A Physiologically Based Pharmacokinetic Approach to Recommend an Individual Dose of Tacrolimus in Adult Heart Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Collection
2.3. Sample Collection and Analysis
2.4. Population PK Analysis
2.5. Model Development
2.6. Model Evaluation
2.7. Evaluation of the Impact of Potential Covariates
2.8. Dosage Recommendations
2.9. Statistical Methods
3. Results
3.1. Population PK Study
3.2. Model Development
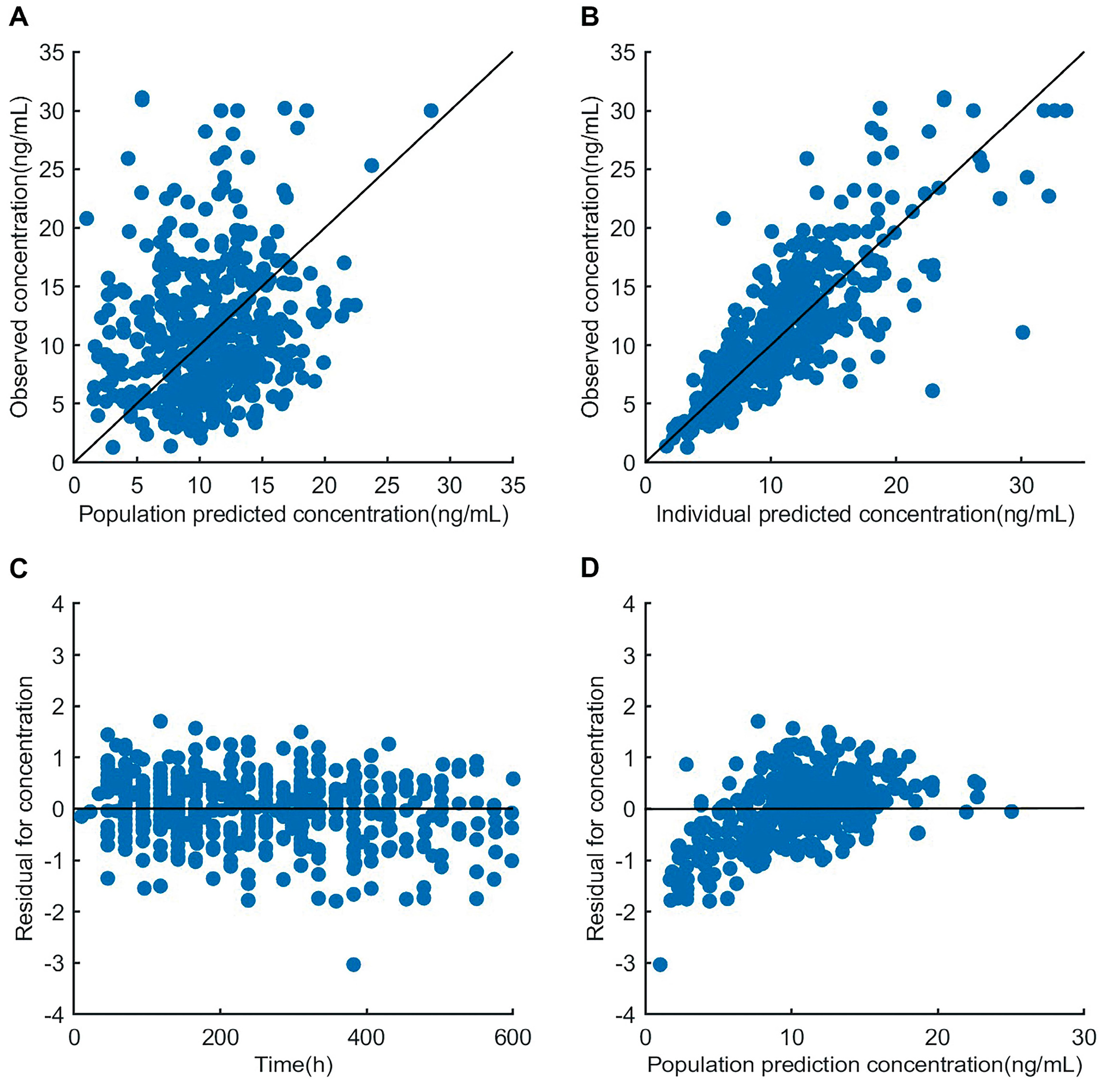
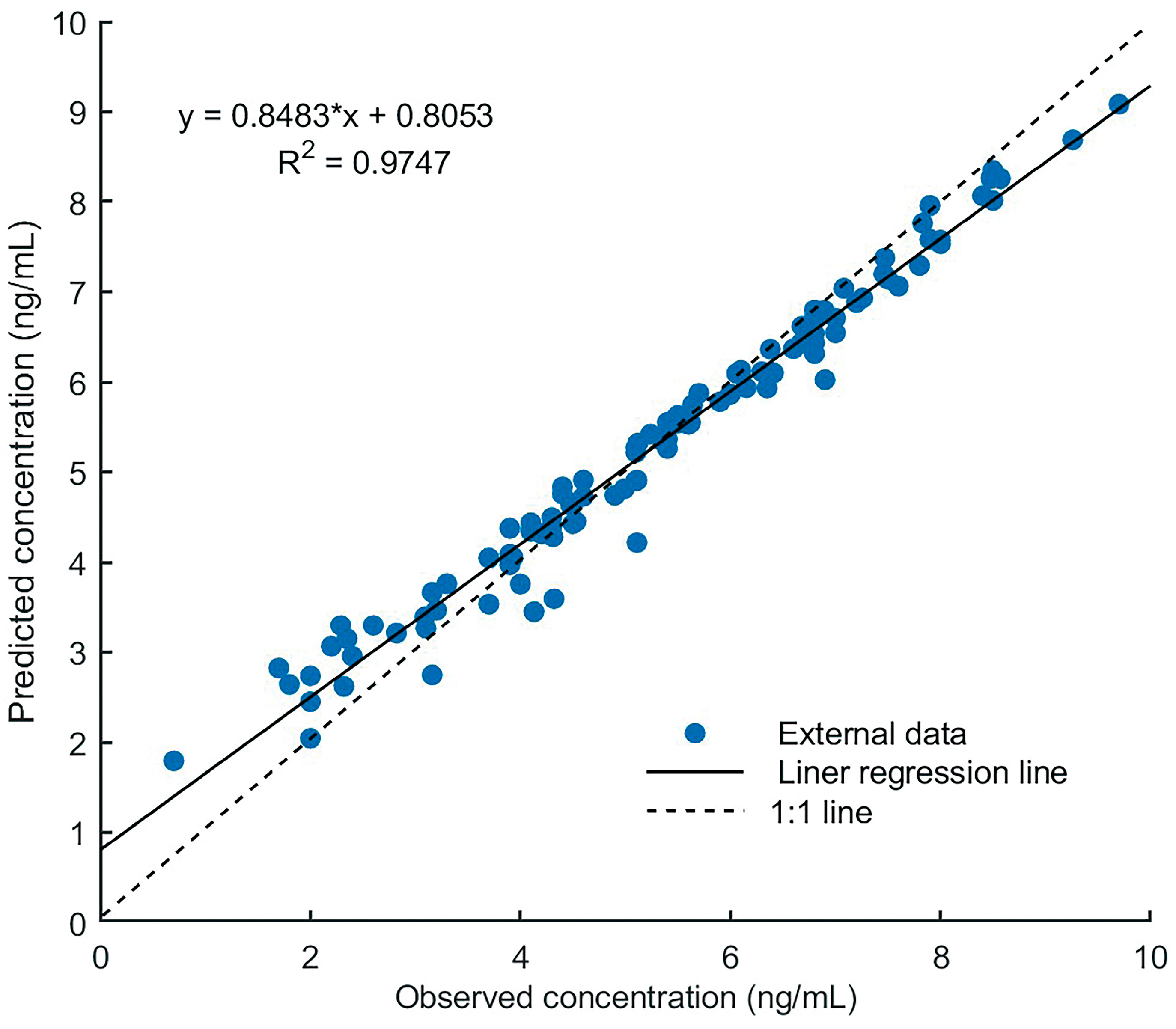
3.3. Model Evaluation
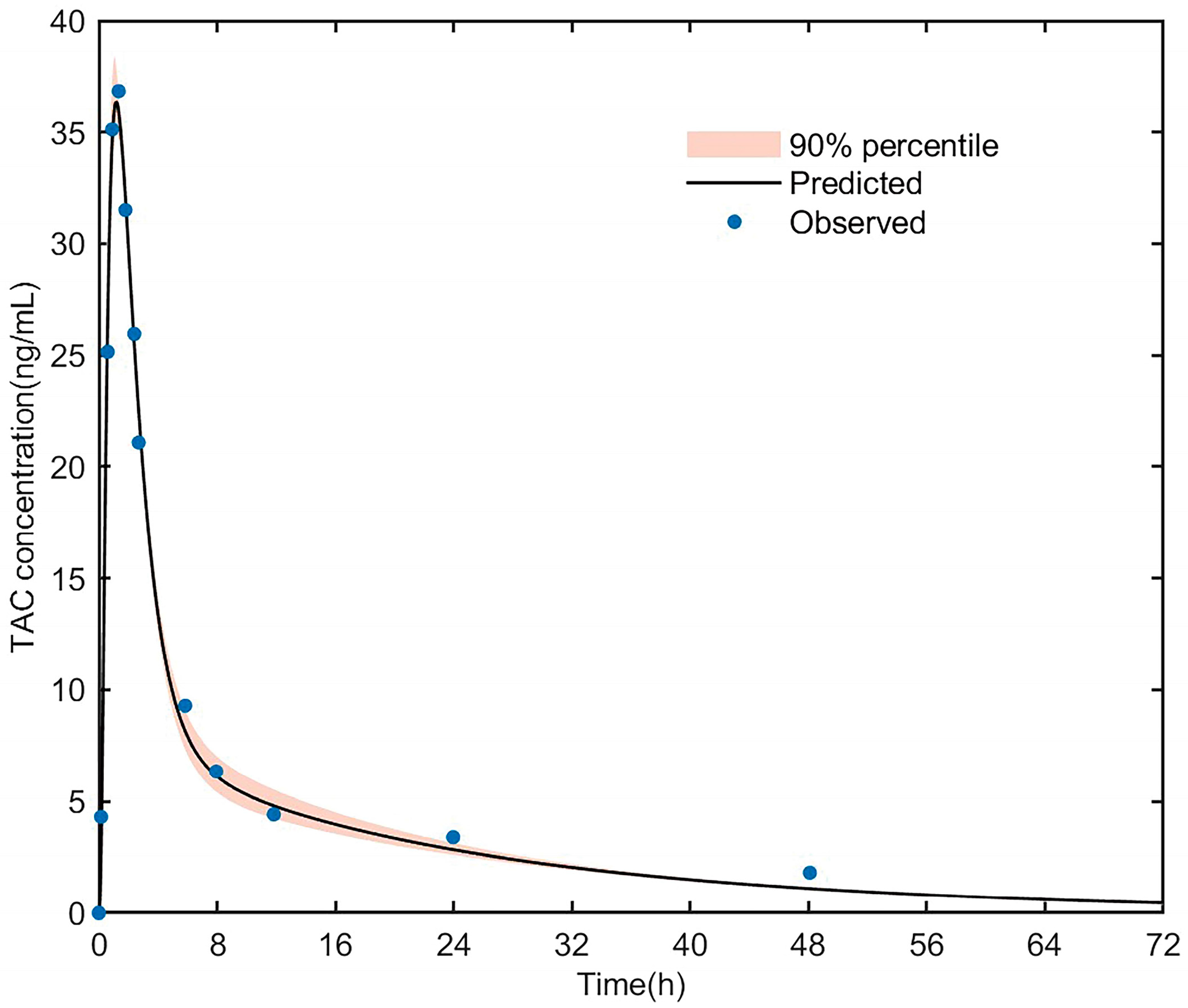
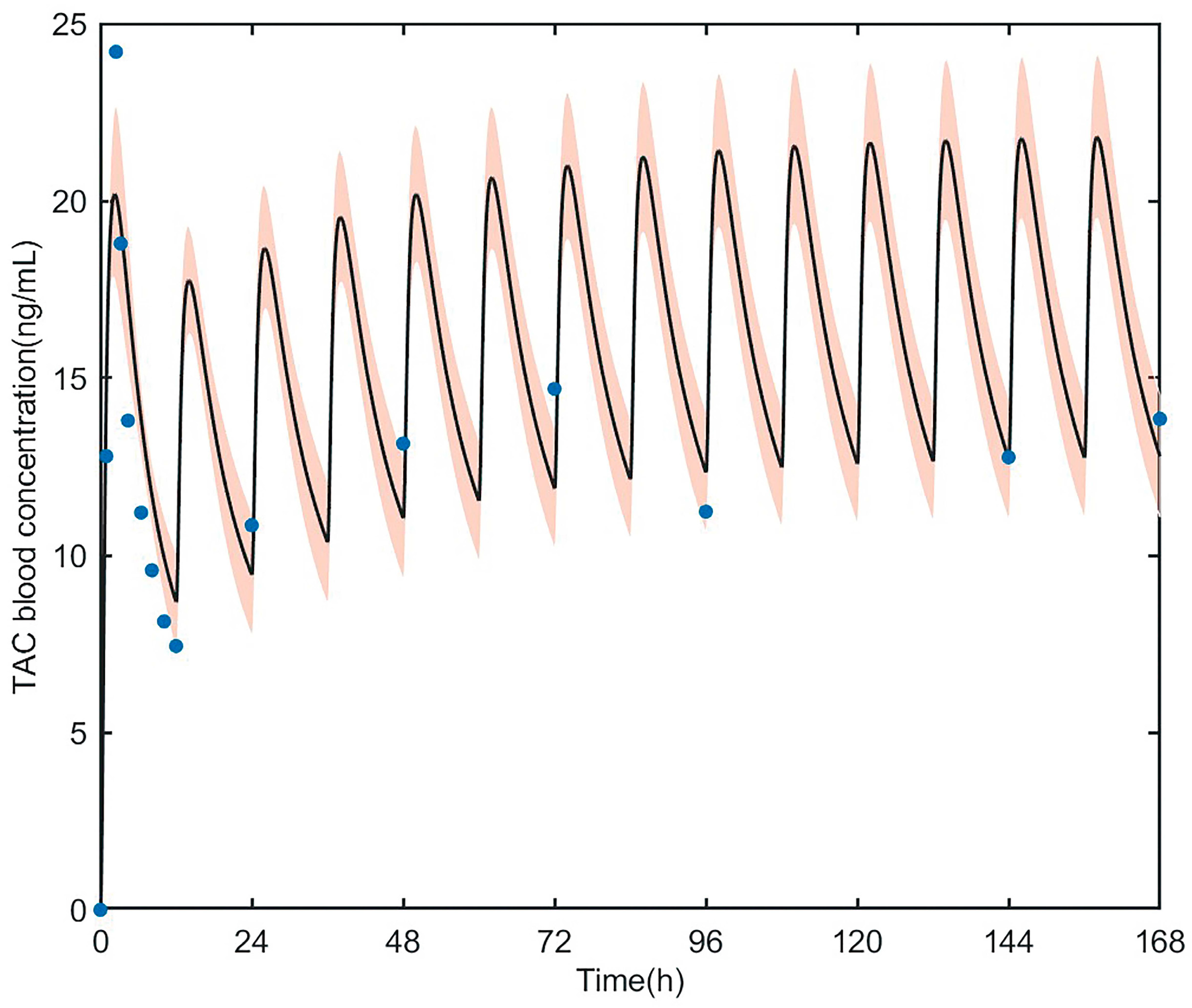
3.3.1. PK Simulation of Tacrolimus in Healthy Adult
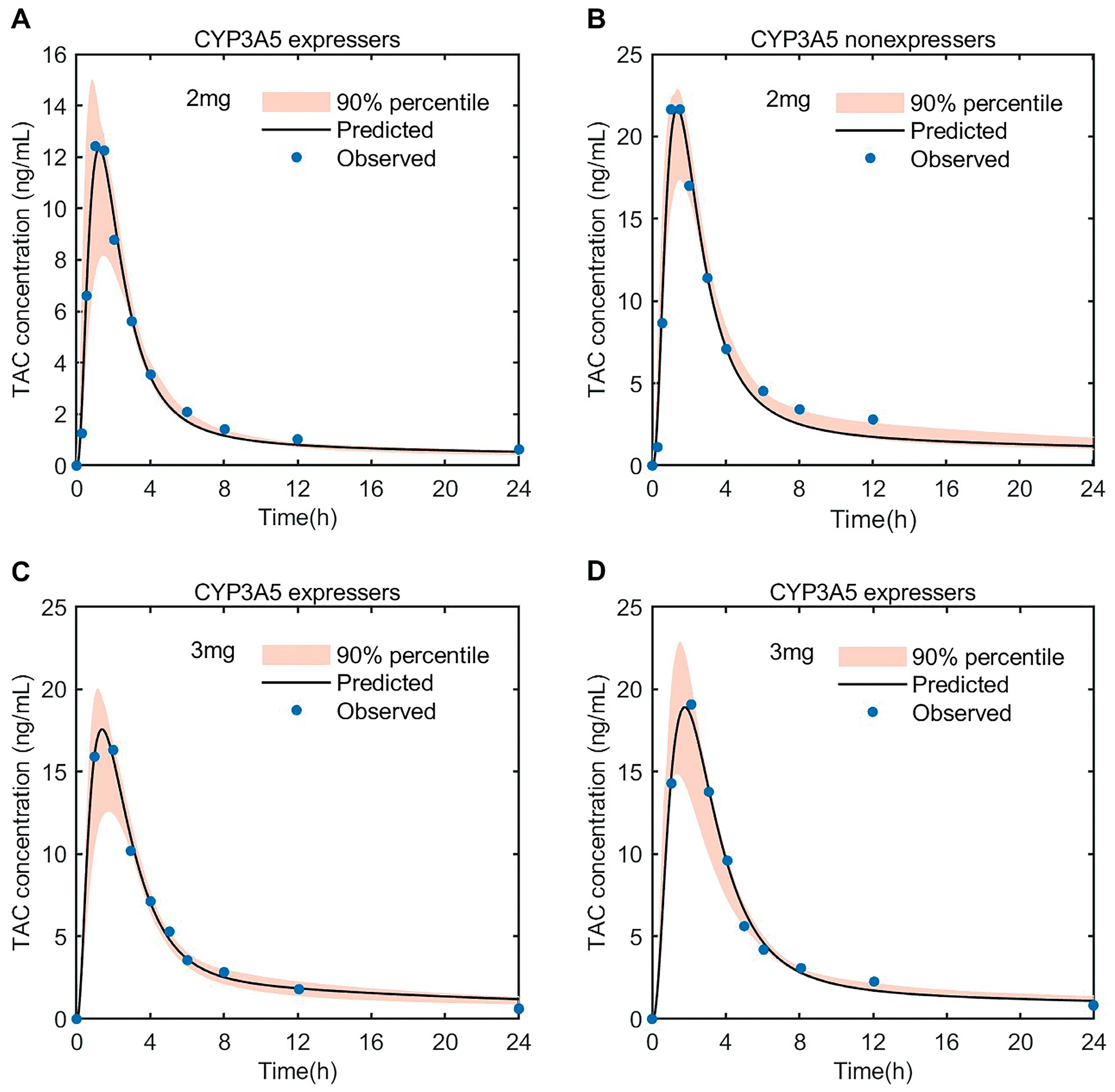
3.3.2. PK Simulation of Tacrolimus in Adult Heart Transplant Patients
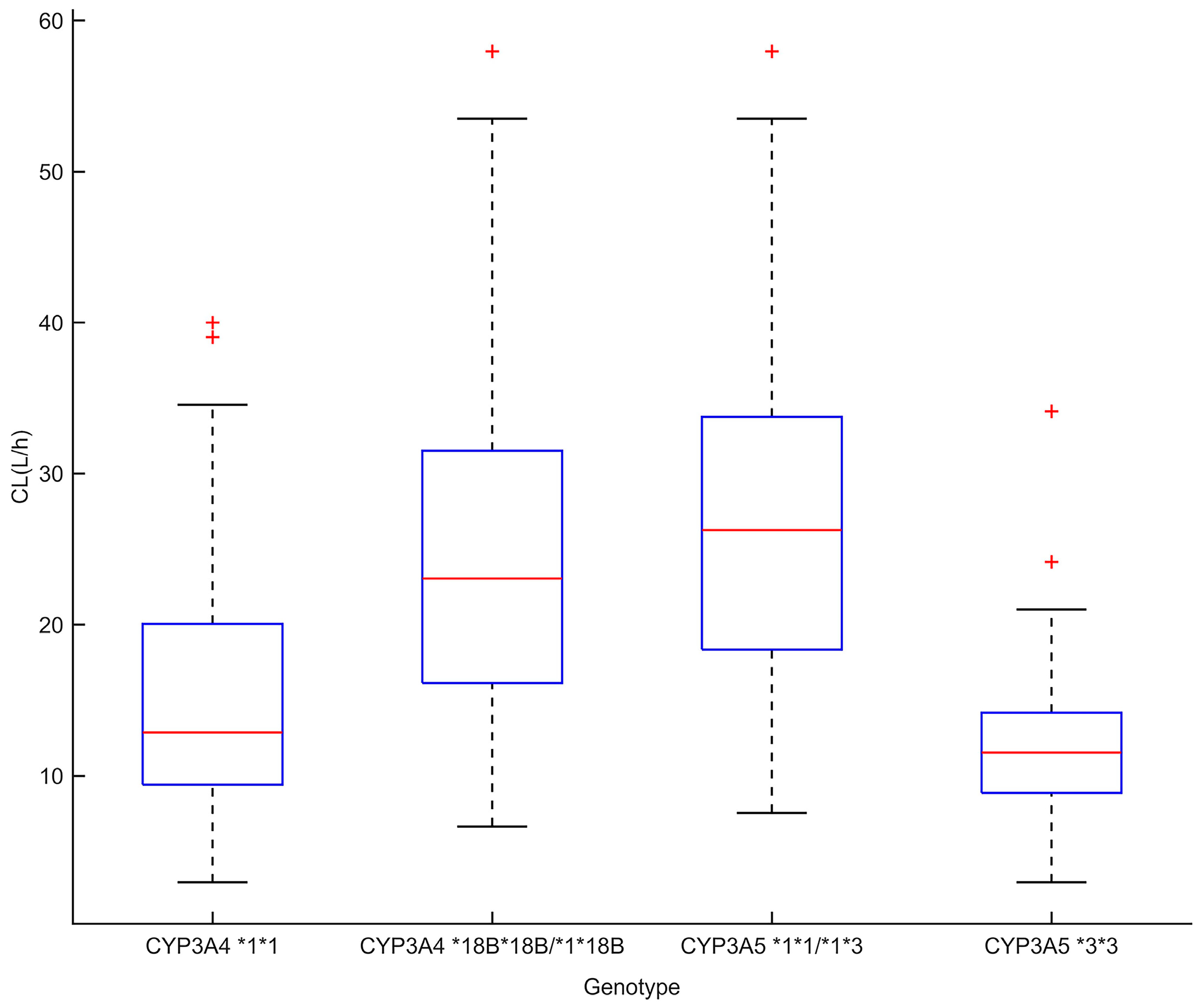
3.4. Impact of Covariates on Tacrolimus PKs
3.5. Proposal of Initial Dosing Regimen of Tacrolimus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Gregori, S.; De Silvestri, A.; Cattadori, B.; Rapagnani, A.; Albertini, R.; Novello, E.; Concardi, M.; Arbustini, E.; Pellegrini, C. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy in Heart Transplantation: New Strategies and Preliminary Results in Endomyocardial Biopsies. Pharmaceutics 2022, 14, 1247. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Schutte-Nutgen, K.; Tholking, G.; Suwelack, B.; Reuter, S. Tacrolimus—Pharmacokinetic Considerations for Clinicians. Curr. Drug Metab. 2018, 19, 342–350. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Modigraf. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/modigraf-epar-product-information_en.pdf (accessed on 26 May 2023).
- U.S. Food and Drug Administration. Prograf. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/050708s053,050709s045,210115s005lbl.pdf (accessed on 26 May 2023).
- Cui, Y.-F.; Pan, Y.; Zhu, M.-F.; Jiao, Z. Pharmacokinetic Evaluation of Tacrolimus in Chinese Adult Patients during the Early Stages Post-Lung Transplantation. J. Pers. Med. 2023, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Sikma, M.A.; Hunault, C.C.; Huitema, A.D.R.; De Lange, D.W.; Van Maarseveen, E.M. Clinical Pharmacokinetics and Impact of Hematocrit on Monitoring and Dosing of Tacrolimus Early After Heart and Lung Transplantation. Clin. Pharmacokinet. 2020, 59, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Deininger, K.M.; Vu, A.; Page, R.L.; Ambardekar, A.V.; Lindenfeld, J.; Aquilante, C.L. CYP3A Pharmacogenetics and Tacrolimus Disposition in Adult Heart Transplant Recipients. Clin. Transplant. 2016, 30, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Patel, T.; Miller, J.A.; Torrice, C.D.; Aggarwal, M.; Sketch, M.R.; Alexander, M.D.; Armistead, P.M.; Coghill, J.M.; Grgic, T.; et al. Influence of Germline Genetics on Tacrolimus Pharmacokinetics and Pharmacodynamics in Allogeneic Hematopoietic Stem Cell Transplant Patients. Int. J. Mol. Sci. 2020, 21, 858. [Google Scholar] [CrossRef]
- Birdwell, K.; Decker, B.; Barbarino, J.; Peterson, J.; Stein, C.; Sadee, W.; Wang, D.; Vinks, A.; He, Y.; Swen, J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef]
- Jeong, H.; Chiou, W.L. Role of P-Glycoprotein in the Hepatic Metabolism of Tacrolimus. Xenobiotica Fate Foreign Compd. Biol. Syst. 2006, 36, 1–13. [Google Scholar] [CrossRef]
- Liu, S.; Chen, R.-X.; Li, J.; Zhang, Y.; Wang, X.-D.; Fu, Q.; Chen, L.-Y.; Liu, X.-M.; Huang, H.-B.; Huang, M.; et al. The POR Rs1057868-Rs2868177 GC-GT Diplotype Is Associated with High Tacrolimus Concentrations in Early Post-Renal Transplant Recipients. Acta Pharmacol. Sin. 2016, 37, 1251–1258. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, X.; Zhang, L.; Tang, L.; Fang, Y.; Chen, H.; Zhang, L.; Shen, A. The Impact of IL-10 and CYP3A5 Gene Polymorphisms on Dose-Adjusted Trough Blood Tacrolimus Concentrations in Early Post-Renal Transplant Recipients. Pharmacol. Rep. PR 2021, 73, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wei, F.; Liu, H.; Wang, X.; Wang, W.; Ouyang, Y.; Liu, J.; Chen, D.; Zang, Y. Association of the IL-6 Rs1800796 SNP with Concentration/Dose Ratios of Tacrolimus and Donor Liver Function after Transplantation. Immunol. Investig. 2021, 50, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Lu, C. PBPK Modeling and Simulation in Drug Research and Development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Rowland-Yeo, K. Basic Concepts in Physiologically Based Pharmacokinetic Modeling in Drug Discovery and Development. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, C.; Li, R.; Wang, B.; Li, J.; Niu, W.; Zhang, L.; Zhong, M.; Wang, Z.; Qiu, X. Individual Dose Recommendations for Drug Interaction Between Tacrolimus and Voriconazole in Adult Liver Transplant Recipients. Eur. J. Pharm. Sci. 2023, 184, 106405. [Google Scholar] [CrossRef] [PubMed]
- Itohara, K.; Yano, I.; Tsuzuki, T.; Uesugi, M.; Nakagawa, S.; Yonezawa, A.; Okajima, H.; Kaido, T.; Uemoto, S.; Matsubara, K. A Minimal Physiologically-Based Pharmacokinetic Model for Tacrolimus in Living-Donor Liver Transplantation: Perspectives Related to Liver Regeneration and the Cytochrome P450 3A5 (CYP3A5) Genotype. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Paschier, A.; Destere, A.; Monchaud, C.; Labriffe, M.; Marquet, P.; Woillard, J. Tacrolimus Population Pharmacokinetics in Adult Heart Transplant Patients. Br. J. Clin. Pharmacol. 2023, bcp.15857. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, J.; Lin, X.; Qiu, H.; Zhang, J. Population Pharmacokinetic Analysis for Model-Based Therapeutic Drug Monitoring of Tacrolimus in Chinese Han Heart Transplant Patients. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 89–100. [Google Scholar] [CrossRef]
- Déri, M.; Szakál-Tóth, Z.; Fekete, F.; Mangó, K.; Incze, E.; Minus, A.; Merkely, B.; Sax, B.; Monostory, K. CYP3A-Status Is Associated with Blood Concentration and Dose-Requirement of Tacrolimus in Heart Transplant Recipients. Sci. Rep. 2021, 11, 21389. [Google Scholar] [CrossRef]
- Emoto, C.; Johnson, T.N.; Hahn, D.; Christians, U.; Alloway, R.R.; Vinks, A.A.; Fukuda, T. A Theoretical Physiologically-Based Pharmacokinetic Approach to Ascertain Covariates Explaining the Large Interpatient Variability in Tacrolimus Disposition. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 273–284. [Google Scholar] [CrossRef]
- Kirubakaran, R.; Stocker, S.L.; Hennig, S.; Day, R.O.; Carland, J.E. Population Pharmacokinetic Models of Tacrolimus in Adult Transplant Recipients: A Systematic Review. Clin. Pharmacokinet. 2020, 59, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G. PKQuest: A General Physiologically Based Pharmacokinetic Model. Introduction and Application to Propranolol. BMC Clin. Pharmacol. 2002, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G.; Schnider, T.W. Human Physiologically Based Pharmacokinetic Model for Propofol. BMC Anesthesiol. 2005, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Stocco, J.; Hulin, A.; Blanchet, B.; Verstuyft, C.; Durand, F.; Conti, F.; Duvoux, C.; Tod, M. Determination of the Most Influential Sources of Variability in Tacrolimus Trough Blood Concentrations in Adult Liver Transplant Recipients: A Bottom-Up Approach. AAPS J. 2014, 16, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Espié, P.; Tytgat, D.; Sargentini-Maier, M.-L.; Poggesi, I.; Watelet, J.-B. Physiologically Based Pharmacokinetics (PBPK). Drug Metab. Rev. 2009, 41, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, T.; Rowland, M. Physiologically Based Pharmacokinetic Modelling 2: Predicting the Tissue Distribution of Acids, Very Weak Bases, Neutrals and Zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef] [PubMed]
- El-Khateeb, E.; Chinnadurai, R.; Al Qassabi, J.; Scotcher, D.; Darwich, A.S.; Kalra, P.A.; Rostami-Hodjegan, A. Using Prior Knowledge on Systems Through PBPK to Gain Further Insight into Routine Clinical Data on Trough Concentrations: The Case of Tacrolimus in Chronic Kidney Disease. Ther. Drug Monit. 2023; publish ahead of print. [Google Scholar] [CrossRef]
- Lu, X.-F.; Zhan, J.; Zhou, Y.; Bi, K.-S.; Chen, X.-H. Use of a Semi-Physiological Pharmacokinetic Model to Investigate the Influence of Itraconazole on Tacrolimus Absorption, Distribution and Metabolism in Mice. Xenobiotica Fate Foreign Compd. Biol. Syst. 2017, 47, 752–762. [Google Scholar] [CrossRef]
- Wilkinson, G.R.; Shand, D.G. Commentary: A Physiological Approach to Hepatic Drug Clearance. Clin. Pharmacol. Ther. 1975, 18, 377–390. [Google Scholar] [CrossRef]
- Gertz, M.; Houston, J.B.; Galetin, A. Physiologically Based Pharmacokinetic Modeling of Intestinal First-Pass Metabolism of CYP3A Substrates with High Intestinal Extraction. Drug Metab. Dispos. Biol. Fate Chem. 2011, 39, 1633–1642. [Google Scholar] [CrossRef]
- Staatz, C.E.; Tett, S.E. Clinical Pharmacokinetics and Pharmacodynamics of Tacrolimus in Solid Organ Transplantation. Clin. Pharmacokinet. 2004, 43, 623–653. [Google Scholar] [CrossRef]
- Tod, M.; Goutelle, S.; Bleyzac, N.; Bourguignon, L. A Generic Model for Quantitative Prediction of Interactions Mediated by Efflux Transporters and Cytochromes: Application to P-Glycoprotein and Cytochrome 3A4. Clin. Pharmacokinet. 2019, 58, 503–523. [Google Scholar] [CrossRef] [PubMed]
- Hooker, A.C.; Staatz, C.E.; Karlsson, M.O. Conditional Weighted Residuals (CWRES): A Model Diagnostic for the FOCE Method. Pharm. Res. 2007, 24, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Song, Y.-K.; Choi, J.-S.; Ji, H.Y.; Yang, E.; Park, J.S.; Kim, H.S.; Kim, M.-J.; Cho, I.-K.; Chung, S.-J.; et al. Physiologically Based Pharmacokinetic Modelling to Predict Pharmacokinetics of Enavogliflozin, a Sodium-Dependent Glucose Transporter 2 Inhibitor, in Humans. Pharmaceutics 2023, 15, 942. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, L.M.; Frassetto, L.; Floren, L.C.; Dressler, D.; Carrier, S.; Bekersky, I.; Benet, L.Z.; Christians, U. The Pharmacokinetics and Metabolic Disposition of Tacrolimus: A Comparison across Ethnic Groups. Clin. Pharmacol. Ther. 2001, 69, 24–31. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Bu, F.; Zhang, H.; Wang, Q.; Tang, Z.; Yuan, J.; Lin, H.-S.; Xiang, X. Investigation of the Impact of CYP3A5 Polymorphism on Drug-Drug Interaction between Tacrolimus and Schisantherin A/Schisandrin A Based on Physiologically-Based Pharmacokinetic Modeling. Pharmaceuticals 2021, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Imamura, C.K.; Furihata, K.; Okamoto, S.; Tanigawara, Y. Impact of Cytochrome P450 2C19 Polymorphisms on the Pharmacokinetics of Tacrolimus When Coadministered with Voriconazole. J. Clin. Pharmacol. 2016, 56, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Regazzi, M.B.; Rinaldi, M.; Molinaro, M.; Pellegrini, C.; Calvi, M.; Arbustini, E.; Bellotti, E.; Bascapè, V.; Martinelli, L.; Viganò, M. Clinical Pharmacokinetics of Tacrolimus in Heart Transplant Recipients. Ther. Drug Monit. 1999, 21, 2–7. [Google Scholar] [CrossRef]
- Nakagita, K.; Wada, K.; Terada, Y.; Matsuda, S.; Terakawa, N.; Oita, A.; Takada, M. Effect of Fluconazole on the Pharmacokinetics of Everolimus and Tacrolimus in a Heart Transplant Recipient. Int. J. Clin. Pharmacol. Ther. 2018, 56, 270–276. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, M.; Sun, Y.; Li, J.; Lu, Y.; Li, X. Population Pharmacokinetic Analysis of Tacrolimus in Chinese Cardiac Transplant Recipients. Eur. J. Hosp. Pharm. 2020, 27, e12–e18. [Google Scholar] [CrossRef]
- Jusko, W.J.; Piekoszewski, W.; Klintmalm, G.B.; Shaefer, M.S.; Hebert, M.F.; Piergies, A.A.; Lee, C.C.; Schechter, P.; Mekki, Q.A. Pharmacokinetics of Tacrolimus in Liver Transplant Patients. Clin. Pharmacol. Ther. 1995, 57, 281–290. [Google Scholar] [CrossRef]
- Qu, J.; Bian, R.; Liu, B.; Chen, J.; Zhai, J.; Teng, F.; Guo, W.; Wei, H. The Pharmacokinetic Study of Tacrolimus and Wuzhi Capsule in Chinese Liver Transplant Patients. Front. Pharmacol. 2022, 13, 956166. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Yamano, K.; Otsuka, Y. In Vitro-in Vivo Extrapolations to Evaluate the Effect of Concomitant Drugs on Tacrolimus (FK506) Exposure. Biopharm. Drug Dispos. 2015, 36, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, H.; Cai, J.; Huang, J.; Zhang, J.; Shi, S.-J.; Liu, Y.-N.; Zhang, Y. Prediction of Tacrolimus Dosage in the Early Period after Heart Transplantation: A Population Pharmacokinetic Approach. Pharmacogenomics 2019, 20, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Tornatore, K.M.; Meaney, C.J.; Attwood, K.; Brazeau, D.A.; Wilding, G.E.; Consiglio, J.D.; Gundroo, A.; Chang, S.S.; Gray, V.; Cooper, L.M.; et al. Race and Sex Associations with Tacrolimus Pharmacokinetics in Stable Kidney Transplant Recipients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2022, 42, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.C.; Hall, S.D.; Becker, P.; Affrime, M.B.; Cutler, D.L.; Haehner-Daniels, B. In Vivo Effects of Interleukin-10 on Human Cytochrome P450 Activity. Clin. Pharmacol. Ther. 2000, 67, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, J.; Fan, J.; Zhang, T.; Li, Y.; Xie, B.; Zhang, W.; Lin, S.; Ye, L.; Liu, Y.; et al. Influence of IL-18 and IL-10 Polymorphisms on Tacrolimus Elimination in Chinese Lung Transplant Patients. Dis. Markers 2017, 2017, 7834035. [Google Scholar] [CrossRef] [PubMed]
- Bogacz, A.; Polaszewska, A.; Bartkowiak-Wieczorek, J.; Tejchman, K.; Dziewanowski, K.; Ostrowski, M.; Czerny, B.; Grześkowiak, E.; Sieńko, M.; Machaliński, B.; et al. The Effect of Genetic Variations for Interleukin-10 (IL-10) on the Efficacy of Immunosuppressive Therapy in Patients after Kidney Transplantation. Int. Immunopharmacol. 2020, 89, 107059. [Google Scholar] [CrossRef]
- Khaleel, B.; Yousef, A.-M.; Al-Zoubi, M.S.; Al-Ulemat, M.; Masadeh, A.A.; Abuhaliema, A.; Al-Batayneh, K.M.; Al-Trad, B. Impact of Genetic Polymorphisms at the Promoter Area of IL-10 Gene on Tacrolimus Level in Jordanian Renal Transplantation Recipients. J. Med. Biochem. 2022, 41, 327–334. [Google Scholar] [CrossRef]
- Lesche, D.; Sigurdardottir, V.; Setoud, R.; Oberhänsli, M.; Carrel, T.; Fiedler, G.M.; Largiadèr, C.R.; Mohacsi, P.; Sistonen, J. CYP3A5*3 and POR*28 Genetic Variants Influence the Required Dose of Tacrolimus in Heart Transplant Recipients. Ther. Drug Monit. 2014, 36, 710–715. [Google Scholar] [CrossRef]
- Op den Buijsch, R.A.M.; Christiaans, M.H.L.; Stolk, L.M.L.; de Vries, J.E.; Cheung, C.Y.; Undre, N.A.; van Hooff, J.P.; van Dieijen-Visser, M.P.; Bekers, O. Tacrolimus Pharmacokinetics and Pharmacogenetics: Influence of Adenosine Triphosphate-Binding Cassette B1 (ABCB1) and Cytochrome (CYP) 3A Polymorphisms. Fundam. Clin. Pharmacol. 2007, 21, 427–435. [Google Scholar] [CrossRef]
- Hesselink, D.A.; van Schaik, R.H.N.; van der Heiden, I.P.; van der Werf, M.; Gregoor, P.J.H.S.; Lindemans, J.; Weimar, W.; van Gelder, T. Genetic Polymorphisms of the CYP3A4, CYP3A5, and MDR-1 Genes and Pharmacokinetics of the Calcineurin Inhibitors Cyclosporine and Tacrolimus. Clin. Pharmacol. Ther. 2003, 74, 245–254. [Google Scholar] [CrossRef]
- Shi, X.-J.; Geng, F.; Jiao, Z.; Cui, X.-Y.; Qiu, X.-Y.; Zhong, M.-K. Association of ABCB1, CYP3A4*18B and CYP3A5*3 Genotypes with the Pharmacokinetics of Tacrolimus in Healthy Chinese Subjects: A Population Pharmacokinetic Analysis. J. Clin. Pharm. Ther. 2011, 36, 614–624. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Y.; Zhang, J.; Xiang, H.; Mei, H.; Liu, L.; Tong, L.; Zeng, F.; Huang, Y.; Zhou, H.; et al. The Importance of CYP2C19 Genotype in Tacrolimus Dose Optimization When Concomitant with Voriconazole in Heart Transplant Recipients. Br. J. Clin. Pharmacol. 2022, 88, 4515–4525. [Google Scholar] [CrossRef]
- Dong, J.; Liu, S.; Rasheduzzaman, J.M.; Huang, C.; Miao, L. Development of Physiology Based Pharmacokinetic Model to Predict the Drug Interactions of Voriconazole and Venetoclax. Pharm. Res. 2022, 39, 1921–1933. [Google Scholar] [CrossRef]

| Parameter | Value | Reference |
|---|---|---|
| Molecular weight (g/mol) | 804.02 | Drug label |
| pKa | Neutral | Drug label |
| Log P | 3.3 | [26] |
| fup | 0.013 | [17] |
| fmCYP3A4 | 0.35 | Calculated |
| fmCYP3A5 | 0.55 | Calculated |
| Characteristics | Median | Range |
|---|---|---|
| Age (years) | 52.00 | 46.00, 61.00 |
| Weight (kg) | 67.50 | 57.50, 75.00 |
| BMI (kg/m2) | 23.30 | 20.53, 25.36 |
| POD (day) | 23.00 | 19.00, 29.00 |
| Tacrolimus daily dose (mg) | 5.00 | 4.00,6.00 |
| RBC (T/L) | 3.46 | 3.12, 3.93 |
| WBC (T/L) | 10.69 | 9.13, 13.19 |
| HGB (g/L) | 103.25 | 95.26, 115.46 |
| HCT (%) | 31.98 | 29.72, 34.96 |
| ALT (U/L) | 27.56 | 17.37, 39.49 |
| AST (U/L) | 19.24 | 15.92, 23.62 |
| ALP (U/L) | 79.18 | 62.16, 104.62 |
| LDH (U/L) | 353.72 | 294.90, 475.11 |
| CK (U/L) | 76.20 | 50.78, 105.43 |
| ALB (g/L) | 36.74 | 34.48, 38.68 |
| APTT (s) | 29.45 | 28.23, 31.56 |
| PT (s) | 13.00 | 12.51, 13.60 |
| TT (s) | 17.55 | 17.01, 18.14 |
| INR | 1.14 | 1.09, 1.20 |
| TBIL (μmol/L) | 15.90 | 12.13, 21.10 |
| BUN (μmol/L) | 10.70 | 9.19, 15.45 |
| SCR (μmol/L) | 86.65 | 70.94, 128.53 |
| Glucose (μmol/L) | 6.61 | 5.91, 7.96 |
| Recipient Genotype | Count (Percentage) | p-value |
| CYP3A5*3 (rs776746) | 0.33 | |
| CC | 38 (44.2%) | |
| CT | 45 (52.3%) | |
| TT | 3 (3.5%) | |
| CYP3A4*18B (rs2242480) | 0.30 | |
| CC | 45 (52.3%) | |
| CT | 40 (46.5%) | |
| TT | 1 (1.2%) | |
| IL-10 (rs1800896) | 0.20 | |
| TT | 63 (73.3%) | |
| CT | 23 (26.7%) |
| Model Parameter | Healthy Adult Dataset (n = 5) | Model Building Dataset (n = 115) | External Evaluation Dataset (n = 100) | |||
|---|---|---|---|---|---|---|
| Estimate Value | Standard Error | Estimate Value | Standard Error | Estimate Value | Standard Error | |
| Ka (1/h) | 4.4 | 0.6 | 1.9 | 0.2 | 1.4 | 0.1 |
| KD (ng/mL) | 6.8 | 0.5 | 7.2 | 0.7 | 5.9 | 1.7 |
| Bmax (ng/mL) | 204.8 | 1.7 | 145.9 | 18.4 | 176.8 | 45.6 |
| CLint (L/h) | 20,706.0 | 69.7 | 11,535.0 | 506.3 | 10,256.2 | 314.5 |
| Dose | AUC0–12h | Cmax | Reference | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD Pred (ng × h/mL) | Obs (ng × h/mL) | FE | Mean ± SD Pred (ng/mL) | Obs (ng/mL) | FE | ||
| 1.0 mg | 50.6 ± 18.7 | 70.6 | 0.72 | 7.3 ± 3.6 | 10.7 | 0.68 | [41] |
| 2.0 mg | 101.3 ± 27.5 | 116.7 | 0.87 | 14.6 ± 4.9 | 16.3 | 0.90 | [40] |
| 3.0 mg | 151.9 ± 33.1 | 167.2 | 0.91 | 21.9 ± 2.5 | 18.0 | 1.22 | [40] |
| 3.5 mg | 177.3 ± 39.6 | 152.6 | 1.16 | 25.6 ± 4.7 | 24.7 | 1.04 | [40] |
| 4.5 mg | 227.9 ± 54.2 | 230.0 | 0.99 | 32.9 ± 5.3 | 34.7 | 0.95 | [40] |
| Input Parameter | Sensitivity Value for AUC0-last | Sensitivity Value for Cmax | Sensitivity Value for Ctrough |
|---|---|---|---|
| Fraction unbound in plasma | −1.15 | −0.66 | −1.66 |
| Hematocrit | 0.91 | 0.67 | 1.06 |
| Intrinsic clearance (L/h) | −0.74 | −0.47 | −1.09 |
| Fraction of activity of CYP3A5 | −0.30 | −0.17 | −0.45 |
| Fraction of activity of CYP3A4 | −0.15 | −0.09 | −0.22 |
| Bodyweight (kg) | −0.04 | −0.4 | −0.13 |
| Proportion of adipose tissue | 0.02 | 0.17 | 0.05 |
| Dose Regimen | Population | AUC0–24 | Cmax | Ctrough |
|---|---|---|---|---|
| Pred (ng × h/mL) | Pred (ng/mL) | Pred (ng/mL) | ||
| Tacrolimus, 2.5 mg, single dose | EM | 119.29 ± 34.00 | 14.27 ± 3.44 | - |
| PM | 191.91 ± 40.74 | 20.32 ± 3.55 | - | |
| Tacrolimus, 2.5 mg, q12h | EM | - | 23.08 ± 3.86 | 9.62 ± 2.47 |
| PM | - | 40.37 ± 5.13 | 21.00 ± 3.24 |
| BW < 50 kg | PM | EM | ||
| Drug combination | Hematocrit | Hematocrit | Hematocrit | Hematocrit |
| (0.2–0.3) | (0.3–0.4) | (0.2–0.3) | (0.3–0.4) | |
| Without voriconazole | 2.5 (92.5) * | 1.8 (90.7) * | 4.0 (94.8) * | 2.8 (96.1) * |
| With voriconazole | 1.0 (91.8) * | 0.5 (92.4) * | 2.0 (90.7) * | 1.0 (94.5) * |
| BW = 50–80 kg | PM | EM | ||
| Drug combination | Hematocrit (0.2–0.3) | Hematocrit (0.3–0.4) | Hematocrit (0.2–0.3) | Hematocrit (0.3–0.4) |
| Without voriconazole | 3.0 (93.8) * | 2.5 (96.3) * | 4.5 (93.3) * | 3.0 (95.7) * |
| With voriconazole | 1.5 (92.9) * | 1.0 (94.1) * | 2.5 (90.5) * | 1.5 (91.9) * |
| BW > 80 kg | PM | EM | ||
| Drug combination | Hematocrit | Hematocrit | Hematocrit | Hematocrit |
| (0.2–0.3) | (0.3–0.4) | (0.2–0.3) | (0.3–0.4) | |
| Without voriconazole | 3.5 (93.2) * | 2.5 (91.8) * | 5.0 (95.3) * | 3.2 (94.2) * |
| With voriconazole | 1.5 (92.5) * | 1.0 (94.4) * | 2.5 (93.7) * | 2.0 (94.0) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, L.; Li, R.; Zhou, H.; Du, W.; Gu, Y.; Jiang, Y.; Wang, Y.; Chen, X.; Sun, J.; Zhu, J. A Physiologically Based Pharmacokinetic Approach to Recommend an Individual Dose of Tacrolimus in Adult Heart Transplant Recipients. Pharmaceutics 2023, 15, 2580. https://doi.org/10.3390/pharmaceutics15112580
Pei L, Li R, Zhou H, Du W, Gu Y, Jiang Y, Wang Y, Chen X, Sun J, Zhu J. A Physiologically Based Pharmacokinetic Approach to Recommend an Individual Dose of Tacrolimus in Adult Heart Transplant Recipients. Pharmaceutics. 2023; 15(11):2580. https://doi.org/10.3390/pharmaceutics15112580
Chicago/Turabian StylePei, Ling, Run Li, Hong Zhou, Wenxin Du, Yajie Gu, Yingshuo Jiang, Yongqing Wang, Xin Chen, Jianguo Sun, and Junrong Zhu. 2023. "A Physiologically Based Pharmacokinetic Approach to Recommend an Individual Dose of Tacrolimus in Adult Heart Transplant Recipients" Pharmaceutics 15, no. 11: 2580. https://doi.org/10.3390/pharmaceutics15112580






