Effect of E. cava and C. indicum Complex Extract on Phorbol 12-Myristate 13-Acetate (PMA)-Stimulated Inflammatory Response in Human Pulmonary Epithelial Cells and Particulate Matter (PM)2.5-Induced Pulmonary Inflammation in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cells and Chemicals
2.1.2. Preparation of ED
2.1.3. Preparation of PM2.5
2.2. Methods
2.2.1. A549 Cell Culture
2.2.2. Cell Viability Assay
2.2.3. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.2.4. Western Blot Analysis
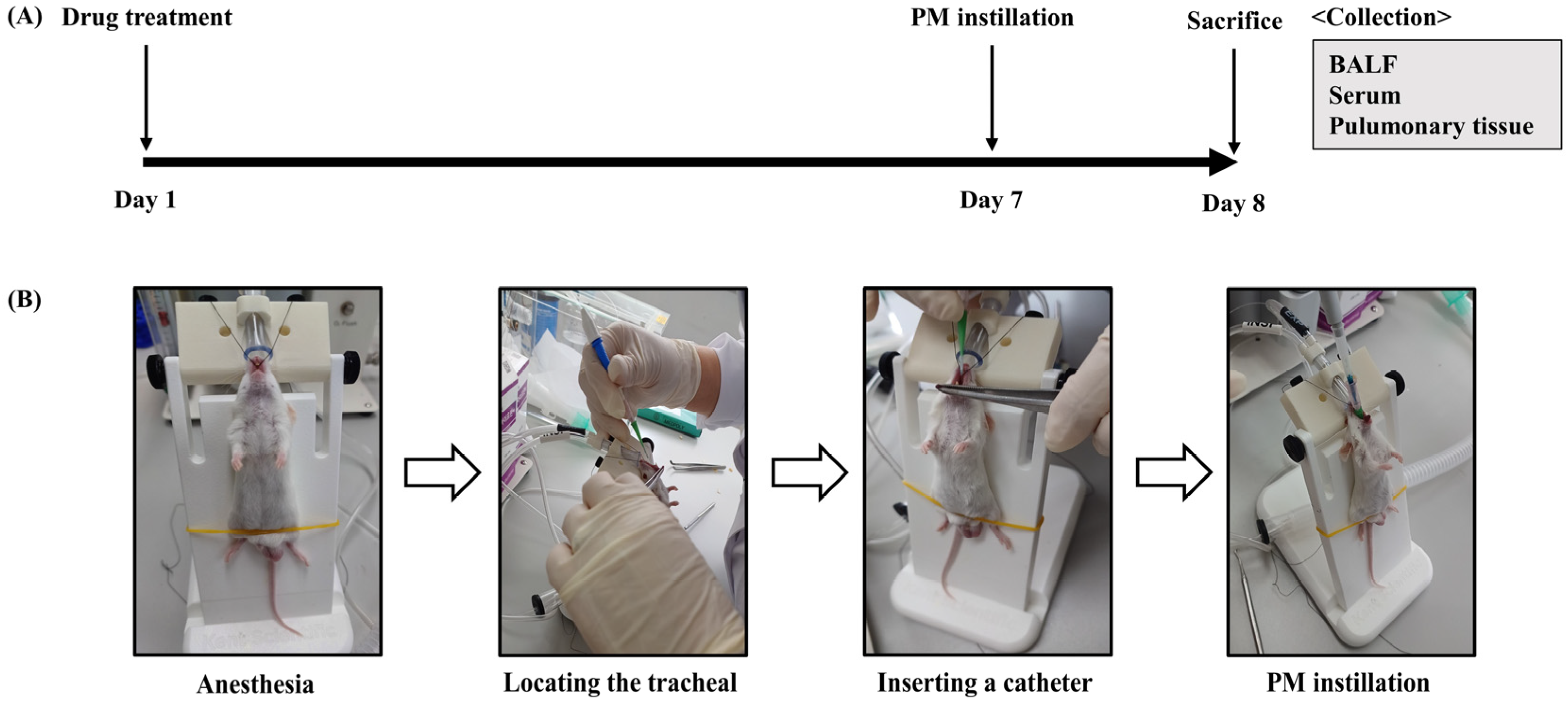
2.2.5. PM2.5-Induced Pulmonary Inflammation Mouse Model and Experimental Design
2.2.6. Analysis of Cell Counting in BALF
2.2.7. Histopathological Analysis
2.2.8. ELISA Analysis for TNF-α and IL-6 in Serum
2.2.9. Statistical Analysis
3. Results
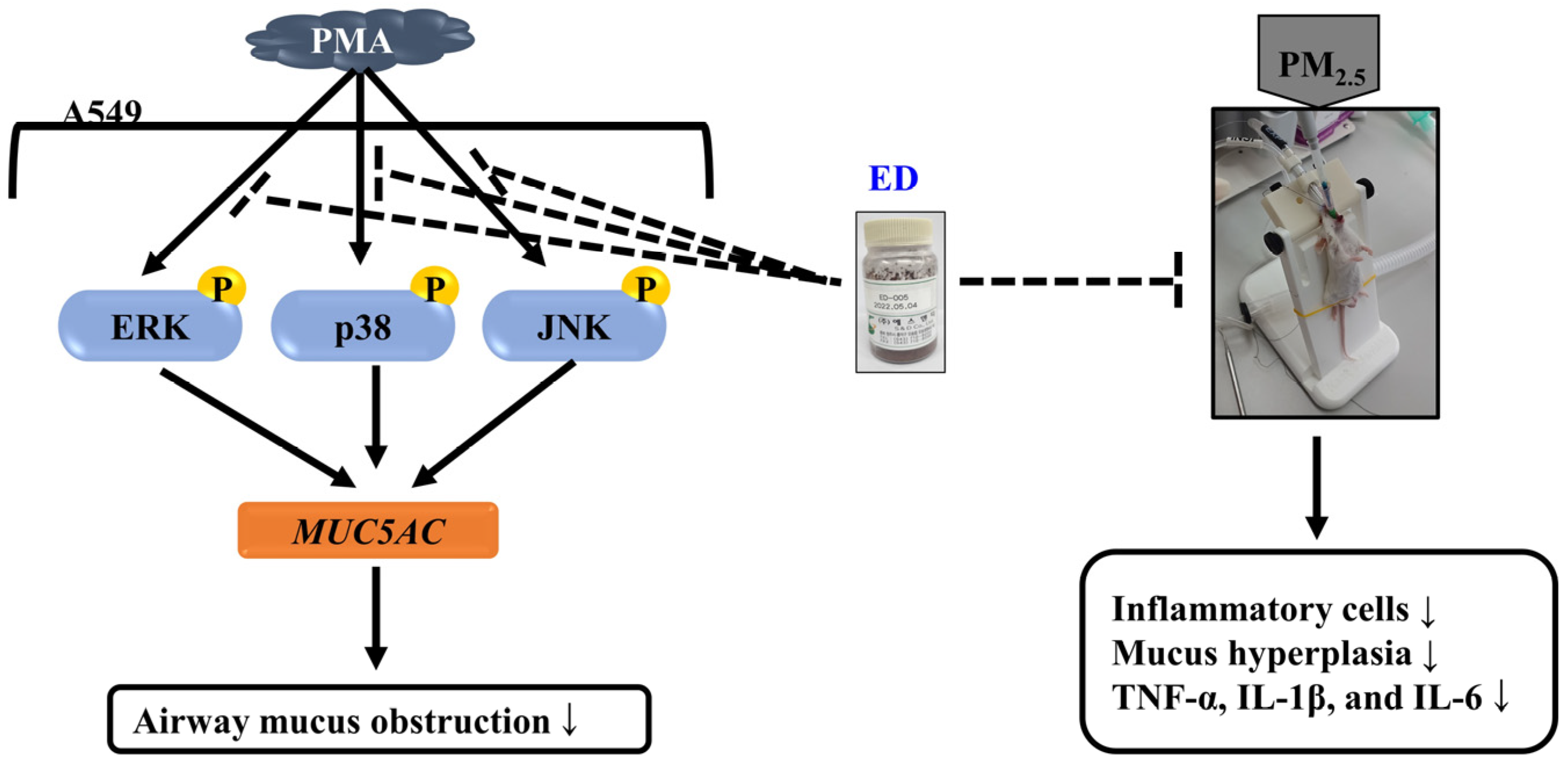
3.1. Effects of ED and Dieckol on MUC5AC Gene Expression in PMA-Stimulated A549 Cells
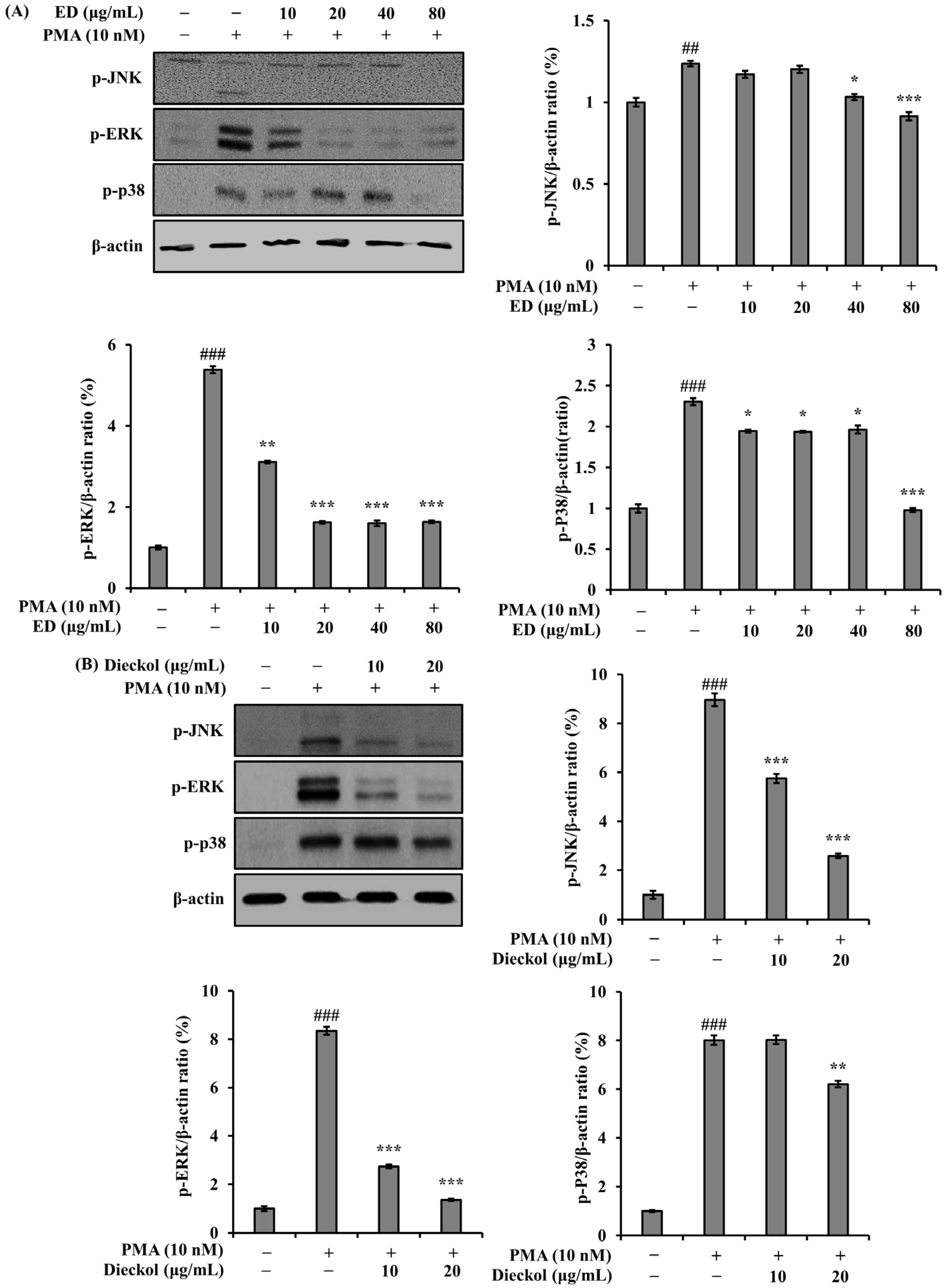
3.2. Effect of ED and Dieckol on the Phosphorylation of MAPKs in PMA-Stimulated A549 Cells
3.3. Effect of ED on Cells in BALF in the PM2.5-Induced Pulmonary Inflammation Mouse Model
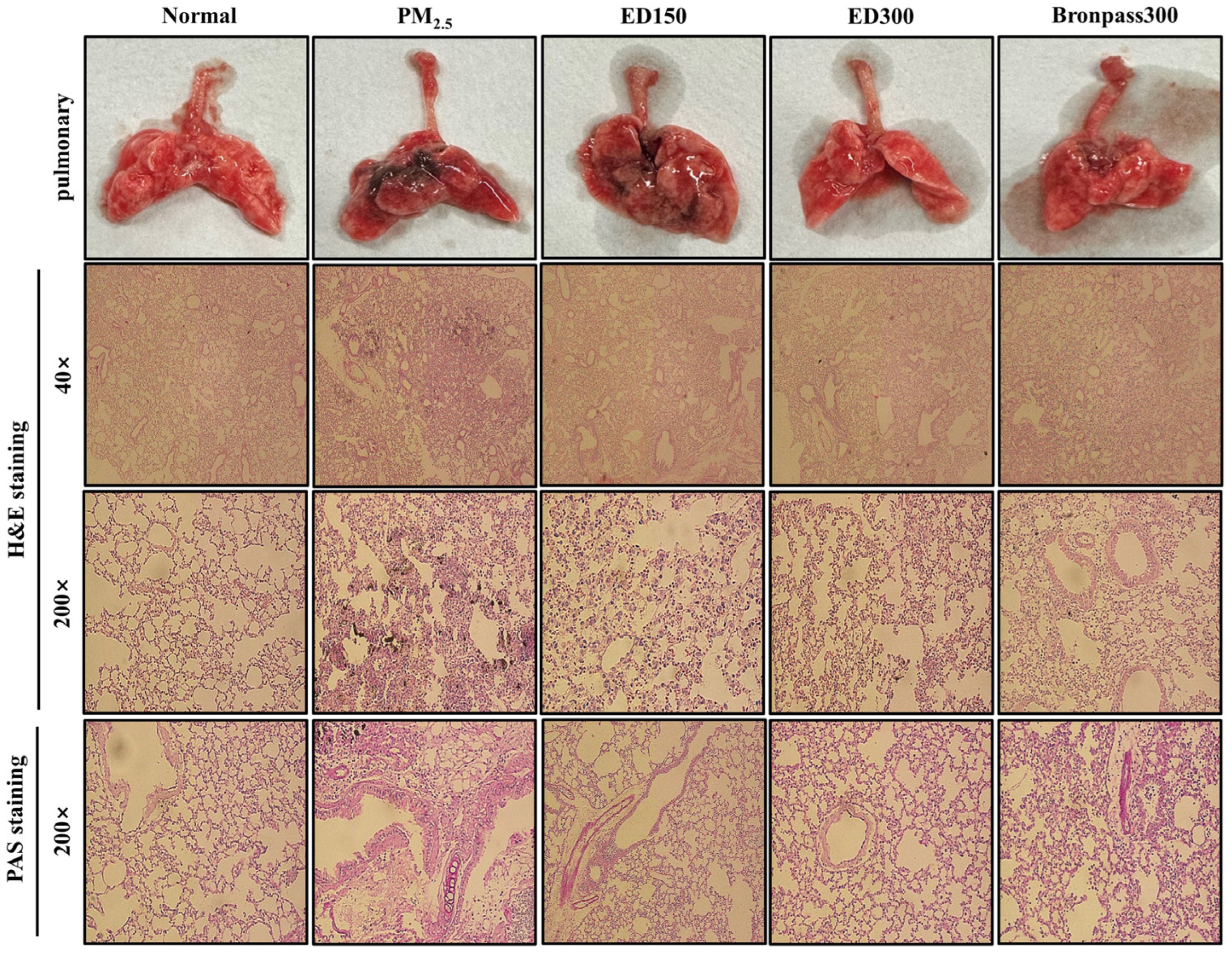
3.4. Effect of ED on Pulmonary Damage in the PM2.5-Induced Pulmonary Inflammation Mouse Model
3.5. Effects of ED on Inflammatory Cytokine Levels in the Serum of a PM2.5-Induced Pulmonary Inflammation Mouse Model
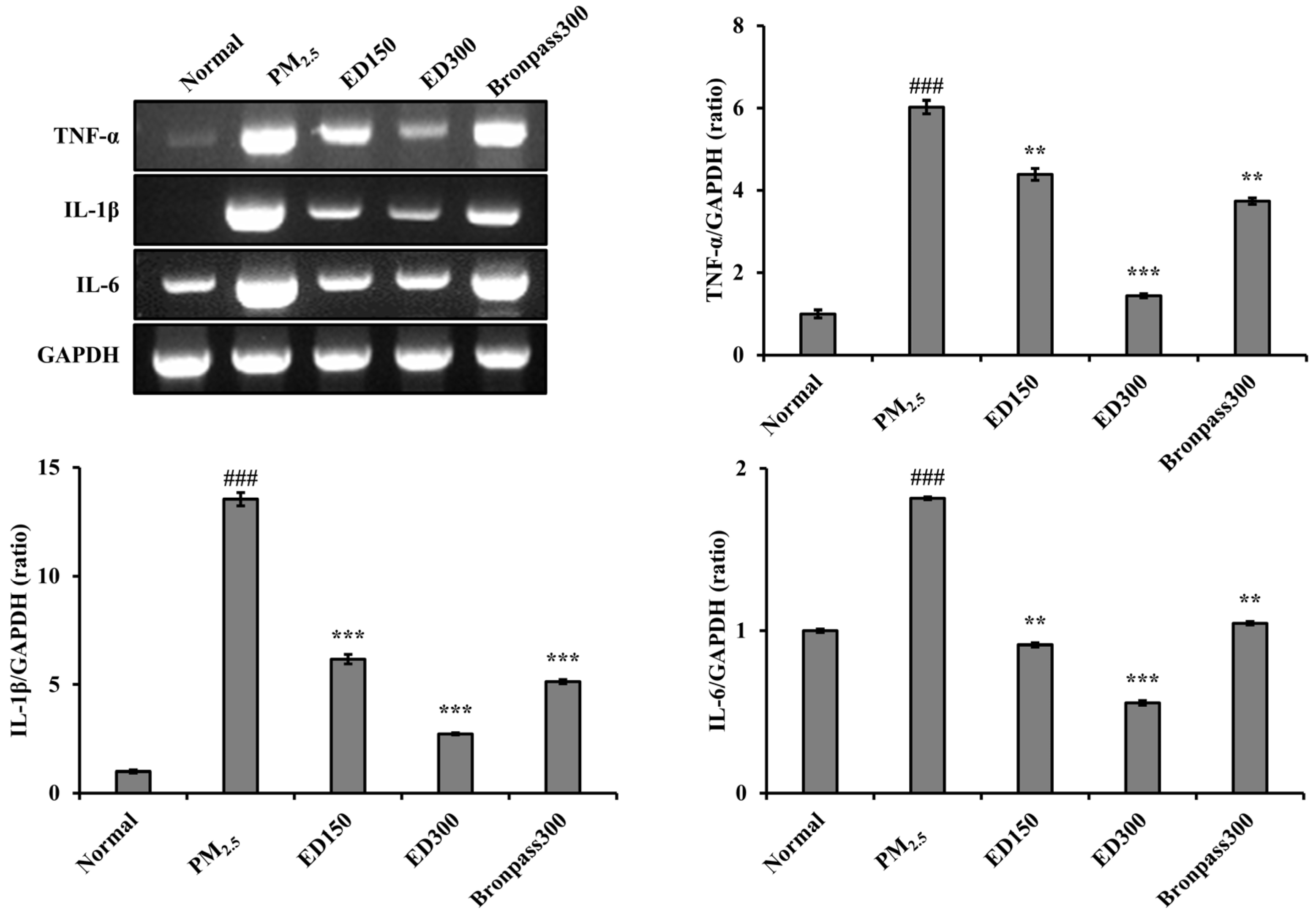
3.6. Effects of ED on Inflammatory Cytokine Levels in Lung Tissue of the PM2.5-Induced Pulmonary Inflammation Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory effects of black ginseng on particulate matter-induced pulmonary injury. Am. J. Chin. Med. 2019, 47, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Park, S.W.; Lee, H.I.; Lee, S.W. Health effects caused by particulate matter and guidelines for health care. Public Health Wkly. Rep. 2018, 11, 458–462. [Google Scholar]
- Song, H.W.; Ji, K.Y.; Kim, B.K.; Yang, W.K.; Han, C.K.; Shin, H.J.; Park, Y.C.; Hwang, J.S.; Kang, H.S.; Kim, S.H. Respiratory Protective Effect of Salvia plebeia R. Br. extracts against ambient particulate matter-induced airway onflammation. Korean J. Med. Crop Sci. 2017, 25, 269–281. [Google Scholar] [CrossRef]
- Brandt, E.B.; Kovacic, M.B.; Lee, G.B.; Gibson, A.M.; Acciani, T.H.; Le Cras, T.D.; Ryan, P.H.; Budelsky, A.L.; Hershey, G.K.K. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J. Allergy Clin. Immunol. 2013, 132, 1194–1204. [Google Scholar] [CrossRef]
- Doeing, D.C.; Solway, J. Airway smooth muscle in the pathophysiology and treatment of asthma. J. Appl. Physiol. 2013, 114, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Fuks, K.; Moebus, S.; Hertel, S.; Viehmann, A.; Nonnemacher, M.; Dragano, N.; Möhlenkamp, S.; Jakobs, H.; Kessler, C.; Erbel, R.; et al. Long-term urban particulate air pollution, traffic, noise and arterial blood pressure. Environ. Health Perspect. 2011, 119, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Reid, A.; Fritschi, L.; de Klerk, N.; Musk, A.W. Long-term effects of aluminium dust inhalation. J. Occup. Environ. Med. 2013, 70, 864–868. [Google Scholar] [CrossRef]
- Wang, G.; Huang, L.; Gao, S.; Gao, S.; Wang, L. Measurements of PM10 and PM2.5 in urban area of Nanjing, China and the assessment of pulmonary deposition of particle mass. Chemosphere 2002, 48, 689–695. [Google Scholar] [CrossRef]
- Bohadana, A.B.; Massin, N.; Wild, P.; Toamain, J.P.; Engel, S.; Goutet, P. Symptoms, airway responsiveness, and exposure to dust in beech and oak wood workers. J. Occup. Environ. Med. 2000, 57, 268–273. [Google Scholar] [CrossRef]
- Park, Y.B.; Rhee, C.K.; Yoon, H.K.; Oh, Y.M.; Lim, S.Y.; Lee, J.H.; Yoo, K.H.; Ahn, J.H. COPD clinical practice guideline of the Korean Academy of Tuberculosis and Respiratory Disease: A summary. Tuberc. Respir. Dis. 2018, 81, 261–273. [Google Scholar] [CrossRef]
- Kim, T.H.; Yang, W.K.; Lee, S.W.; Kim, S.H.; Lyu, Y.R.; Park, Y.C. Inhibitory Effects of GGX on Lung Injury of Chronic Obstructive Lung Disease (COPD) Mice Model. J. Korean Med. 2021, 42, 56–71. [Google Scholar] [CrossRef]
- West, J.B. Respiratory Physiology: The Essentials, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- McDonald, J.A. Lung Growth and Development; Dekkar, M., Ed.; Lung Biology in Health and Disease: New York, NY, USA, 1997; Volume 100. [Google Scholar]
- Vestbo, J.; Prescott, E.; Lange, P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity: Copenhagen City Heart Study Group. Am. J. Respir. Crit. Care Med. 1996, 153, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Hovenberg, H.W.; Davies, J.R.; Herrmann, A.; Linden, C.J.; Carlstedt, I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj. J. 1996, 13, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.J.; Gould, S.; Harris, A. Developmental expression of mucin genes in the human respiratory tract. Am. J. Respir. Cell Mol. Biol. 1997, 17, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Groneberg, D.A.; Eynott, P.R.; Lim, S.; Oates, T.; Wu, R.; Carlstedt, I.; Roberts, P.; McCann, B.; Nicholson, A.G.; Harrison, B.D.; et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 2002, 40, 367–373. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, S.I.; Choi, S.H.; Song, C.H.; Park, S.J.; Shin, Y.K.; Han, C.H.; Lee, Y.J.; Ku, S.K. Single oral dose toxicity test of blue honeysuckle concentrate in mice. Toxicol. Res. 2015, 31, 61–68. [Google Scholar] [CrossRef]
- Pozzi, R.; De Berardis, B.; Paoletti, L.; Guastadisegni, C. Infammatory mediators induced by coarse (PM2.5-10) and fne (PM2.5) urban air particles in RAW 264.7 cells. Toxicology 2003, 183, 1–3. [Google Scholar] [CrossRef]
- Wang, F.; Wang, R.; Liu, H. The acute pulmonary toxicity in mice induced by Staphylococcus aureus, particulate matter, and their combination. Exp. Anim. 2019, 68, 159–168. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Andrade, K.A.M.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharm. 2021, 142, 111988. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, Y.T.; Jeon, Y.J. Antioxidant dieckol downregulates the Rac1/ROS signaling pathway and inhibits Wiskott-Aldrich syndrome protein (WASP)-family verprolin-homologous protein 2 (WAVE2)-mediated invasive migration of B16 mouse melanoma cells. Mol. Cells 2012, 33, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yayeh, T.; Im, E.J.; Kwon, T.H.; Roh, S.S.; Kim, S.; Kim, J.H.; Hong, S.B.; Cho, J.Y.; Park, N.H.; Rhee, M.H. Hemeoxygenase 1 partly mediates the anti-inflammatory effect of dieckol in lipopolysaccharide stimulated murine macrophages. Int. Immunopharmacol. 2014, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Ryu, Y.B.; Kim, Y.M.; Song, N.; Kim, C.Y.; Rho, M.C.; Jeong, J.H.; Cho, K.O.; Lee, W.S.; Park, S.J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg. Med. Chem. 2013, 21, 4706–4713. [Google Scholar] [CrossRef]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine alga Ecklonia cava extract and dieckol attenuate prostaglandin E2 production in HaCaT keratinocytes exposed to airborne particulate matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, H.S.; Jayawardena, T.U.; Ryu, B.; Yang, H.W.; Ahn, G.; Lee, W.; Jeon, Y.J. Dieckol: An algal polyphenol attenuates urban fine dust-induced inflammation in RAW 264.7 cells via the activation of anti-inflammatory and antioxidant signaling pathways. J. Appl. Phycol. 2019, 32, 2387–2396. [Google Scholar] [CrossRef]
- Kang, H.; Park, C.H.; Kwon, S.O.; Lee, S.G. ED formula, a complex of Ecklonia cava and Chrysanthemum indicum, ameliorates airway inflammation in lipopolysaccharide-stimulated RAW macrophages and ovalbumin-induced asthma mouse model. Pharmaceuticals 2023, 21, 1185. [Google Scholar] [CrossRef]
- Lee, S.G.; Kwon, S.O. Ecklonia cava-Hizikia fusiformis complex extract alleviates inflammation in human lung epithelia. J. Plant Biotechnol. 2022, 49, 90–98. [Google Scholar] [CrossRef]
- Myou, S.; Leff, A.R.; Myo, S.; Boetticher, E.; Tong, J.; Meliton, A.Y.; Liu, J.; Munoz, N.M.; Zhu, X. Blockade of inflammation and airway hyperresponsiveness in immunesensitized mice by dominant-negative phosphoinositide-3-kinase-TAT. J. Exp. Med. 2003, 198, 1573–1582. [Google Scholar] [CrossRef]
- Freeman, C.M.; Crudgington, S.; Stolberg, V.R.; Brown, J.P.; Sonstein, J.; Alexis, N.E.; Doerschuk, C.M.; Basta, P.V.; Carretta, E.E.; Couper, D.J.; et al. Design of a multi-center immunophenotyping analysis of peripheral blood, sputum and bronchoalveolar lavage fluid in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). J. Transl. Med. 2015, 13, 192015. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Borm, P.J.; Jimenez, L.A.; Gilmour, P.S.; Schins, R.P.; Knaapen, A.M.; Rahman, I.; Faux, S.P.; Brown, D.M.; et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic. Biol. Med. 2003, 34, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Jang, G.T.; Kim, J.H. A study on chronic or recurrent respiratory symptoms. Korean J. Orient Pediatr. 2002, 16, 83–99. [Google Scholar]
- Basbaum, C.; Welsh, M.J. Mucus secretion and ion transport in airways. In Textbook of Respiratory Medicine; W.B. Saunders: Philadelphia, PA, USA, 2000; pp. 327–348. [Google Scholar]
- Park, S.H.; Lee, H.J.; Ryu, J.; Son, K.H.; Kwon, S.Y.; Lee, S.K.; Kim, Y.S.; Hong, J.H.; Seok, J.H.; Lee, C.J. Effects of ophiopogonin D and spicatoside A derived from Liriope Tuber on secretion and production of mucin from airway epithelial cells. Phytomedicine 2014, 21, 172–176. [Google Scholar] [CrossRef]
- Park, S.J.; Kang, S.Y.; Kim, N.S.; Kim, H.M. Phosphatidylinositol 3-kinase regulates PMA-induced differentiation and superoxide production in HL-60 cells. Immunopharmacol. Immunotoxicol. 2002, 24, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Hewson, C.A.; Edbrooke, M.R.; Johnston, S.L. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J. Mol. Biol. 2004, 344, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.F.; Barnes, P.J. Treatment of airway mucus hypersecretion. Ann. Med. 2006, 38, 116–125. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, 69–74. [Google Scholar]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, composition, and lung diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Ho, C.; Tsai, M.; Chen, Y.; Kuo, C.; Lin, P. Persistent elevation of blood pressure by ambient coarse particulate matter after recovery from pulmonary inflammation in mice. Environ. Toxicol. 2019, 34, 814–824. [Google Scholar] [CrossRef]
- Misiukiewicz-Stepien, P.; Paplinska-Goryca, M. Biological effect of PM10 on airway epithelium-focus on obstructive lung diseases. Clin. Immunol. 2021, 227, 108754. [Google Scholar] [CrossRef]
- Lee, Y.; Min, D.; Park, S.; Lee, J.; Bae, H. Standardized herbal extract PM014 alleviates fine dust-induced lung inflammation in mice. BMC Complement. Med. Ther. 2020, 20, 270. [Google Scholar] [CrossRef] [PubMed]



| Gene | Size (bp) | Sequence | Origin |
|---|---|---|---|
| MUC5AC | 458 | F 1: 5′-TGATCATCCAGCAGGGCT-3′ R 2: 5′-CCGAGCTCAGAGGACATATGGG-3′ | Human |
| Rig/S15 | 361 | F: 5′-TTCCGCAAGTTCACCTACC-3′ R: 5′-CGGGCCGGCCATGCTTTACG-3′ | Human |
| TNF-α | 390 | F: 5′-TTCGAGTGACAAGCCTGTAGC-3′ R: 5′-AGATTGACCTCAGCGCTGAGT-3′ | Mouse |
| IL-1β | 385 | F: 5′-CATATGAGCTGAAAGCTCTCCA-3′ R: 5′-GACACAGATTCCATGGTGAAGTC-3′ | Mouse |
| IL-6 | 435 | F: 5′-GGAGGCTTAAITACACATGTT-3′ R: 5′-TGATTCAAGATGAATTGGAT-3′ | Mouse |
| GAPDH | 378 | F: 5′-CCAGTATGACTCCACTCACG-3′ R: 5′-CCTTCCACAATGCCAAGTT-3′ | Mouse |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-G.; Park, C.-H.; Kang, H. Effect of E. cava and C. indicum Complex Extract on Phorbol 12-Myristate 13-Acetate (PMA)-Stimulated Inflammatory Response in Human Pulmonary Epithelial Cells and Particulate Matter (PM)2.5-Induced Pulmonary Inflammation in Mice. Pharmaceutics 2023, 15, 2621. https://doi.org/10.3390/pharmaceutics15112621
Lee S-G, Park C-H, Kang H. Effect of E. cava and C. indicum Complex Extract on Phorbol 12-Myristate 13-Acetate (PMA)-Stimulated Inflammatory Response in Human Pulmonary Epithelial Cells and Particulate Matter (PM)2.5-Induced Pulmonary Inflammation in Mice. Pharmaceutics. 2023; 15(11):2621. https://doi.org/10.3390/pharmaceutics15112621
Chicago/Turabian StyleLee, Sung-Gyu, Chan-Hwi Park, and Hyun Kang. 2023. "Effect of E. cava and C. indicum Complex Extract on Phorbol 12-Myristate 13-Acetate (PMA)-Stimulated Inflammatory Response in Human Pulmonary Epithelial Cells and Particulate Matter (PM)2.5-Induced Pulmonary Inflammation in Mice" Pharmaceutics 15, no. 11: 2621. https://doi.org/10.3390/pharmaceutics15112621





