Modeling and Simulation as a Tool to Assess Voriconazole Exposure in the Central Nervous System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
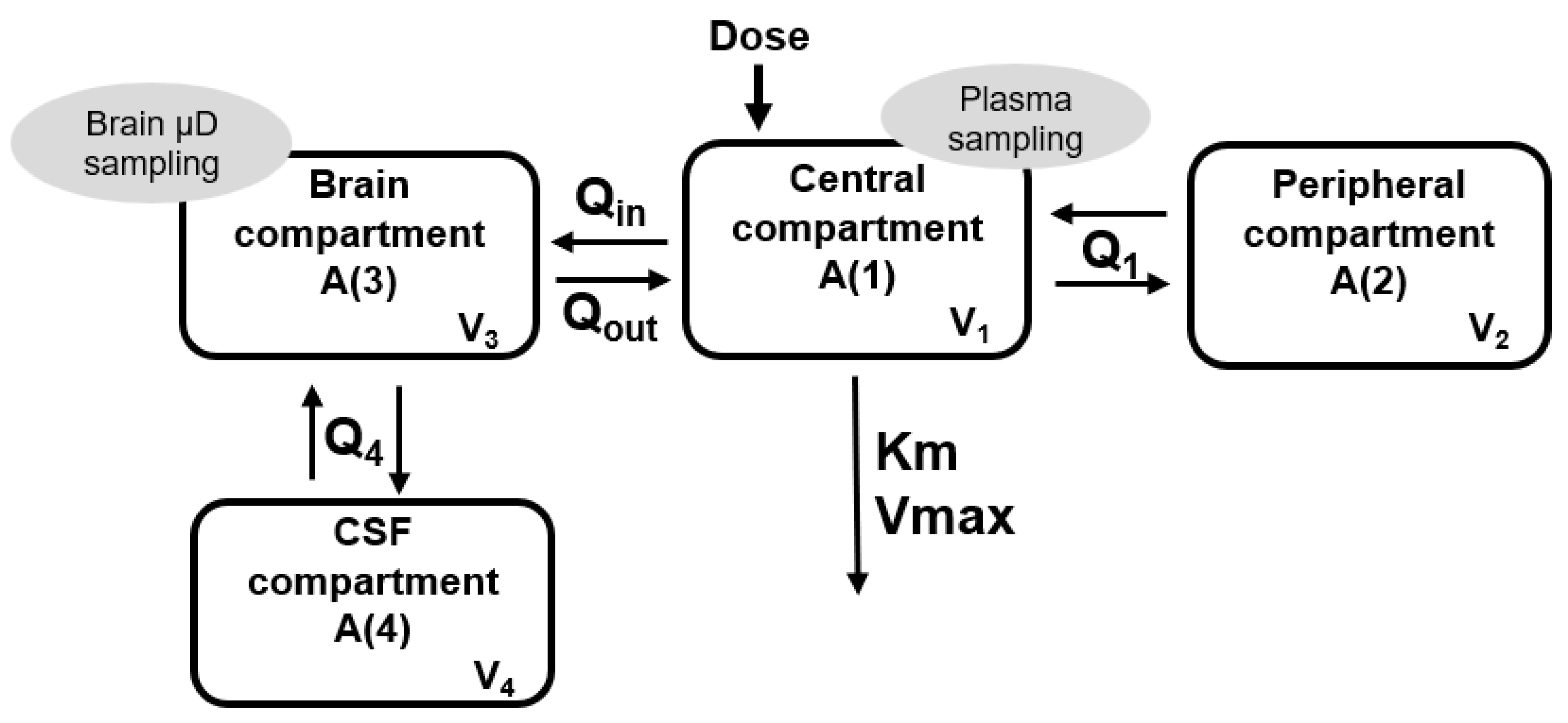
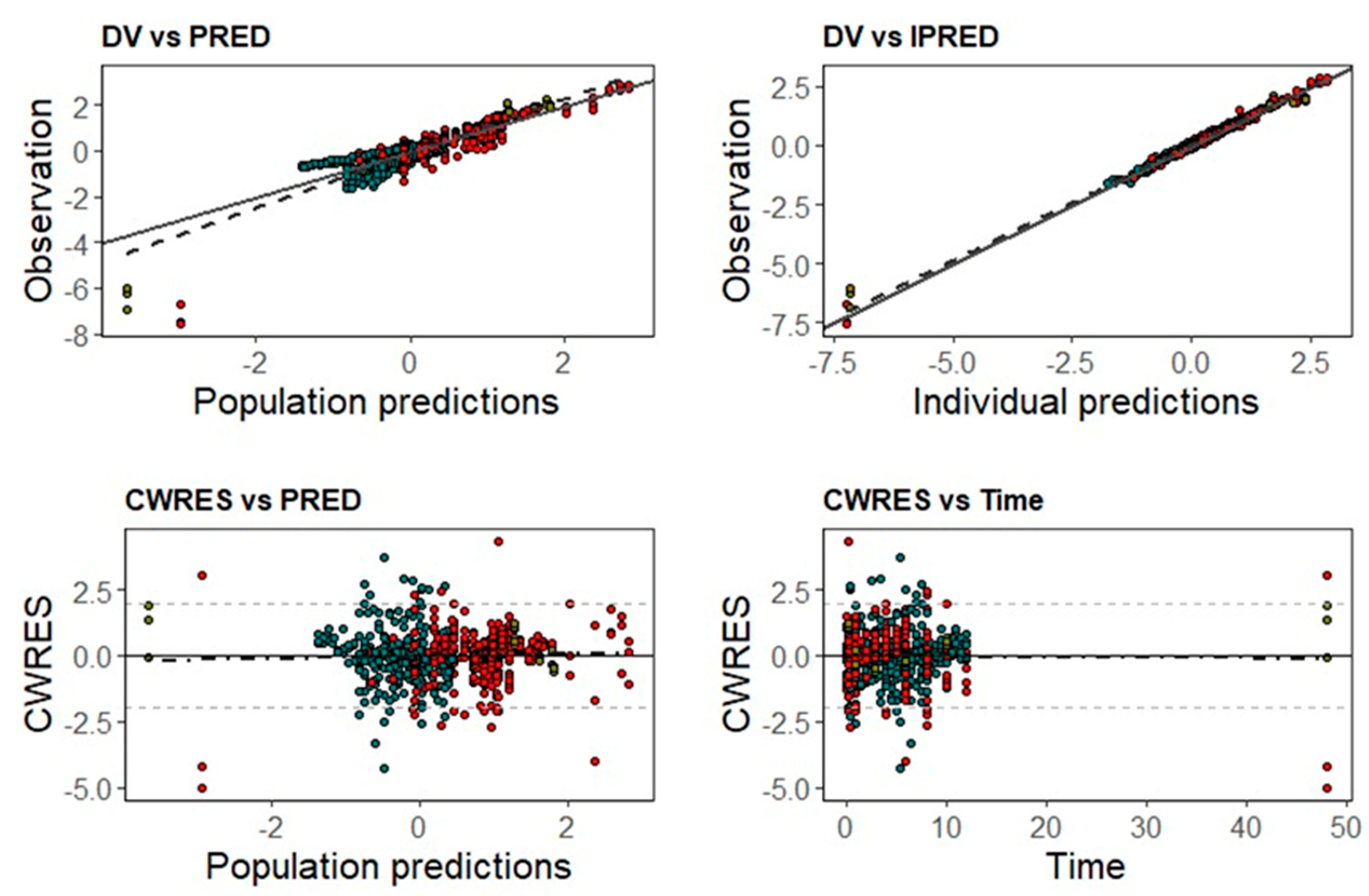
2.2. Population Pharmacokinetic Analysis
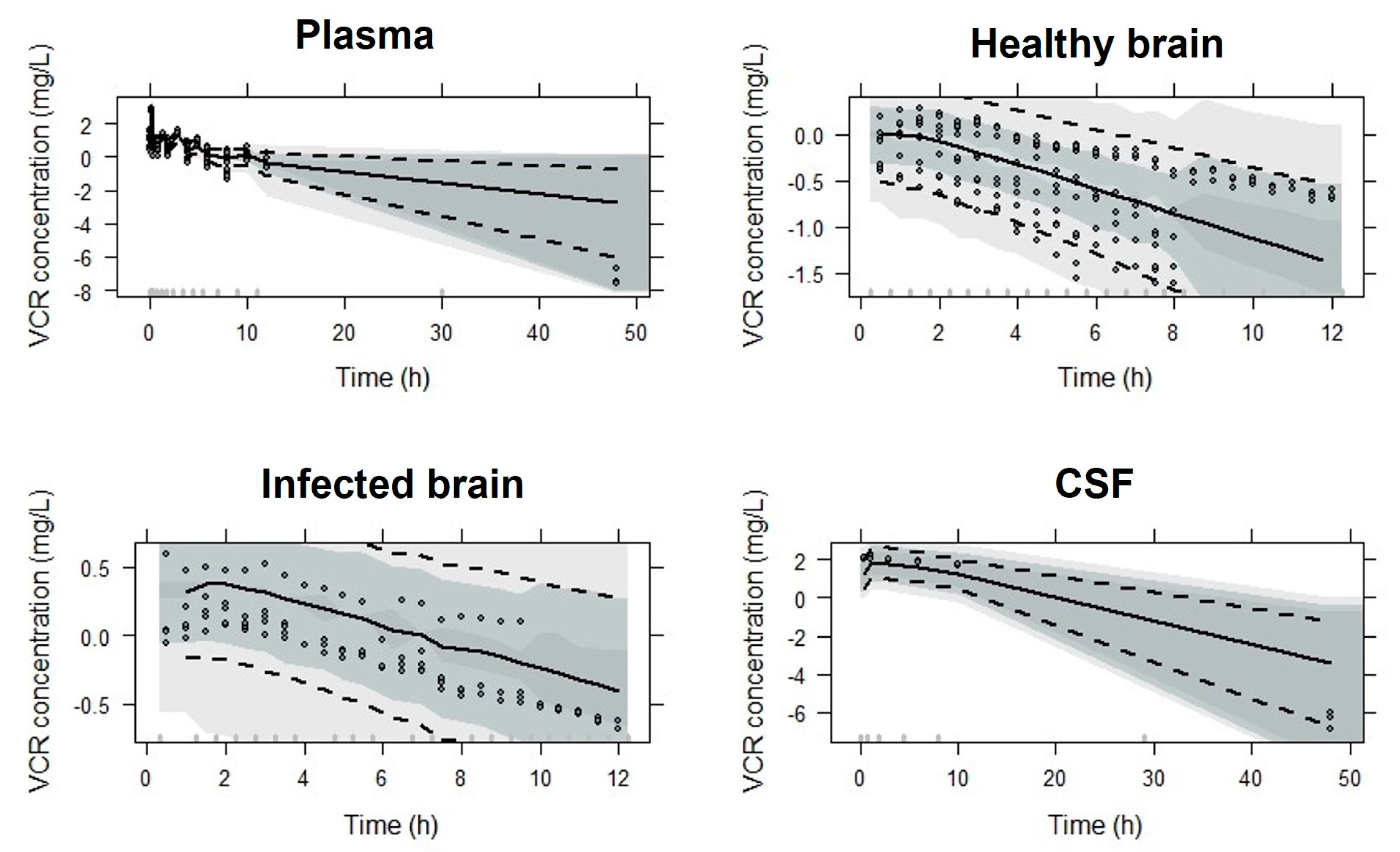
2.3. Simulations
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, K.M.; Montrief, T.; Ramzy, M.; Koyfman, A.; Long, B. Cryptococcal meningitis: A review for emergency clinicians. Intern. Emerg. Med. 2021, 16, 1031–1042. [Google Scholar] [CrossRef]
- Williamson, P.R.; Jarvis, J.N.; Panackal, A.A.; Fisher, M.C.; Molloy, S.F.; Loyse, A.; Harrison, T.S. Cryptococcal meningitis: Epidemiology, immunology, diagnosis and therapy. Nat. Rev. Neurol. 2016, 13, 13–24. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Fungal infections of the central nervous system. In CNS Infections; García-Moncó, J., Ed.; Springer: Cham, Switzerland, 2018; pp. 129–156. [Google Scholar] [CrossRef]
- Kon, A.S. Consenso Em Criptococose 2008. Rev. Soc. Bras. Med. Trop. 2008, 41, 524–544. [Google Scholar]
- Tenforde, M.W.; Shapiro, E.A.; Rouse, B.; Jarvis, J.N.; Li, T.; Eshun-Wilson, I.; Ford, N. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst. Rev. 2018, 2018, CD005647. [Google Scholar] [CrossRef]
- Zavala, S.; Baddley, J.W. Cryptococcosis. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2020. [Google Scholar]
- Abdel-Hafez, Y.; Siaj, H.; Janajri, M.; Abu-Baker, Y.; Nazzal, Z.; Hamdan, Z.; Adwan, R.; Aiesh, B.M.; Anaya, A.I. Tolerability and epidemiology of nephrotoxicity associated with conventional amphotericin B therapy: A retrospective study in tertiary care centers in Palestine. BMC Nephrol. 2022, 23, 132. [Google Scholar] [CrossRef]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- Ngan, N.T.; Thanh Hoang Le, N.; Vi Vi, N.N.; Van, N.T.; Mai, N.T.; Van Anh, D.; Trieu, P.H.; Lan, N.P.; Phu, N.H.; Chau, N.V.; et al. An open label randomized controlled trial of tamoxifen combined with amphotericin B and Fluconazole for cryptococcal meningitis. eLife 2021, 10, e68929. [Google Scholar] [CrossRef]
- Zhao, H.-Z.; Wang, R.-Y.; Wang, X.; Jiang, Y.-K.; Zhou, L.-H.; Cheng, J.-H.; Huang, L.-P.; Harrison, T.S.; Zhu, L.-P. High dose fluconazole in salvage therapy for HIV-uninfected cryptococcal meningitis. BMC Infect. Dis. 2018, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.D. Antifungal drugs: Special problems treating central nervous system infections. J. Fungi. 2019, 5, 97. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, J.; Su, X.; Yang, L.; Wang, Y.; Wang, A.; Xu, X.; Li, M.; Jiang, Y.; Peng, F. Amphotericin B plus fluorocytosine combined with voriconazole for the treatment of non-HIV and non-transplant-associated cryptococcal meningitis: A retrospective study. BMC Neurol. 2022, 22, 274. [Google Scholar] [CrossRef]
- Nakao, M.; Fujita, K.; Arakawa, S.; Hayashi, S.; Tomita, S.; Sato, H.; Muramatsu, H. Treatment of cryptococcus gattii infection using voriconazole. Intern. Med. 2021, 60, 3635–3638. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, J.-T.; Yan, B.; Gao, T.; Xing, X.-W.; Tian, C.-L.; Huang, X.-S.; Yu, S.-Y. Voriconazole: A novel treatment option for cryptococcal meningitis. Infect. Dis. 2015, 47, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Geist, M.J.P.; Egerer, G.; Burhenne, J.; Riedel, K.D.; Weiss, J.; Mikus, G. Steady-state pharmacokinetics and metabolism of voriconazole in patients. J. Antimicrob. Chemother. 2013, 68, 2592–2599. [Google Scholar] [CrossRef] [Green Version]
- Hope, W.W. Population pharmacokinetics of voriconazole in adults. Antimicrob. Agents Chemother. 2012, 56, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Muto, C.; Shoji, S.; Tomono, Y.; Liu, P. Population pharmacokinetic analysis of voriconazole from a pharmacokinetic study with immunocompromised Japanese pediatric subjects. Antimicrob. Agents Chemother. 2015, 59, 3216–3223. [Google Scholar] [CrossRef] [Green Version]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef] [Green Version]
- Lutsar, I.; Roffey, S.; Troke, P. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin. Infect. Dis. 2003, 37, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Pennick, G.J.; Dorsey, S.A.; Furmaga, W.; Lewis, J.S.; Patterson, T.F.; Sutton, D.A.; Fothergill, A.W. A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations withinthe bloodstream and cerebral spinal fluid. Antimicrob. Agents Chemother. 2014, 58, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, R.; Sano, H.; Kishimoto, K.; Suzuki, D.; Yasuda, K.; Kobayashi, K. Voriconazole concentrations in cerebrospinal fluid during prophylactic use in children with acute myelogenous leukemia. Pediatr. Infect. Dis. J. 2016, 35, 297–298. [Google Scholar] [CrossRef]
- Alves, I.A.; Staudt, K.J.; De Miranda Silva, C.; De Araujo Lock, G.; Costa, T.D.; De Araujo, B.V. Influence of experimental cryptococcal meningitis in wistar rats on voriconazole brain penetration assessed by microdialysis. Antimicrob. Agents Chemother. 2017, 61, e00321-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammarlund-Udenaes, M.; Fridén, M.; Syvänen, S.; Gupta, A. On the rate and extent of drug delivery to the brain. Pharm. Res. 2008, 25, 1737–1750. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Matsumiya, K.; Tohyama, K.; Kosugi, Y. Translational CNS Steady-State Drug Disposition Model in Rats, Monkeys, and Humans for Quantitative Prediction of Brain-to-Plasma and Cerebrospinal Fluid-to-Plasma Unbound Concentration Ratios. AAPS J. 2021, 23, 81. [Google Scholar] [CrossRef]
- Li, Y. Pharmacokinetics/Pharmacodynamics of Voriconazole. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2008. [Google Scholar]
- Lelièvre, B.; Briet, M.; Godon, C.; Legras, P.; Riou, J.; Vandeputte, P.; Diquet, B.; Bouchara, J.P. Impact of infection status and cyclosporine on voriconazole pharmacokinetics in an experimental model of cerebral scedosporiosis. J. Pharmacol. Exp. Ther. 2018, 365, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Tunblad, K.; Hammarlund-Udenaes, M.; Jonsson, E.N. An integrated model for the analysis of pharmacokinetic data from microdialysis experiments. Pharm. Res. 2004, 21, 1698–1707. [Google Scholar] [CrossRef]
- KuKanich, B.; Huff, D.; Riviere, J.E.; Papich, M.G. Naïve averaged, naïve pooled, and population pharmacokinetics of orally administered marbofloxacin in juvenile harbor seals. J. Am. Vet. Med. Assoc. 2007, 230, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Boxenbaum, H. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J. Pharmacokinet. Biopharm. 1982, 10, 201–227. [Google Scholar] [CrossRef]
- Wang, C.; Allegaert, K.; Peeters, M.Y.M.; Tibboel, D.; Danhof, M.; Knibbe, C.A.J. The allometric exponent for scaling clearance varies with age: A study on seven propofol datasets ranging from preterm neonates to adults. Br. J. Clin. Pharmacol. 2014, 77, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachaly, J.R.; Terapêutica Por Extrapolação Alométrica. Tratado Animais Selvagens 2006, 1215–1223. Available online: https://www.evz.ufg.br/up/66/o/Dr._Pachaly_-_Extrapolação_Alométrica_-_2007.pdf?1331574034 (accessed on 10 January 2023).
- Grensemann, J.; Pfaffendorf, C.; Wicha, S.G.; König, C.; Roedl, K.; Jarczak, D.; Iwersen-Bergmann, S.; Manthey, C.; Kluge, S.; Fuhrmann, V. Voriconazole pharmacokinetics are not altered in critically ill patients with acute-on-chronic liver failure and continuous renal replacement therapy: An observational study. Microorganisms 2021, 9, 2087. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Zheng, J.; Zhang, Y.; Yang, L.; He, N.; Zhai, S. Predicting the Outcome of Voriconazole Individualized Medication Using Integrated Pharmacokinetic/Pharmacodynamic Model. Front. Pharmacol. 2021, 12, 711187. [Google Scholar] [CrossRef] [PubMed]
- Resztak, M.; Sobiak, J.; Czyrski, A. Recent advances in therapeutic drug monitoring of voriconazole, mycophenolic acid, and vancomycin: A literature review of pediatric studies. Pharmaceutics 2021, 13, 1991. [Google Scholar] [CrossRef]
- Nielsen, E.I.; Cars, O.; Friberg, L.E. Pharmacokinetic/Pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: A step toward model-based dose optimization. Antimicrob. Agents Chemother. 2011, 55, 4619–4630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, D.K.; Betts, A.M. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J. Pharmacokinet. Pharmacodyn. 2012, 39, 67–86. [Google Scholar] [CrossRef]
- Serraj, K.; Alaoui, H.; El Oumri, A.A.; Barrimi, M.; Bachir, H. Effective Voriconazole in an Immunocompetent Patient with Amphotericin B Resistant Systemic Cryptococcal Granulomatosis. Cureus 2020, 12, e11101. [Google Scholar] [CrossRef] [PubMed]
- Nau, R.; Sörgel, F.; Eiffert, H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.D.; Artru, A.A.; Adkison, K.K. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv. Drug. Deliv. Rev. 2004, 56, 1825–1857. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Estimate (%RSE) | Bootstrap Median (95% CI) |
|---|---|---|
| Vmax (mg/h) | 1.06 (13) | 1.01 (0.385–1.160) |
| Km (mg/L) | 4.76 (20) | 4.56 (1.128–5.993) |
| Km,infected (mg/L) | 8.13 (44) | 0.68 (0.239–1.324) |
| Q1 (L) | 2.69 (18) | 2.64 (0.360–4.992) |
| V1 (L) | 1.15 (7) | 1.16 (0.953–1.469) |
| V2 (L) | 0.37 (18) | 0.35 (0.042–0.586) |
| Qin (L/h) | 0.81 (13) | 0.83 (0.285–1.983) |
| Qout (L/h) | 0.63 (13) | 0.63 (0.221–1.822) |
| Qout,infected (L/h) | 0.38 (36) | 0.35 (0.117–0.549) |
| V3 (L) | 0.00041FIX | - |
| Q4 (L/h) | 0.33 (19) | 0.35 (0.085–1.170) |
| V4 (L) | 0.13 (17) | 0.13 (0.049–0.310) |
| ωKm (%CV) | 38 (15) | 33 (25–223) |
| ωV1 (%CV) | 29 (13) | 26 (15–38) |
| ωV2 (%CV) | 47 (34) | 52 (26–100) |
| ωQout (%CV) | 39 (17) | 37 (25–47) |
| Plasma log-additive error (mg/L) | 0.107 (5) | 0.107 (0.073–0.137) |
| Microdialysis log-additive error (mg/L) | 0.074 (5) | 0.072 (0.056–0.091) |
| CSF log-additive error (mg/L) | 0.450 (18) | 0.449 (0.239–1.324) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staudt, K.J.; Dias, B.B.; Alves, I.A.; Lelièvre, B.; Bouchara, J.-P.; de Araújo, B.V. Modeling and Simulation as a Tool to Assess Voriconazole Exposure in the Central Nervous System. Pharmaceutics 2023, 15, 1781. https://doi.org/10.3390/pharmaceutics15071781
Staudt KJ, Dias BB, Alves IA, Lelièvre B, Bouchara J-P, de Araújo BV. Modeling and Simulation as a Tool to Assess Voriconazole Exposure in the Central Nervous System. Pharmaceutics. 2023; 15(7):1781. https://doi.org/10.3390/pharmaceutics15071781
Chicago/Turabian StyleStaudt, Keli Jaqueline, Bruna Bernar Dias, Izabel Almeida Alves, Bénédicte Lelièvre, Jean-Philippe Bouchara, and Bibiana Verlindo de Araújo. 2023. "Modeling and Simulation as a Tool to Assess Voriconazole Exposure in the Central Nervous System" Pharmaceutics 15, no. 7: 1781. https://doi.org/10.3390/pharmaceutics15071781





