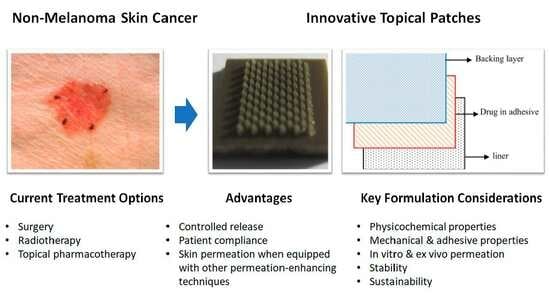
Innovative Topical Patches for Non-Melanoma Skin Cancer: Current Challenges and Key Formulation Considerations
Abstract
:1. Introduction
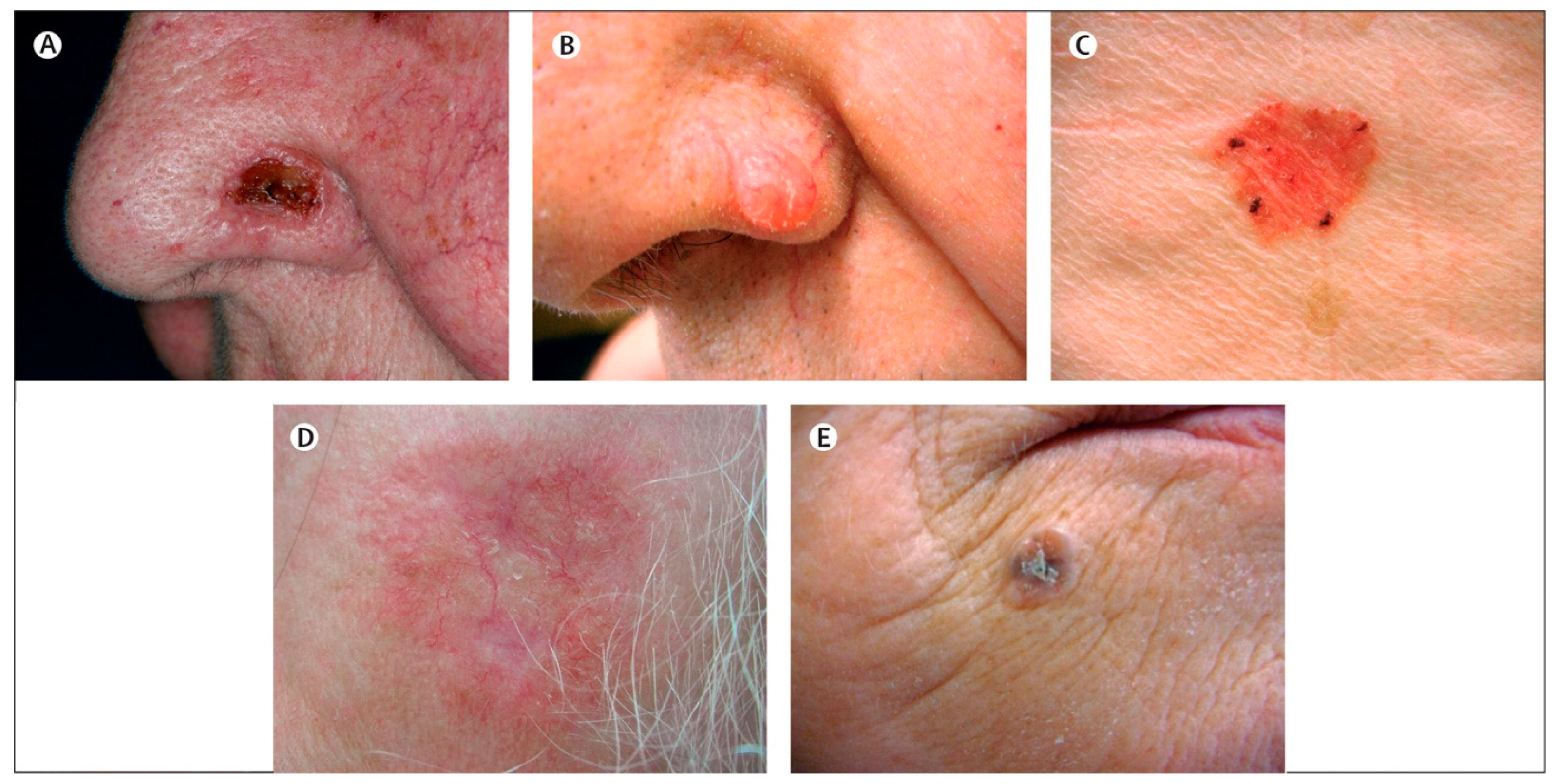
2. Epidemiology of Non-Melanoma Skin Cancer
3. The Current Treatment Landscape for Non-Melanoma Skin Cancer
| Therapeutic Agent | Dosage Form | Strength | Brand Names | Mode of Action | Common Indications | Limitations | Physico-Chemical Properties | Ref. |
|---|---|---|---|---|---|---|---|---|
| 5-Fluorouracil (5-FU) | Cream | 5% | Efudex® Carac® | Interferes with DNA synthesis by blocking thymidylate synthase | Bowen’s disease (SCC in situ); superficial BCC; AK | Skin irritation; photosensitivity | Log P (−0.85); molecular weight (130.078 g/mol); melting point (291.8 °C) | [31,42] |
| Imiquimod (IMQ) | Cream | 5% | Aldara® | Induces immune response against cancer cells | Superficial BCC; genital warts; AK | Local skin reactions; psoriasis | Log P (2.6); molecular weight (240.30 g/mol); melting point (295 °C) | [37,43] |
| Diclofenac sodium | Gel | 3% | Solaraze® | Inhibits COX-2 enzyme, reducing prostaglandin E2 synthesis | AK | Local skin irritation; digestive adverse events | Log P (4.26); molecular weight (318.13 g/mol); melting point (286 °C) | [44] |
| Ingenol mebutate | Gel | 0.015% | Picato® | Induces local lesion cell death; promotes an inflammatory response | AK | Local skin irritation | Log P (3.12); molecular weight (430.5 g/mol); melting point (153.5 °C) | [45] |
| Tirbanibulin | Ointment | 1% | Klisyri® | Disrupts microtubules by direct binding to tubulin | AK | Local skin irritation; sun sensitivity | Log P (N/A); molecular weight (g/mol); melting point (N/A) | [46] |
4. Limitations of Current Topical Treatment for Non-Melanoma Skin Cancer
5. Advances in Topical Patch Technology for Non-Melanoma Skin Cancer Treatment
5.1. Microneedle Array Patches
5.2. Polymeric, Drug-in-Adhesive, and Matrix-Type Patches
5.3. Hydrogels
6. Challenges Associated with Novel Topical Patch Development for Non-Melanoma Skin Cancer Treatment
7. Key Considerations for Novel Patch Development
7.1. Physicochemical Properties of Drugs
7.2. Type of Patches and Polymer Selection
7.3. Prevention of Drug Crystallisation
7.4. Backing Layer and Release Liner Selection
7.5. Mechanical and Adhesive Properties
7.6. In Vitro Drug Release Profile
7.7. In Vitro and Ex Vivo Permeation Profile
7.8. Stability
7.9. Other Considerations
8. Future Perspective
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, W.; Fang, L.; Ni, R.; Zhang, H.; Pan, G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer 2022, 22, 836. [Google Scholar] [CrossRef] [PubMed]
- Brokmeier, L.L.; Diehl, K.; Spähn, B.A.; Jansen, C.; Konkel, T.; Uter, W.; Görig, T. “Well, to Be Honest, I Don’t Have an Idea of What It Might Be”—A Qualitative Study on Knowledge and Awareness Regarding Nonmelanoma Skin Cancer. Curr. Oncol. 2023, 30, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.; Trakatelli, M.; Kalabalikis, D.; Ferrandiz, L.; Ruiz-de-Casas, A.; Moreno-Ramirez, D.; Sotiriadis, D.; Ioannides, D.; Aquilina, S.; Apap, C.; et al. Known and potential new risk factors for skin cancer in European populations: A multicentre case–control study. Br. J. Dermatol. 2012, 167, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fahradyan, A.; Howell, A.C.; Wolfswinkel, E.M.; Tsuha, M.; Sheth, P.; Wong, A.K. Updates on the Management of Non-Melanoma Skin Cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef]
- Madan, V.; Lear, J.; Szeimies, R. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Schadendorf, D. Update in the treatment of non-melanoma skin cancers: The use of PD-1 inhibitors in basal cell carcinoma and cutaneous squamous-cell carcinoma. J. Immunother. Cancer 2022, 10, e005082. [Google Scholar] [CrossRef]
- Souto, E.B.; da Ana, R.; Vieira, V.; Fangueiro, J.F.; Dias-Ferreira, J.; Cano, A.; Zielińska, A.; Silva, A.M.; Staszewski, R.; Karczewski, J. Non-melanoma skin cancers: Physio-pathology and role of lipid delivery systems in new chemotherapeutic treatments. Neoplasia 2022, 30, 100810. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Non-Melanoma Skin Cancer. General Practice Consultations, Hospitalisation and Mortality; AIHW, Department of Health: Canberra, Australia, 2008.
- Khan, N.H.; Mir, M.; Qian, L.; Baloch, M.; Ali Khan, M.F.; Rehman, A.-u.; Ngowi, E.E.; Wu, D.-D.; Ji, X.-Y. Skin cancer biology and barriers to treatment: Recent applications of polymeric micro/nanostructures. J. Adv. Res. 2022, 36, 223–247. [Google Scholar] [CrossRef]
- Micali, G.; Lacarrubba, F.; Nasca, M.R.; Ferraro, S.; Schwartz, R.A. Topical pharmacotherapy for skin cancer: Part II. Clinical applications. J. Am. Acad. Dermatol. 2014, 70, 979.e1–979.e12, quiz 9912. [Google Scholar] [CrossRef]
- Chen, M.; Shamim, M.A.; Shahid, A.; Yeung, S.; Andresen, B.T.; Wang, J.; Nekkanti, V.; Meyskens, F.L.; Kelly, K.M.; Huang, Y. Topical Delivery of Carvedilol Loaded Nano-Transfersomes for Skin Cancer Chemoprevention. Pharmaceutics 2020, 12, 1151. [Google Scholar] [CrossRef]
- Hasan, N.; Imran, M.; Nadeem, M.; Jain, D.; Haider, K.; Moshahid Alam Rizvi, M.; Sheikh, A.; Kesharwani, P.; Kumar Jain, G.; Jalees Ahmad, F. Formulation and development of novel lipid-based combinatorial advanced nanoformulation for effective treatment of non-melanoma skin cancer. Int. J. Pharm. 2023, 632, 122580. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Mignot, M.; Mondon, K.; Moller, M.; Gurny, R.; Kalia, Y.N. Self-assembled mPEG-hexPLA polymeric nanocarriers for the targeted cutaneous delivery of imiquimod. Eur. J. Pharm. Biopharm. 2019, 142, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, S.; Tagne, J.B.; Wilson, T.A.; Nicolosi, R.J. A nanoemulsion formulation of dacarbazine reduces tumor size in a xenograft mouse epidermoid carcinoma model compared to dacarbazine suspension. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Costa-Almeida, R.; Bogas, D.; Fernandes, J.R.; Timochenco, L.; Silva, F.A.L.S.; Meneses, J.; Gonçalves, I.C.; Magalhães, F.D.; Pinto, A.M. Near-Infrared Radiation-Based Mild Photohyperthermia Therapy of Non-Melanoma Skin Cancer with PEGylated Reduced Nanographene Oxide. Polymers 2020, 12, 1840. [Google Scholar] [CrossRef]
- Krishnan, V.; Mitragotri, S. Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; He, X.; Yang, F.; Han, R.; Yang, C.; Li, W.; Qian, Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Zawislak, A.A.; Woolfson, A.D. Design and physicochemical characterisation of a bioadhesive patch for dose-controlled topical delivery of imiquimod. Int. J. Pharm. 2006, 307, 318–325. [Google Scholar] [CrossRef]
- Marzi, M.; Rostami Chijan, M.; Zarenezhad, E. Hydrogels as promising therapeutic strategy for the treatment of skin cancer. J. Mol. Struct. 2022, 1262, 133014. [Google Scholar] [CrossRef]
- Kim, S.; Abdella, S.; Abid, F.; Afinjuomo, F.; Youssef, S.H.; Holmes, A.; Song, Y.; Vaidya, S.; Garg, S. Development and Optimization of Imiquimod-Loaded Nanostructured Lipid Carriers Using a Hybrid Design of Experiments Approach. Int. J. Nanomed. 2023, 18, 1007–1029. [Google Scholar] [CrossRef]
- Guillot, A.J.; Martínez-Navarrete, M.; Zinchuk-Mironova, V.; Melero, A. Microneedle-assisted transdermal delivery of nanoparticles: Recent insights and prospects. Wiley Interdiscip. Rev. 2023, 15, e1884. [Google Scholar] [CrossRef]
- Leow, L.J.; Teh, N. Clinical clearance of complex basal cell carcinoma in patients receiving sonidegib: A case series. Dermatol. Ther. 2022, 35, e15217. [Google Scholar] [CrossRef] [PubMed]
- Welsch, M.J.; Troiani, B.M.; Hale, L.; DelTondo, J.; Helm, K.F.; Clarke, L.E. Basal cell carcinoma characteristics as predictors of depth of invasion. J. Am. Acad. Dermatol. 2012, 67, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Granada, C.; Rodriguez-Waitkus, P. Cutaneous squamous cell carcinoma and related entities: Epidemiology, clinical and histological features, and basic science overview. Curr. Probl. Cancer 2015, 39, 206–215. [Google Scholar] [CrossRef]
- Reichrath, J.; Saternus, R.; Vogt, T. Endocrine actions of vitamin D in skin: Relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol. Cell. Endocrinol. 2017, 453, 96–102. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Migden, M.R.; Chang, A.L.S.; Dirix, L.; Stratigos, A.J.; Lear, J.T. Emerging trends in the treatment of advanced basal cell carcinoma. Cancer Treat. Rev. 2018, 64, 1–10. [Google Scholar] [CrossRef]
- Lee, E.H.; Klassen, A.F.; Lawson, J.L.; Cano, S.J.; Scott, A.M.; Pusic, A.L. Patient experiences and outcomes following facial skin cancer surgery: A qualitative study. Aust. J. Dermatol. 2016, 57, e100–e104. [Google Scholar] [CrossRef]
- Ravitskiy, L.; Brodland, D.G.; Zitelli, J.A. Cost Analysis: Mohs Micrographic Surgery. Dermatol. Surg. 2012, 38, 585–594. [Google Scholar] [CrossRef]
- Khong, J.; Gorayski, P.; Roos, D. Non-melanoma skin cancer in general practice: Radiotherapy is an effective treatment option. Aust. J. Gen. Pract. 2020, 49, 496–499. [Google Scholar] [CrossRef]
- Bagatin, E. 5-fluorouracil for actinic keratoses. Expert. Rev. Dermatol. 2010, 5, 131–139. [Google Scholar] [CrossRef]
- Gross, K.; Kircik, L.; Kricorian, G. 5% 5-Fluorouracil cream for the treatment of small superficial Basal cell carcinoma: Efficacy, tolerability, cosmetic outcome, and patient satisfaction. Dermatol. Surg. 2007, 33, 433–439; discussion 440. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Schön, M. Imiquimod: Mode of action. Br. J. Dermatol. 2007, 157, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, V.; Keating, G.M.; Perry, C.M. Imiquimod. Am. J. Clin. Dermatol. 2005, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Gebauer, K.; Shumack, S.; Amies, M.; Bryden, J.; Fox, T.L.; Owens, M.L. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: Results of a multicenter 6-week dose-response trial. . J. Am. Acad. Dermatol. 2001, 44, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Geisse, J.; Caro, I.; Lindholm, J.; Golitz, L.; Stampone, P.; Owens, M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: Results from two phase III, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol. 2004, 50, 722–733. [Google Scholar] [CrossRef]
- Hanna, E.; Abadi, R.; Abbas, O. Imiquimod in dermatology: An overview. Int. J. Deramtol. 2016, 55, 831–844. [Google Scholar] [CrossRef]
- Huber, L.; Pereira, T.; Ramos, D.; Rezende, L.; Emery, F.; Sobral, L.; Leopoldino, A.; Lopez, R. Topical Skin Cancer Therapy Using Doxorubicin-Loaded Cationic Lipid Nanoparticles and Iontophoresis. J. Biomed. Nanotechnol. 2015, 11, 1975–1988. [Google Scholar] [CrossRef]
- Paolino, D.; Celia, C.; Trapasso, E.; Cilurzo, F.; Fresta, M. Paclitaxel-loaded ethosomes®: Potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur. J. Pharm. Biopharm. 2012, 81, 102–112. [Google Scholar] [CrossRef]
- Sonavane, K.; Phillips, J.; Ekshyyan, O.; Moore-Medlin, T.; Roberts Gill, J.; Rong, X.; Lakshmaiah, R.R.; Abreo, F.; Boudreaux, D.; Clifford, J.L.; et al. Topical Curcumin-Based Cream Is Equivalent to Dietary Curcumin in a Skin Cancer Model. J. Skin Cancer 2012, 2012, 147863. [Google Scholar] [CrossRef]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical nanostructured lipid carrier gel of quercetin and resveratrol: Formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef]
- Mutalik, S.; Shetty, P.K.; Kumar, A.; Kalra, R.; Parekh, H.S. Enhancement in deposition and permeation of 5-fluorouracil through human epidermis assisted by peptide dendrimers. Drug Deliv. 2014, 21, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Nazerdeylami, S.; Ghasemi, J.B.; Mohammadi Ziarani, G.; Amiri, A.; Badiei, A. Direct monitoring of diclofenac using a supramolecular fluorescent approach based on β-cyclodextrin nanosponge. J. Mol. Liqids 2021, 336, 116104. [Google Scholar] [CrossRef]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency (EMA). Klisyri. 2021. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/klisyri (accessed on 30 October 2023).
- Siegel, J.A.; Korgavkar, K.; Weinstock, M.A. Current perspective on actinic keratosis: A review. Br. J. Dermatol. 2017, 177, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Micali, G.; Lacarrubba, F.; Bhatt, K.; Nasca, M.R. Medical approaches to non-melanoma skin cancer. Expert Rev. Anticancer Ther. 2013, 13, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Bath-Hextall, F.; Ozolins, M.; Armstrong, S.J.; Colver, G.B.; Perkins, W.; Miller, P.S.J.; Williams, H.C. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): A multicentre, non-inferiority, randomised controlled trial. Lancet Oncol. 2014, 15, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Pandit, J.; Sultana, Y.; Sultana, S.; Ali, A.; Aqil, M.; Chauhan, M. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: In vitro characterization and in vivo study. Drug Deliv. 2015, 22, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Fouladian, P.; Afinjuomo, F.; Song, Y.; Youssef, S.H.; Vaidya, S.; Garg, S. Effect of plasticizers on drug-in-adhesive patches containing 5-fluorouracil. Int. J. Pharm. 2022, 611, 121316. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Du, W.; Sun, M.; Ling, G.; Zhang, P. Microneedle-mediated treatment for superficial tumors by combining multiple strategies. Drug Deliv. Transl. Res. 2023, 13, 1600–1620. [Google Scholar] [CrossRef]
- Layek, B.; Rahman Nirzhor, S.S.; Rathi, S.; Kandimalla, K.K.; Wiedmann, T.S.; Prabha, S. Design, Development, and Characterization of Imiquimod-Loaded Chitosan Films for Topical Delivery. AAPS PharmSciTech 2019, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Z.; Zhang, M.; Ling, G.; Zhang, P. Research progress of microneedles in the treatment of melanoma. J. Control. Release 2022, 348, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Jain, A.K.; Lee, C.H.; Gill, H.S. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J. Control. Release 2016, 239, 72–81. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, M.; Zhang, T.; Peng, J.; Jia, Y.; Cao, Y.; Qian, Z. Novel Approach of Using Near-Infrared Responsive PEGylated Gold Nanorod Coated Poly(l-lactide) Microneedles to Enhance the Antitumor Efficiency of Docetaxel-Loaded MPEG-PDLLA Micelles for Treating an A431 Tumor. ACS Appl. Mater. Interfaces 2017, 9, 15317–15327. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.F.; Douroumis, D. 3D printed microneedles for anticancer therapy of skin tumours. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110248. [Google Scholar] [CrossRef]
- Sabri, A.H.; Cater, Z.; Gurnani, P.; Ogilvie, J.; Segal, J.; Scurr, D.J.; Marlow, M. Intradermal delivery of imiquimod using polymeric microneedles for basal cell carcinoma. Int. J. Pharm. 2020, 589, 119808. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Controll. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef]
- Cilurzo, F.; Gennari, C.G.; Minghetti, P. Adhesive properties: A critical issue in transdermal patch development. Expert. Opin. Drug Deliv. 2012, 9, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.D.; Deen, G.R.; Bates, J.S.; Maiti, C.; Lam, C.Y.K.; Pachauri, A.; AlAnsari, R.; Bělský, P.; Yoon, J.; Dodda, J.M. Smart Skin-Adhesive Patches: From Design to Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2213560. [Google Scholar] [CrossRef]
- Carcamo-Martinez, A.; Dominguez-Robles, J.; Mallon, B.; Raman, M.T.; Cordeiro, A.S.; Bell, S.E.J.; Larraneta, E.; Donnelly, R.F. Potential of Polymeric Films Loaded with Gold Nanorods for Local Hyperthermia Applications. Nanomaterials 2020, 10, 582. [Google Scholar] [CrossRef]
- Kim, S.; Youssef, S.H.; Song, Y.; Garg, S. Development and application of a chromatographic method for simultaneous quantification of 5-fluorouracil and imiquimod in drug-in-adhesive topical patches. Sustain. Chem. Pharm. 2022, 27, 100711. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Luca, A.; Nacu, I.; Tanasache, S.; Peptu, C.A.; Butnaru, M.; Verestiuc, L. New Methacrylated Biopolymer-Based Hydrogels as Localized Drug Delivery Systems in Skin Cancer Therapy. Gels 2023, 9, 371. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Kleesz, P.; Darlenski, R.; Fluhr, J.W. Full-Body Skin Mapping for Six Biophysical Parameters: Baseline Values at 16 Anatomical Sites in 125 Human Subjects. Skin. Pharmacol. Physiol. 2011, 25, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Khavari, P.A. Modelling cancer in human skin tissue. Nat. Rev. Cancer 2006, 6, 270–280. [Google Scholar] [CrossRef]
- Oosterhoff, D.; Heusinkveld, M.; Lougheed, S.M.; Kosten, I.; Lindstedt, M.; Bruijns, S.C.M.; van Es, T.; van Kooyk, Y.; van der Burg, S.H.; de Gruijl, T.D. Intradermal Delivery of TLR Agonists in a Human Explant Skin Model: Preferential Activation of Migratory Dendritic Cells by Polyribosinic-Polyribocytidylic Acid and Peptidoglycans. J. Immun. 2013, 190, 3338–3345. [Google Scholar] [CrossRef]
- Tuan-Mahmood, T.-M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef]
- Sartawi, Z.; Blackshields, C.; Faisal, W. Dissolving microneedles: Applications and growing therapeutic potential. J. Control. Release 2022, 348, 186–205. [Google Scholar] [CrossRef]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Halder, J.; Gupta, S.; Kumari, R.; Gupta, G.D.; Rai, V.K. Microneedle Array: Applications, Recent Advances, and Clinical Pertinence in Transdermal Drug Delivery. J. Pharm. Innov. 2021, 16, 558–565. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef] [PubMed]
- Margetts, L.; Sawyer, R. Transdermal drug delivery: Principles and opioid therapy. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 171–176. [Google Scholar] [CrossRef]
- Subedi, R.K.; Oh, S.Y.; Chun, M.-K.; Choi, H.-K. Recent advances in transdermal drug delivery. Arch. Pharmacal Res. 2010, 33, 339–351. [Google Scholar] [CrossRef]
- Venkatraman, S.; Gale, R. Skin adhesives and skin adhesion: 1. Transdermal drug delivery systems. Biomaterials 1998, 19, 1119–1136. [Google Scholar] [CrossRef]
- Minghetti, P.; Cilurzo, F.; Pagani, S.; Casiraghi, A.; Assandri, R.; Montanari, L. Formulation Study of Oxybutynin Patches. Pharm. Dev. Technol. 2007, 12, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Bhattaccharjee, S.A.; Zhang, W.; Clark, M.; Singh, O.; Doncel, G.F.; Banga, A.K. Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV. Pharmaceutics 2019, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Bozorg, B.D.; Banga, A.K. Effect of Different Pressure-Sensitive Adhesives on Performance Parameters of Matrix-Type Transdermal Delivery Systems. Pharmaceutics 2020, 12, 209. [Google Scholar] [CrossRef]
- Ganti, S.S.; Bhattaccharjee, S.A.; Murnane, K.S.; Blough, B.E.; Banga, A.K. Formulation and evaluation of 4-benzylpiperidine drug-in-adhesive matrix type transdermal patch. Int. J. Pharm. 2018, 550, 71–78. [Google Scholar] [CrossRef]
- Jain, P.; Banga, A.K. Inhibition of crystallization in drug-in-adhesive-type transdermal patches. Int. J. Pharm. 2010, 394, 68–74. [Google Scholar] [CrossRef]
- Tahir, M.A.; Ali, M.E.; Lamprecht, A. Nanoparticle formulations as recrystallization inhibitors in transdermal patches. Int. J. Pharm. 2020, 575, 118886. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Ortenzi, M.A.; Gennari, C.G.M.; Casiraghi, A.; Minghetti, P.; Cilurzo, F. Design of pressure-sensitive adhesive suitable for the preparation of transdermal patches by hot-melt printing. Int. J. Pharm. 2020, 586, 119607. [Google Scholar] [CrossRef] [PubMed]
- Rizi, K.; Xu, K.; Begum, T.; Faull, J.; Bhakta, S.; Murdan, S. A drug-in-adhesive anti-onychomycotic nail patch: Influence of drug and adhesive nature on drug release, ungual permeation, in vivo residence in human and anti-fungal efficacy. Int. J. Pharm. 2022, 614, 121437. [Google Scholar] [CrossRef]
- Akram, M.R.; Ahmad, M.; Abrar, A.; Sarfraz, R.M.; Mahmood, A. Formulation design and development of matrix diffusion controlled transdermal drug delivery of glimepiride. Drug Des. Dev. Ther. 2018, 12, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.; Mujtaba, A.; Akhter, M.H.; Zafar, A.; Kohli, K. Optimisation of ethosomal nanogel for topical nano-CUR and sulphoraphane delivery in effective skin cancer therapy. J. Microencapsul. 2020, 37, 91–108. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Das, P.J.; Pal, P.; Mazumder, B. Topical delivery of paclitaxel for treatment of skin cancer. Drug Dev. Ind. Pharm. 2016, 42, 1482–1494. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Neamatallah, T.; Alshehri, S.; Mujtaba, M.A.; Riadi, Y.; Radhakrishnan, A.K.; Khalilullah, H.; Gupta, M.; Akhter, M.H. Development, Characterization, and Evaluation of alpha-Mangostin-Loaded Polymeric Nanoparticle Gel for Topical Therapy in Skin Cancer. Gels 2021, 7, 230. [Google Scholar] [CrossRef]
- Aggarwal, G.; Dhawan, S.; Harikumar, S.L. Formulation, in vitro, and in vivo evaluation of matrix-type transdermal patches containing olanzapine. Pharm. Dev. Technol. 2013, 18, 916–925. [Google Scholar] [CrossRef]
- Sabir, F.; Qindeel, M.; Rehman, A.u.; Ahmad, N.M.; Khan, G.M.; Csoka, I.; Ahmed, N. An efficient approach for development and optimisation of curcumin-loaded solid lipid nanoparticles’ patch for transdermal delivery. J. Microencapsul. 2021, 38, 233–248. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Debeer, S.; Le Luduec, J.-B.; Kaiserlian, D.; Laurent, P.; Nicolas, J.-F.; Dubois, B.; Kanitakis, J. Comparative histology and immunohistochemistry of porcine versus human skin. Eur. J. Dermatol. 2013, 23, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A Mechanistic Study to Determine the Structural Similarities Between Artificial Membrane Strat-M™ and Biological Membranes and Its Application to Carry Out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of Formulation Parameters on Permeation of Ibuprofen from Topical Formulations Using Strat-M® Membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, F.; Snorradottir, B.S.; Gunnarsson, S.; Georgsdottir, E.; Sigurdsson, S. Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling. Pharmaceutics 2022, 14, 1880. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.B.; Petersson, K.; Nielsen, H.M. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur. J. Pharm. Biopharm. 2011, 79, 68–75. [Google Scholar] [CrossRef]
- Schafer, N.; Balwierz, R.; Biernat, P.; Ochędzan-Siodłak, W.; Lipok, J. Natural Ingredients of Transdermal Drug Delivery Systems as Permeation Enhancers of Active Substances through the Stratum Corneum. Mol. Pharm. 2023, 20, 3278–3297. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Yamane, M.A.; Williams, A.C.; Barry, B.W. Effects of terpenes and oleic acid as skin penetration enhancers towards 5-fluorouracil as assessed with time; permeation, partitioning and differential scanning calorimetry. Int. J. Pharm. 1995, 116, 237–251. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, R.B.; Singh, J. Effects of ionization and penetration enhancers on the transdermal delivery of 5-fluorouracil through excised human stratum corneum. Int. J. Pharm. 2005, 298, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel chemical permeation enhancers for transdermal drug delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. [Google Scholar] [CrossRef]
- Kovacik, A.; Kopecna, M.; Vavrova, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert. Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Shah, H.S.; Gotecha, A.; Jetha, D.; Rajput, A.; Bariya, A.; Panchal, S.; Butani, S. Gamma oryzanol niosomal gel for skin cancer: Formulation and optimization using quality by design (QbD) approach. AAPS Open 2021, 7, 9. [Google Scholar] [CrossRef]
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016, 33, 372–380. [Google Scholar] [CrossRef]
- Ugur Kaplan, A.B.; Cetin, M.; Orgul, D.; Taghizadehghalehjoughi, A.; Hacımuftuoglu, A.; Hekimoglu, S. Formulation and in vitro evaluation of topical nanoemulsion and nanoemulsion-based gels containing daidzein. J. Drug Dev. Sci. Techol. 2019, 52, 189–203. [Google Scholar] [CrossRef]
- Han, H.H.; Kim, S.-J.; Kim, J.; Park, W.; Kim, C.; Kim, H.; Hahn, S.K. Bimetallic Hyaluronate-Modified Au@Pt Nanoparticles for Noninvasive Photoacoustic Imaging and Photothermal Therapy of Skin Cancer. ACS Appl. Mater. Interfaces 2023, 15, 11609–11620. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Ahlin Grabnar, P.; Kristl, J. The manufacturing techniques of drug-loaded polymeric nanoparticles from preformed polymers. J. Microencapsul. 2011, 28, 323–335. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef] [PubMed]
- Borgheti-Cardoso, L.N.; Viegas, J.S.R.; Silvestrini, A.V.P.; Caron, A.L.; Praca, F.G.; Kravicz, M.; Bentley, M. Nanotechnology approaches in the current therapy of skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Ewert de Oliveira, B.; Junqueira Amorim, O.H.; Lima, L.L.; Rezende, R.A.; Mestnik, N.C.; Bagatin, E.; Leonardi, G.R. 5-Fluorouracil, innovative drug delivery systems to enhance bioavailability for topical use. J. Drug Dev. Sci. Techol. 2021, 61, 102155. [Google Scholar] [CrossRef]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: Development, in Vitro Characterization, and in Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol. Pharm. 2018, 15, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Imran, M.; Sheikh, A.; Tiwari, N.; Jaimini, A.; Kesharwani, P.; Jain, G.K.; Ahmad, F.J. Advanced multifunctional nano-lipid carrier loaded gel for targeted delivery of 5-flurouracil and cannabidiol against non-melanoma skin cancer. Environ. Res. 2023, 233, 116454. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, P. Chapter 15—In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 563–627. [Google Scholar]
- ICH. Stability Testing of New Drug Substances and Products Q1A (R2); ICH: Geneva, Switzerland, 2003. [Google Scholar]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef]
- European Medicine Agency (EMA). Guideline on Quality of Transdermal Patches; EMA/CHMP/QWP/608924/2014; European Medicine Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zuin, V.G.; Eilks, I.; Elschami, M.; Kümmerer, K. Education in green chemistry and in sustainable chemistry: Perspectives towards sustainability. Green Chem. 2021, 23, 1594–1608. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Mohamed, H.M.; Kurowska-Susdorf, A.; Dewani, R.; Fares, M.Y.; Andruch, V. Green analytical chemistry as an integral part of sustainable education development. Curr. Opin. Green Sustain. Chem. 2021, 31, 100508. [Google Scholar] [CrossRef]
- Blum, C.; Bunke, D.; Hungsberg, M.; Roelofs, E.; Joas, A.; Joas, R.; Blepp, M.; Stolzenberg, H.-C. The concept of sustainable chemistry: Key drivers for the transition towards sustainable development. Sustain. Chem. Pharm. 2017, 5, 94–104. [Google Scholar] [CrossRef]
- Ondo, A.L.; Mings, S.M.; Pestak, R.M.; Shanler, S.D. Topical combination therapy for cutaneous squamous cell carcinoma in situ with 5-fluorouracil cream and imiquimod cream in patients who have failed topical monotherapy. J. Am. Acad. Dermatol. 2006, 55, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Maurizii, G.; Moroni, S.; Khorshid, S.; Aluigi, A.; Tiboni, M.; Casettari, L. 3D-printed EVA-based patches manufactured by direct powder extrusion for personalized transdermal therapies. Int. J. Pharm. 2023, 635, 122720. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef] [PubMed]

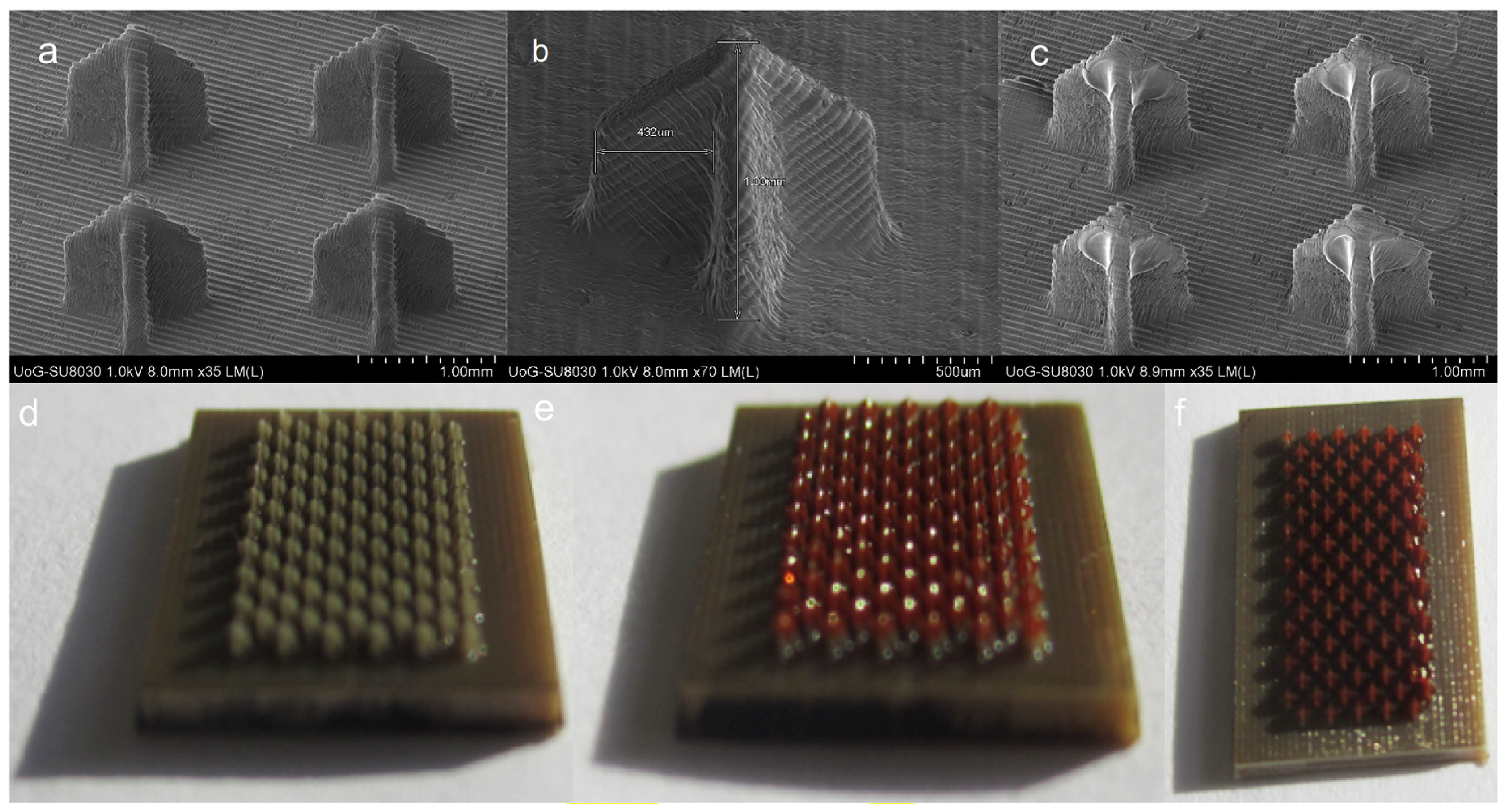
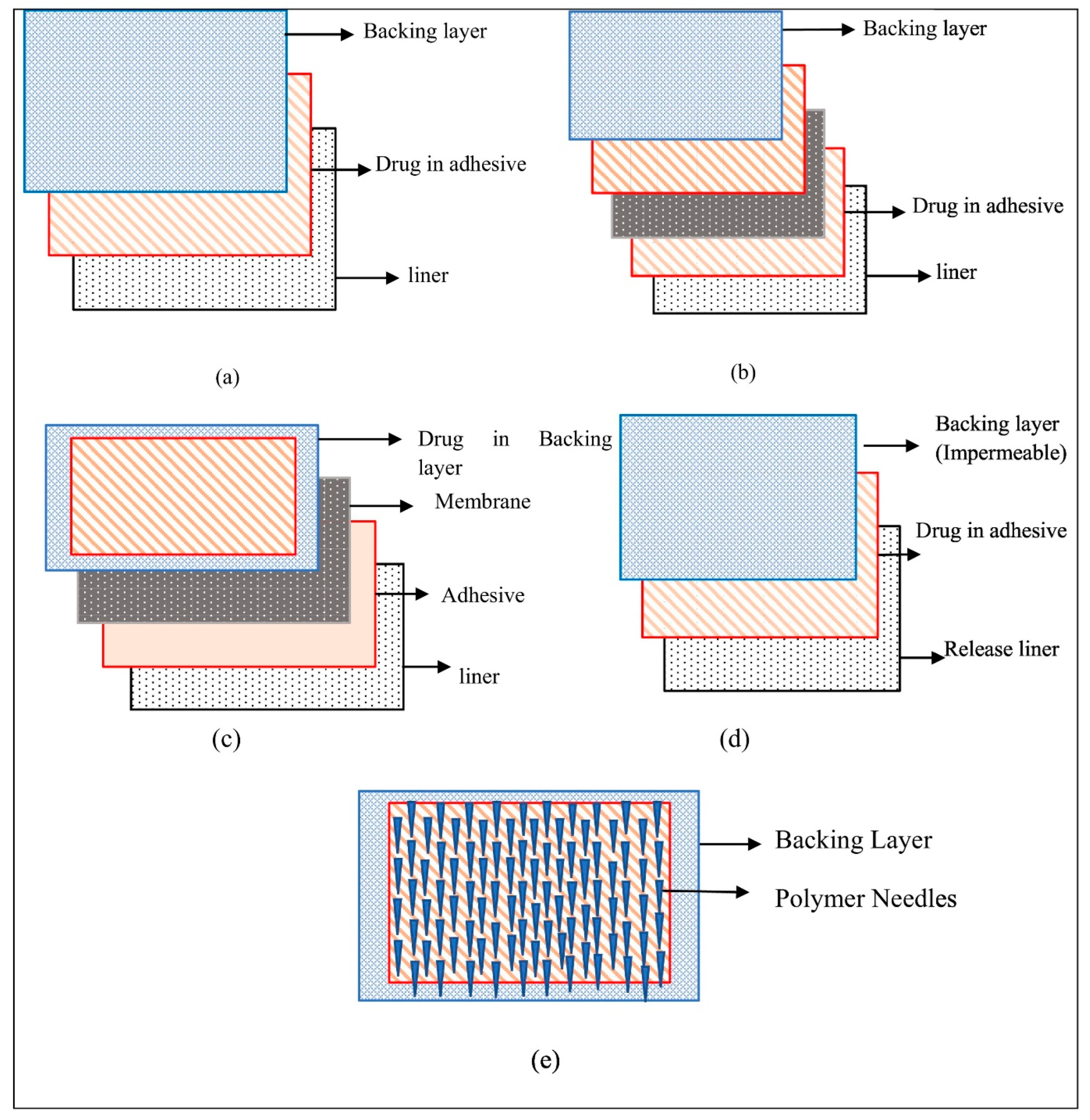


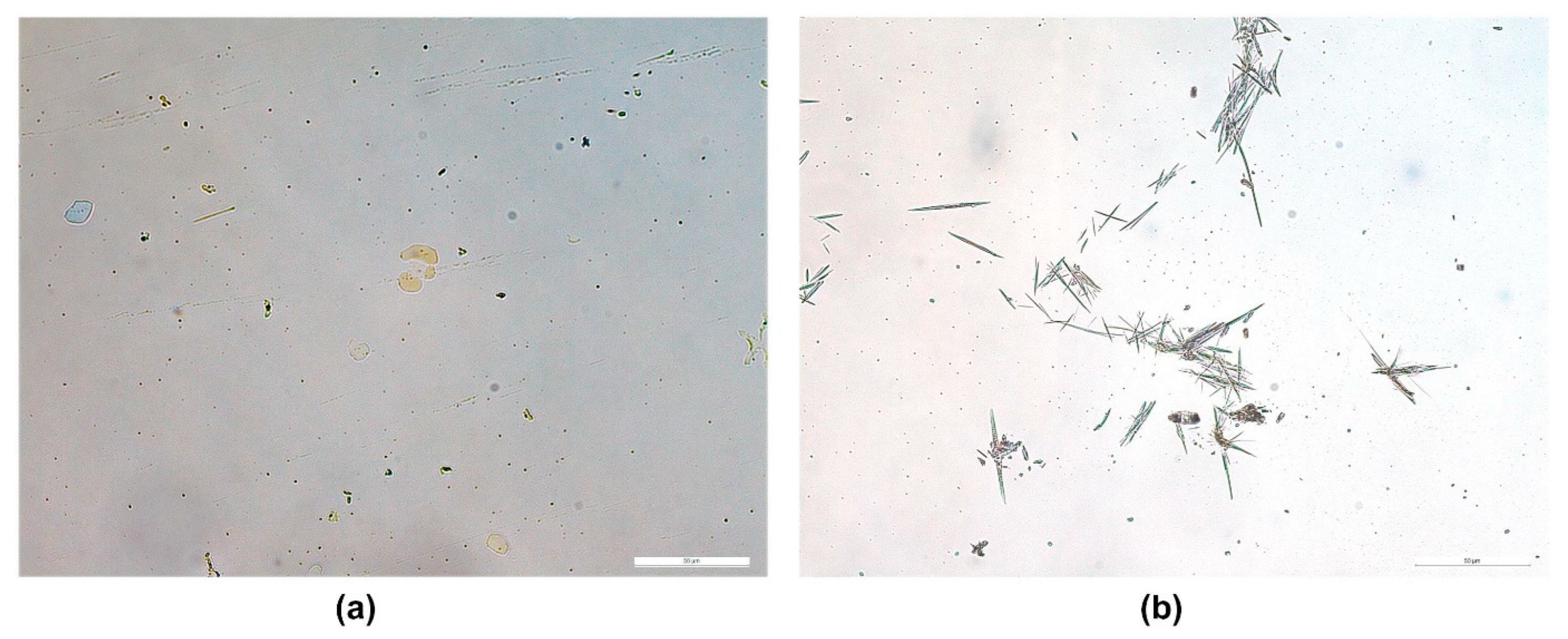
| Therapeutic Agent | Materials Used for Microneedle Preparation | Incorporated Nanosystem | Skin Cancer Model | Key Findings | Ref. |
|---|---|---|---|---|---|
| 5-Aminolevulinic acid (5-ALA) | Stainless steel | N/A | A20 tumour-bearing Balb/cA nude mice | Stainless steel microneedles coated with 5-ALA achieved much deeper skin penetration (~480 µm) compared to its topical cream counterpart (~150 µm). The microneedles significantly reduced subcutaneous tumour growth by about 57%. Conversely, the topical cream containing 5-ALA (5 mg) failed to suppress the tumour volume. | [60] |
| Gold | Poly(L-lactide) | PEGylated Gold Nanorod | Female A431 tumour-bearing Balb/cA nude mice | The GNR-PEG@MN demonstrated excellent skin penetration capabilities with a height of 480 μm whilst achieving effective heat transfer in vivo, with the tumour sites reaching 50 °C within 5 min. The combination of low-dose MPEG-PDLLA-DTX micelles and GNR-PEG@MNs eliminated the A431 tumour in vivo with no recurrence. | [61] |
| 5-FU Indocyanine green (ICG) | Hyaluronic acid | Monomethoxy-poly (ethylene glycol)-polycaprolactone nanoparticle | A431 tumour-bearing Balb/cA nude mice | 5-Fu-ICGMPEG-PCL@HA MN demonstrated a good skin penetration of 600 µm with a rapid heating transfer efficacy to 60 °C in 5 min upon 808 nm near-infrared laser. The microneedles also demonstrated tumour inhibition capability without recurrence. | [17] |
| Cisplatin | Biocompatible photopolymer resin | N/A | A431 human squamous carcinoma xenografts in Balb/c nude mice | The microneedles were fabricated using biocompatible photopolymer resin via stereolithography 3D printing. Cisplatin was coated on the needle surface via inkjet printing. 3D-printed microneedles had good skin penetration, achieving 80% penetration depth (737.7 ± 63.7 µm). Rapid cisplatin release rates (80–90%) were observed in the first 1 h. In vivo evaluation demonstrated that the cisplatin permeated sufficiently, exhibiting high anticancer activity and resulting in 100% tumour regression. | [62] |
| IMQ | Polyvinylpyrrolidone and vinyl acetate (PVPVA); Polyethylene glycol 400 | N/A | Microneedles loaded with IMQ, utilising a polyvinylpyrrolidone-co-vinyl acetate polymer achieved a penetration depth of 426 ± 72 µm. Despite the microneedle containing an IMQ load six times lower than the clinical dose of Aldara®, it achieved a similar level of IMQ intradermal delivery. | [63] |
| Patch Type | Therapeutic Agent | Materials Used for Patch Preparation | Incorporated Nanosystem | Key Findings | Ref. |
|---|---|---|---|---|---|
| Matrix (referred to as polymeric by the authors) | IMQ | PMVE/MA, tripropyleneglycol methyl ether | N/A | Polymeric patches containing IMQ of 4.75, 9.50, and 12.50 mg cm−2 were developed. The patches released significantly more drug through a Cuprophan® dialysis membrane than the commercial cream, Aldara® over 6 h. | [18] |
| Matrix (referred to as polymeric by the authors) | Gold | Gantrez® S-97 (copolymer of methyl vinyl ether and maleic acid, Mw = 1,200,000 | Functionalised gold nanorods with thiolated poly(ethylene) glycol | Polymeric film containing gold nanorods (GnRs) for use in local hyperthermia applications was developed. The GnR-loaded films were able to heat the skin model over 40 °C, demonstrating its potential for non-invasive local hyperthermia applications against NMSC. | [68] |
| Drug-in-adhesive (DIA) | 5-FU | Dimethylaminoethyl methacrylate, butyl methacrylate and methyl methacrylate (2:1:1) (Eudragit® E) | N/A | DIA patches containing 5-FU were developed for the first time using Eudragit® E as an adhesive polymer matrix. The patches containing 40% (relative to the polymer ratio) triethyl citrate, dibutyl sebacate, or triacetin as a plasticiser achieved adhesive properties similar to several marketed patches. A controlled release of 5-FU was achieved, suggesting its potential application in skin cancer treatment. | [51] |
| DIA | 5-FU and IMQ | Eudragit® E | N/A | DIA patches using Eudragit® E and triacetin were developed containing both 5-FU and IMQ. The in vitro release rate of 5-FU was quicker than that of IMQ, with about 75% of the drug content released within the initial 50 min and 120 min, respectively. | [69] |
| Matrix | IMQ | hydroxypropyl methylcellulose (HPMC) K4M, propylene glycol | Nanostructured lipid carriers (NLCs) | NLCs containing IMQ were developed by Design of Experiments. The optimised formulation was then incorporated into a matrix-type topical patch consisting of hydroxypropyl methylcellulose (HPMC) K4M and propylene glycol. The ex vivo deposition study demonstrated that the IMQ-NLCs patch significantly increased IMQ deposition in the deeper skin layers than Aldara® cream. | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Day, C.M.; Song, Y.; Holmes, A.; Garg, S. Innovative Topical Patches for Non-Melanoma Skin Cancer: Current Challenges and Key Formulation Considerations. Pharmaceutics 2023, 15, 2577. https://doi.org/10.3390/pharmaceutics15112577
Kim S, Day CM, Song Y, Holmes A, Garg S. Innovative Topical Patches for Non-Melanoma Skin Cancer: Current Challenges and Key Formulation Considerations. Pharmaceutics. 2023; 15(11):2577. https://doi.org/10.3390/pharmaceutics15112577
Chicago/Turabian StyleKim, Sangseo, Candace M. Day, Yunmei Song, Amy Holmes, and Sanjay Garg. 2023. "Innovative Topical Patches for Non-Melanoma Skin Cancer: Current Challenges and Key Formulation Considerations" Pharmaceutics 15, no. 11: 2577. https://doi.org/10.3390/pharmaceutics15112577