Essential Oil Electrospun Nanofibers Designed for Medical Wound Dressings. Pharmaceutics 2021, 13, 1939. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Multifluid Electrospinning for Multi-Drug Delivery Systems: Pros and Cons, Challenges, and Future Directions. Biomater. Sci. 2022, 11, 37–61. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, E.K.; Schindler, M.; Lukas, D. AC Electrospinning: Impact of High Voltage and Solvent on the Electrospinnability and Productivity of Polycaprolactone Electrospun Nanofibrous Scaffolds. Mater. Today Chem. 2022, 26, 101025. [Google Scholar] [CrossRef]
- Ajalli, N.; Pourmadadi, M.; Yazdian, F.; Abdouss, M.; Rashedi, H.; Rahdar, A. PVA Based Nanofiber Containing GO Modified with Cu Nanoparticles and Loaded Curcumin; High Antibacterial Activity with Acceleration Wound Healing. Curr. Drug Deliv. 2023, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-X.; Wu, J.-W.; Wong, S.-C.; Qu, J.-P.; Srivatsan, T.S. The Technique of Electrospinning for Manufacturing Core-Shell Nanofibers. Mater. Manuf. Process. 2018, 33, 202–219. [Google Scholar] [CrossRef]
- Coimbra, P.; Freitas, J.P.; Gonçalves, T.; Gil, M.H.; Figueiredo, M. Preparation of Gentamicin Sulfate Eluting Fiber Mats by Emulsion and by Suspension Electrospinning. Mater. Sci. Eng. C 2019, 94, 86–93. [Google Scholar] [CrossRef]
- Xin, J.; Qin, M.; Ye, G.; Gong, H.; Li, M.; Sui, X.; Liu, B.; Fu, Q.; He, Z. Hydrophobic Ion Pairing-Based Self-Emulsifying Drug Delivery Systems: A New Strategy for Improving the Therapeutic Efficacy of Water-Soluble Drugs. Expert Opin. Drug Deliv. 2023, 20, 1–11. [Google Scholar] [CrossRef]
- Hu, J.; Prabhakaran, M.P.; Tian, L.; Ding, X.; Ramakrishna, S. Drug-Loaded Emulsion Electrospun Nanofibers: Characterization, Drug Release and In Vitro Biocompatibility. RSC Adv. 2015, 5, 100256–100267. [Google Scholar] [CrossRef]
- Pinheiro Bruni, G.; de Oliveira, J.P.; Gómez-Mascaraque, L.G.; Fabra, M.J.; Guimarães Martins, V.; Zavareze, E.d.R.; López-Rubio, A. Electrospun β-Carotene–Loaded SPI:PVA Fiber Mats Produced by Emulsion-Electrospinning as Bioactive Coatings for Food Packaging. Food Packag. Shelf Life 2020, 23, 100426. [Google Scholar] [CrossRef]
- Shibata, T.; Yoshimura, N.; Kobayashi, A.; Ito, T.; Hara, K.; Tahara, K. Emulsion-Electrospun Polyvinyl Alcohol Nanofibers as a Solid Dispersion System to Improve Solubility and Control the Release of Probucol, a Poorly Water-Soluble Drug. J. Drug Deliv. Sci. Technol. 2022, 67, 102953. [Google Scholar] [CrossRef]
- Zupančič, Š. Core-Shell Nanofibers as Drug Delivery Systems. Acta Pharm. 2019, 69, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Al-Enizi, A.; Aldalbahi, A.; El-Hamshary, H.; El-Newehy, M.H. Core-Shell Nanofibers from Poly(Vinyl Alcohol) Based Biopolymers Using Emulsion Electrospinning as Drug Delivery System for Cephalexin Drug. J. Macromol. Sci. Part A 2021, 58, 130–144. [Google Scholar] [CrossRef]
- Zhan, F.; Yan, X.; Li, J.; Sheng, F.; Li, B. Encapsulation of Tangeretin in PVA/PAA Crosslinking Electrospun Fibers by Emulsion-Electrospinning: Morphology Characterization, Slow-Release, and Antioxidant Activity Assessment. Food Chem. 2021, 337, 127763. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.-R.; Ghasemi-Mobarakeh, L.; Itel, F.; Schoeller, J.; Fashandi, H.; Borzi, A.; Neels, A.; Fortunato, G.; Rossi, R.M. Emulsion Electrospinning of Sodium Alginate/Poly(ε-Caprolactone) Core/Shell Nanofibers for Biomedical Applications. Nanoscale Adv. 2022, 4, 2929–2941. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Y.; Zhang, S.; Li, T.; Ramakrishna, S.; Liu, Y. Progress of Improving Mechanical Strength of Electrospun Nanofibrous Membranes. Macromol. Mater. Eng. 2020, 305, 2000230. [Google Scholar] [CrossRef]
- Abdelhakim, H.E.; Coupe, A.; Tuleu, C.; Edirisinghe, M.; Craig, D.Q.M. Utilising Co-Axial Electrospinning as a Taste-Masking Technology for Paediatric Drug Delivery. Pharmaceutics 2021, 13, 1665. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Lu, W.; Guo, Y. Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications. Nanomaterials 2021, 11, 1316. [Google Scholar] [CrossRef]
- Al-Nimry, S.S.; Khanfar, M.S. Enhancement of the Solubility of Asenapine Maleate through the Preparation of Co-Crystals. Curr. Drug Deliv. 2022, 19, 788–800. [Google Scholar] [CrossRef]
- Partheniadis, I.; Stathakis, G.; Tsalavouti, D.; Heinämäki, J.; Nikolakakis, I. Essential Oil—Loaded Nanofibers for Pharmaceutical and Biomedical Applications: A Systematic Mini-Review. Pharmaceutics 2022, 14, 1799. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Yousefi, M.; Khezerlou, A.; Aman Mohammadi, M.; Jafari, S.M. Application of Different Biopolymers for Nanoencapsulation of Antioxidants via Electrohydrodynamic Processes. Food Hydrocoll. 2019, 97, 105170. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent Advances in Multiaxial Electrospinning for Drug Delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Huang, Z.; Zhang, H.; Ramalingam, M. Preparation and Characterization of Nano-Silver-Loaded Antibacterial Membrane via Coaxial Electrospinning. Biomimetics 2023, 8, 419. [Google Scholar] [CrossRef]
- Wei, Y.-S.; Feng, K.; Li, S.-F.; Hu, T.-G.; Zong, M.-H.; Wu, H. Highly-Hydrophobic Nanofiber Mat for Efficient Colonic Delivery of Lactoferrin: Preparation, Characterization and Release Mechanism. Innov. Food Sci. Emerg. Technol. 2022, 78, 103015. [Google Scholar] [CrossRef]
- Oliveira, A.; Araújo, A.; Rodrigues, L.C.; Silva, C.S.; Reis, R.L.; Neves, N.M.; Leão, P.; Martins, A. Metronidazole Delivery Nanosystem Able to Reduce the Pathogenicity of Bacteria in Colorectal Infection. Biomacromolecules 2022, 23, 2415–2427. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Lo, T.-S.; Lin, Y.-T.; Chien, Y.-H.; Lu, C.-J.; Liu, S.-J. Fabrication of Drug-Eluting Polycaprolactone/Poly(Lactic-Co-Glycolic Acid) Prolapse Mats Using Solution-Extrusion 3D Printing and Coaxial Electrospinning Techniques. Polymers 2021, 13, 2295. [Google Scholar] [CrossRef]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.; Kenawy, E.-R. Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef]
- Gong, M.; Chen, Z.; Zhou, L.; Gao, F.; Cheng, J.; Zou, W. Application of Mesoscale Simulation to Explore the PH Response of Eudragit S100 Used as the Novel Colon-Targeted Powder of Pulsatilla Saponin D. J. Nanomater. 2021, 2021, e9556911. [Google Scholar] [CrossRef]
- Khin, S.Y.; Soe, H.M.S.H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of Fenofibrate/Randomly Methylated β-Cyclodextrin-Loaded Eudragit® RL 100 Nanoparticles for Ocular Delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef]
- Qian, C.; Liu, Y.; Chen, S.; Zhang, C.; Chen, X.; Liu, Y.; Liu, P. Electrospun Core–Sheath PCL Nanofibers Loaded with NHA and Simvastatin and Their Potential Bone Regeneration Applications. Front. Bioeng. Biotechnol. 2023, 11, 1205252. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Wang, L.; Ding, X. Solid Drug Particles Encapsulated Bead-on-String Nanofibers: The Control of Bead Number and Its Corresponding Release Profile. J. Biomater. Sci. Polym. Ed. 2019, 30, 1454–1469. [Google Scholar] [CrossRef]
- Phan, N.T.; Tran, Y.T.H.; Nguyen, L.T.; Hoang, Y.K.; Bui, C.K.; Nguyen, H.D.; Vu, G.T.T. Self Nanoelmusifying Drug Delivery System of Rosuvastatin: Bioavailability Evaluation and In Vitro—In Vivo Correlation. Curr. Drug Deliv. 2022, 21, 734–743. [Google Scholar] [CrossRef]
- Turanlı, Y.; Acartürk, F. Preparation and Characterization of Colon-Targeted PH/Time-Dependent Nanoparticles Using Anionic and Cationic Polymethacrylate Polymers. Eur. J. Pharm. Sci. 2022, 171, 106122. [Google Scholar] [CrossRef]
- Xu, L.; He, H.; Du, Y.; Zhang, S.; Yu, D.-G.; Liu, P. Electrosprayed Core (Cellulose Acetate)–Shell (Polyvinylpyrrolidone) Nanoparticles for Smart Acetaminophen Delivery. Pharmaceutics 2023, 15, 2314. [Google Scholar] [CrossRef]
- Wandling, E.N.; Rhoads, K.; Ohman, D.E.; Heise, R.L. Electrosprayed Mesenchymal Stromal Cell Extracellular Matrix Nanoparticles Accelerate Cellular Wound Healing and Reduce Gram-Negative Bacterial Growth. Pharmaceutics 2023, 15, 1277. [Google Scholar] [CrossRef]
- Zieman, J.; Cohan, M.; Wang, Y.; De La Sancha, A.; Kanungo, M.; Azzouz, R.; Smith, R.; Schmidt, K.; Kumpaty, S.; Chen, J.; et al. Development of Gelatin-Coated Hydrogel Microspheres for Novel Bioink Design: A Crosslinker Study. Pharmaceutics 2023, 15, 90. [Google Scholar] [CrossRef]
- Zhang, X.; Prior, T.J.; Chen, K.; Santoro, O.; Redshaw, C. Ring Opening Polymerization of Lactides and Lactones by Multimetallic Titanium Complexes Derived from the Acids Ph2C(X)CO2H (X = OH, NH2). Catalysts 2022, 12, 935. [Google Scholar] [CrossRef]
- Radisavljevic, A.; Stojanovic, D.B.; Perisic, S.; Djokic, V.; Radojevic, V.; Rajilic-Stojanovic, M.; Uskokovic, P.S. Cefazolin-Loaded Polycaprolactone Fibers Produced via Different Electrospinning Methods: Characterization, Drug Release and Antibacterial Effect. Eur. J. Pharm. Sci. 2018, 124, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Bisht, M.; Bhawna, B.; Pandey, S. Enhanced Solubility and Improved Stability of Curcumin in Novel Water-in-Deep Eutectic Solvent Microemulsions. J. Mol. Liq. 2021, 339, 117037. [Google Scholar] [CrossRef]
- Pires, P.C.; Paiva-Santos, A.C.; Veiga, F. Nano and Microemulsions for the Treatment of Depressive and Anxiety Disorders: An Efficient Approach to Improve Solubility, Brain Bioavailability and Therapeutic Efficacy. Pharmaceutics 2022, 14, 2825. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, J.S.; Choi, H.-G.; Jin, S.G.; Cho, C.-W. Novel Ezetimibe-Loaded Fibrous Microparticles for Enhanced Solubility and Oral Bioavailability by Electrospray Technique. J. Drug Deliv. Sci. Technol. 2021, 66, 102877. [Google Scholar] [CrossRef]
- Gupta, A.; Sood, A.; Dhiman, A.; Shrimali, N.; Singhmar, R.; Guchhait, P.; Agrawal, G. Redox responsive poly (allylamine)/eudragit S-100 nanoparticles for dual drug delivery in colorectal cancer. Biomater. Adv. 2022, 143, 213184. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Geng, C.; Shen, H.; Zhang, Q.; Miao, Y.; Wu, J.; Ouyang, R.; Zhou, S. Two Hawks with One Arrow: A Review on Bifunctional Scaffolds for Photothermal Therapy and Bone Regeneration. Nanomaterials 2023, 13, 551. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Hou, C.; Li, T.; Xin, B. The Release Kinetic of Drug Encapsulated Poly(L-Lactide-Co-ɛ-Caprolactone) Core-Shell Nanofibers Fabricated by Emulsion Electrospinning. J. Macromol. Sci. Part A 2022, 59, 489–503. [Google Scholar] [CrossRef]
- Fan, W.; Peng, H.; Yu, Z.; Wang, L.; He, H.; Ma, Y.; Qi, J.; Lu, Y.; Wu, W. The Long-Circulating Effect of Pegylated Nanoparticles Revisited via Simultaneous Monitoring of Both the Drug Payloads and Nanocarriers. Acta Pharm. Sin. B 2022, 12, 2479–2493. [Google Scholar] [CrossRef]
- Laracuente, M.-L.; Yu, M.H.; McHugh, K.J. Zero-Order Drug Delivery: State of the Art and Future Prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]
- Man, F.; Yang, Y.; He, H.; Qi, J.; Wu, W.; Lu, Y. Establishment of In Vitro Dissolution Based on Similarity with In Vivo Dissolution: A Case Study on Aripiprazole. Mol. Pharm. 2023, 20, 2579–2588. [Google Scholar] [CrossRef]
- Gupta, C.; Naik, I.; Menon, M.; Ambre, P.; Coutinho, E. A Review on Exploring the Opportunities of Polymer Drug Conjugated Systems for Targeted Cancer Treatment. Curr. Drug Deliv. 2023, 20, 8–30. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Raman, S. Recent Trends in Carbon Nanotubes Based Prostate Cancer Therapy: A Biomedical Hybrid for Diagnosis and Treatment. Curr. Drug Deliv. 2022, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ji, X.; Zhang, Y.; Liu, C.; Zhang, Z.; Lv, Y.; Dong, X.; He, H.; Qi, J.; Lu, Y.; et al. Near-Infrared Fluorophores with Absolute Aggregation-Caused Quenching and Negligible Fluorescence Re-Illumination for In Vivo Bioimaging of Nanocarriers. Aggregate 2023, 4, e277. [Google Scholar] [CrossRef]
- He, H.; Liu, C.; Ming, J.; Lv, Y.; Qi, J.; Lu, Y.; Dong, X.; Zhao, W.; Wu, W. Accurate and Sensitive Probing of Onset of Micellization Based on Absolute Aggregation-Caused Quenching Effect. Aggregate 2022, 3, e163. [Google Scholar] [CrossRef]
- Cai, Y.; Qi, J.; Lu, Y.; He, H.; Wu, W. The In Vivo Fate of Polymeric Micelles. Adv. Drug Deliv. Rev. 2022, 188, 114463. [Google Scholar] [CrossRef]







| Number | Shell | Core | Drug Content (MTD) | Flow Rate | Voltage | |||
|---|---|---|---|---|---|---|---|---|
| PCL | PVA | PF-127 | Shell | Core | ||||
| F1 | 15%ESR (100:0) | 10% | 8% | 2% | 2% | 2.0 mL/h | 0.5 mL/h | 12.0 kV |
| F2 | 15%ESR (95:5) | |||||||
| F3 | 15%ESR (90:10) | |||||||
| F4 | 15%ESR (80:20) | |||||||
| Number | Model | HBS | PBS | ||
|---|---|---|---|---|---|
| Equation | R2 | Equation | R2 | ||
| F1 | Zero-order kinetics | Q = 0.290 t + 11.203 | 0.7540 | Q = 0.052 t + 36.248 | 0.9624 |
| First-order kinetics | Q = 38.810(1 − e−0.033 t) | 0.9925 | Q = 68.162(1 − e−0.006 t) | 0.9637 | |
| Higuchi kinetics | Q = 3.779 t1/2 + 1.833 | 0.9617 | Q = 1.975 t1/2 + 19.282 | 0.9867 | |
| Ritger–Peppas kinetics | Q = 5.889 t0.41 | 0.9758 | Q = 8.689 t0.32 | 0.9914 | |
| F2 | Zero-order kinetics | Q = 0.227 t + 19.721 | 0.4573 | Q = 0.026 t + 37.335 | 0.9727 |
| First-order kinetics | Q = 36.314(1 − e−0.098 t) | 0.9891 | Q = 51.824(1 − e−0.010 t) | 0.9062 | |
| Higuchi kinetics | Q = 3.416 t1/2 + 9.769 | 0.7828 | Q = 0.977 t1/2 + 28.952 | 0.9941 | |
| Ritger–Peppas kinetics | Q = 17.691 t0.17 | 0.9867 | Q = 15.999 t0.19 | 0.9987 | |
| F3 | Zero-order kinetics | Q = 0.332 t + 18.118 | 0.6014 | Q = 0.023 t + 46.722 | 0.9551 |
| First-order kinetics | Q = 46.786(1 − e−0.047 t) | 0.9982 | Q = 59.164(1 − e−0.012 t) | 0.8842 | |
| Higuchi kinetics | Q = 4.623 t1/2 + 5.677 | 0.8805 | Q = 0.866 t1/2 + 39.297 | 0.9754 | |
| Ritger–Peppas kinetics | Q = 11.470 t0.31 | 0.9411 | Q = 24.631 t0.14 | 0.9815 | |
| F4 | Zero-order kinetics | Q = 0.398 t + 27.167 | 0.5451 | Q = 0.024 t + 59.590 | 0.9441 |
| First-order kinetics | Q = 59.029(1 − e−0.063 t) | 0.9942 | Q = 72.559(1 − e−0.013 t) | 0.8986 | |
| Higuchi kinetics | Q = 5.736 t1/2 + 11.172 | 0.8532 | Q = 0.910 t1/2 + 51.730 | 0.9788 | |
| Ritger–Peppas kinetics | Q = 20.168 t0.25 | 0.9689 | Q = 34.500 t0.12 | 0.9940 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, L.; Zhu, Y.; Wang, L.; Yu, D.-G.; Liu, L.-y. Tri-Layer Core–Shell Fibers from Coaxial Electrospinning for a Modified Release of Metronidazole. Pharmaceutics 2023, 15, 2561. https://doi.org/10.3390/pharmaceutics15112561
Wang Y, Liu L, Zhu Y, Wang L, Yu D-G, Liu L-y. Tri-Layer Core–Shell Fibers from Coaxial Electrospinning for a Modified Release of Metronidazole. Pharmaceutics. 2023; 15(11):2561. https://doi.org/10.3390/pharmaceutics15112561
Chicago/Turabian StyleWang, Ying, Lin Liu, Yuanjie Zhu, Liangzhe Wang, Deng-Guang Yu, and Li-ying Liu. 2023. "Tri-Layer Core–Shell Fibers from Coaxial Electrospinning for a Modified Release of Metronidazole" Pharmaceutics 15, no. 11: 2561. https://doi.org/10.3390/pharmaceutics15112561