Application of Silver Nanoparticles in Parasite Treatment
Abstract
:1. Introduction
2. Synthesis and Potential Applications of Silver Nanomaterials
2.1. The Properties of Silver Nanomaterials
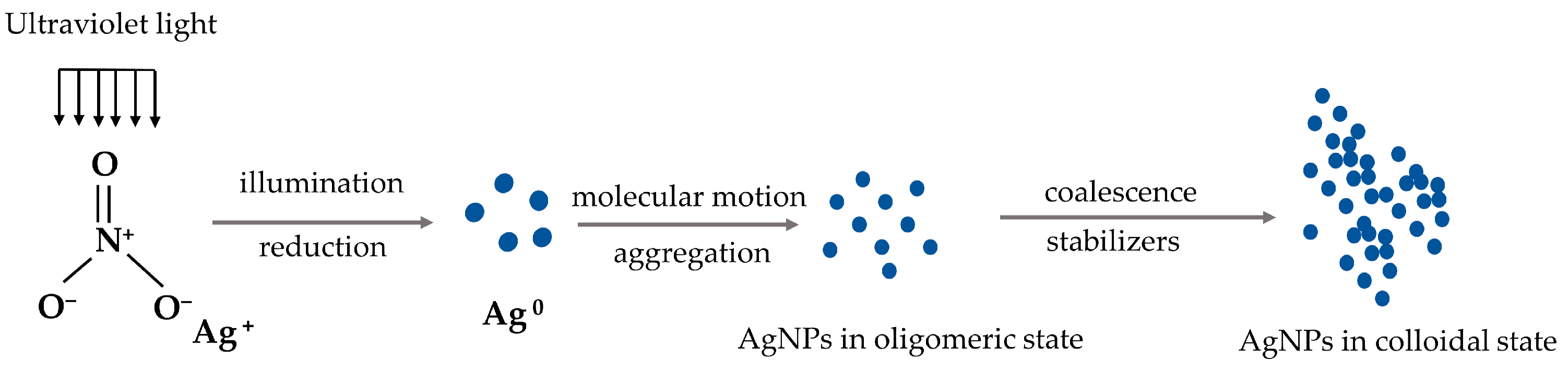
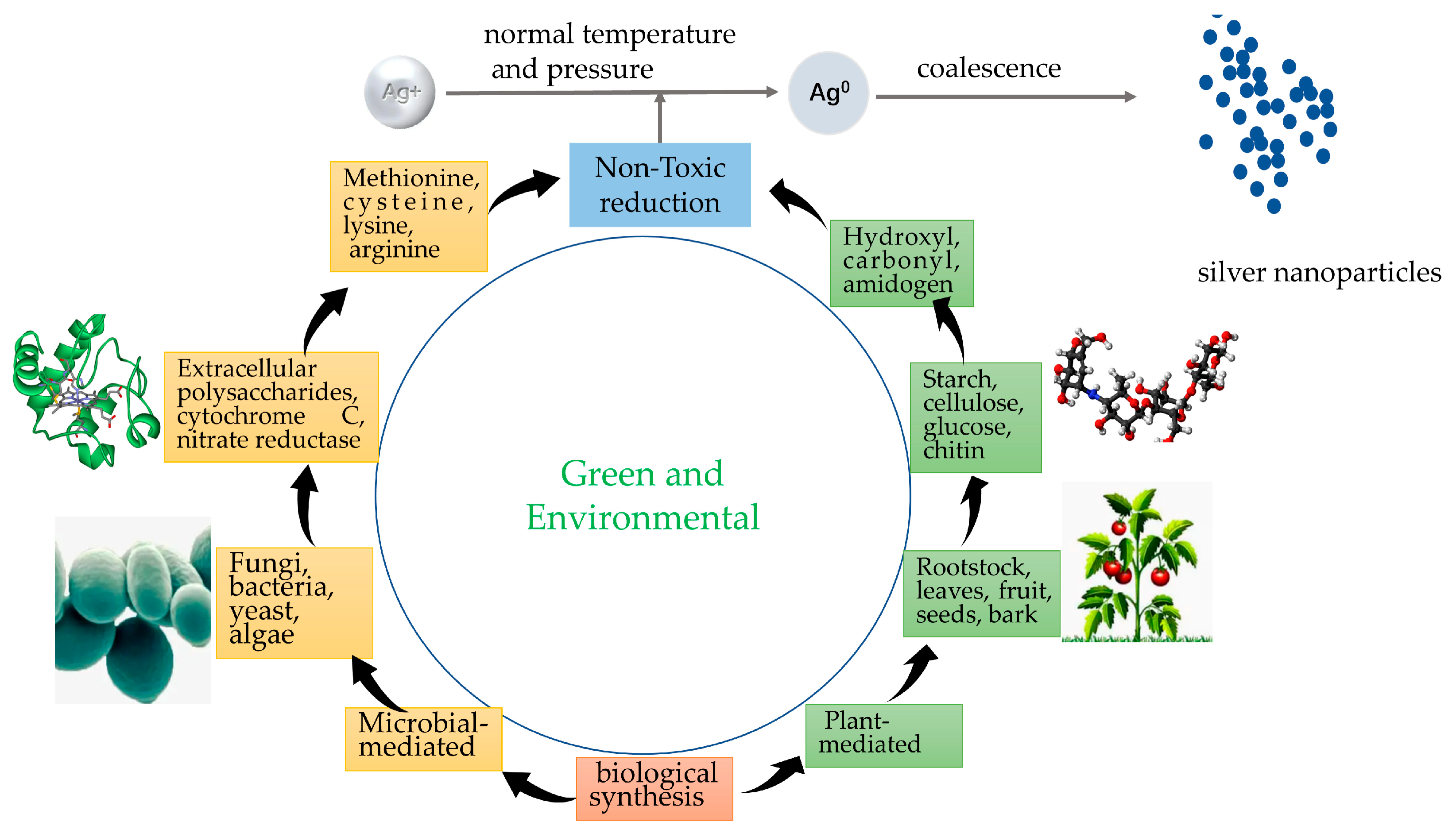
2.2. The Synthesis of Silver Nanoparticles
2.3. Potential Applications of AgNPs
3. Antiprotozoal Effect of AgNPs
3.1. Leishmaniasis
3.2. Flukes
3.3. Toxoplasmosis
3.4. Cryptosporidium
3.5. Haemonchus
3.6. Blastocystis Hominis
3.7. Strongylides
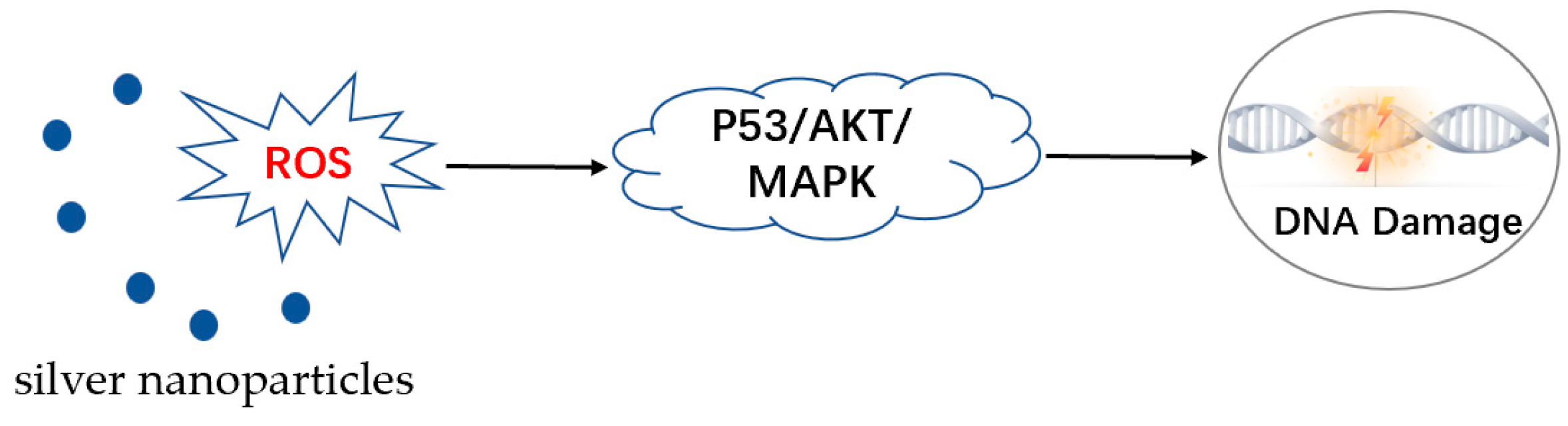
4. Anti-Parasitic Mechanism of AgNPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theel, E.S.; Pritt, B.S. Parasites. Microbiol. Spectr. 2016, 4, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Shivaramaiah, C.; Barta, J.R.; Hernandez-Velasco, X.; Tellez, G.; Hargis, B.M. Coccidiosis: Recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet. Med. 2014, 5, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Ojimelukwe, A.E.; Emedhem, D.E.; Agu, G.O.; Nduka, F.O.; Abah, A.E. Populations of Eimeria tenella express resistance to commonly used anticoccidial drugs in southern Nigeria. Int. J. Vet. Sci. Med. 2018, 6, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Fernandez, I.; Pegg, E.; Macdonald, S.E.; Tomley, F.M.; Blake, D.P.; Marugan-Hernandez, V. Laboratory Growth and Genetic Manipulation of Eimeria tenella. Curr. Protoc. Microbiol. 2019, 53, e81. [Google Scholar] [CrossRef] [Green Version]
- Beaumier, C.M.; Gillespie, P.M.; Hotez, P.J.; Bottazzi, M.E. New vaccines for neglected parasitic diseases and dengue. Transl. Res. 2013, 162, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ali, N. Vaccine Development Against Leishmania donovani. Front. Immunol. 2012, 3, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillespie, P.M.; Beaumier, C.M.; Strych, U.; Hayward, T.; Hotez, P.J.; Bottazzi, M.E. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 2016, 34, 2992–2995. [Google Scholar] [CrossRef]
- Etewa, S.E.; El-Maaty, D.A.A.; Hamza, R.S.; Metwaly, A.S.; Sarhan, M.H.; Abdel-Rahman, S.A.; Fathy, G.M.; El-Shafey, M.A. Assessment of spiramycin-loaded chitosan nanoparticles treatment on acute and chronic toxoplasmosis in mice. J. Parasit. Dis. 2018, 42, 102–113. [Google Scholar] [CrossRef]
- Marei, N.; Elwahy, A.H.M.; Salah, T.A.; El Sherif, Y.; El-Samie, E.A. Enhanced antibacterial activity of Egyptian local insects’ chitosan-based nanoparticles loaded with ciprofloxacin-HCl. Int. J. Biol. Macromol. 2019, 126, 262–272. [Google Scholar] [CrossRef]
- Parthiban, E.; Manivannan, N.; Ramanibai, R.; Mathivanan, N. Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol. Rep. 2019, 21, e00297. [Google Scholar] [CrossRef] [PubMed]
- Cameron, P.; Gaiser, B.K.; Bhandari, B.; Bartley, P.M.; Katzer, F.; Bridle, H. Silver Nanoparticles Decrease the Viability of Cryptosporidium parvum Oocysts. Appl. Environ. Microbiol. 2016, 82, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordienko, M.G.; Palchikova, V.V.; Kalenov, S.V.; Belov, A.A.; Lyasnikova, V.N.; Poberezhniy, D.Y.; Chibisova, A.V.; Sorokin, V.V.; Skladnev, D.A. Antimicrobial activity of silver salt and silver nanoparticles in different forms against microorganisms of different taxonomic groups. J. Hazard. Mater. 2019, 378, 120754. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernandez, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chand, K.; Abro, M.I.; Aftab, U.; Shah, A.H.; Lakhan, M.N.; Cao, D.; Mehdi, G.; Ali Mohamed, A.M. Green synthesis characterization and antimicrobial activity against Staphylococcus aureus of silver nanoparticles using extracts of neem, onion and tomato. RSC Adv. 2019, 9, 17002–17015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbuDalo, M.A.; Al-Mheidat, I.R.; Al-Shurafat, A.W.; Grinham, C.; Oyanedel-Craver, V. Synthesis of silver nanoparticles using a modified Tollens’ method in conjunction with phytochemicals and assessment of their antimicrobial activity. PeerJ 2019, 7, e6413. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Cosar, G.; Abamor, E.S.; Bagirova, M.; Shinwari, Z.K.; Allahverdiyev, A.M. Comparative study on the antileishmanial activities of chemically and biologically synthesized silver nanoparticles (AgNPs). 3 Biotech 2018, 8, 98. [Google Scholar] [CrossRef]
- Hassan, D.; Farghali, M.; Eldeek, H.; Gaber, M.; Elossily, N.; Ismail, T. Antiprotozoal activity of silver nanoparticles against Cryptosporidium parvum oocysts: New insights on their feasibility as a water disinfectant. J. Microbiol. Methods 2019, 165, 105698. [Google Scholar] [CrossRef]
- Fauss, E.K.; MacCuspie, R.I.; Oyanedel-Craver, V.; Smith, J.A.; Swami, N.S. Disinfection action of electrostatic versus steric-stabilized silver nanoparticles on E. coli under different water chemistries. Colloids Surf. B Biointerfaces 2014, 113, 77–84. [Google Scholar] [CrossRef]
- Huang, W.; Zhai, H.J.; Wang, L.S. Probing the interactions of O(2) with small gold cluster anions (Au(n)(-), n = 1–7): Chemisorption vs physisorption. J. Am. Chem. Soc. 2010, 132, 4344–4351. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. Engl. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Abramenko, N.B.; Demidova, T.B.; Abkhalimov capital Ie, C.; Ershov, B.G.; Krysanov, E.Y.; Kustov, L.M. Ecotoxicity of different-shaped silver nanoparticles: Case of zebrafish embryos. J. Hazard. Mater. 2018, 347, 89–94. [Google Scholar] [CrossRef]
- Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int. J. Environ. Res. Public Health 2013, 10, 5221–5238. [Google Scholar] [CrossRef] [Green Version]
- Cavassin, E.D.; de Figueiredo, L.F.; Otoch, J.P.; Seckler, M.M.; de Oliveira, R.A.; Franco, F.F.; Marangoni, V.S.; Zucolotto, V.; Levin, A.S.; Costa, S.F. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J. Nanobiotechnol. 2015, 13, 64. [Google Scholar] [CrossRef]
- Ivask, A.; Elbadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindos, G.; Fernandez, M.J.; Fernandez, M.D. Synthesis, Physical, Mechanical and Antibacterial Properties of Nanocomposites Based on Poly (vinyl alcohol)/Graphene Oxide-Silver Nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Lee, D.K.; Kang, Y.S. Synthesis of Silver Nanocrystallites by a New Thermal Decomposition Method and Their Characterization. ETRI J. 2004, 26, 252–256. [Google Scholar] [CrossRef]
- Jung, J.H.; Oh, H.C.; Noh, H.S.; Ji, J.H.; Kim, S.S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J. Aerosol Sci. 2006, 37, 1662–1670. [Google Scholar] [CrossRef]
- Zhao, F.J.; Liu, L.; Yang, Y.; Zhang, R.L.; Ren, G.H.; Xu, D.L.; Zhou, P.W.; Han, K.L. Effect of the Hydrogen Bond on Photochemical Synthesis of Silver Nanoparticles. J. Phys. Chem. A 2015, 119, 12579–12585. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Kim, E.S.; Larkin, E.L.; Long, L.; Jennings, W.D.; Ahadian, S.; Ghannoum, M.A.; Wnek, G.E. Antimicrobial Activity of Silver Containing Crosslinked Poly (Acrylic Acid) Fibers. Micromachines 2019, 10, 829. [Google Scholar] [CrossRef] [Green Version]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zarate-Trivino, D.; Saldarriaga, J.; Perez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef] [Green Version]
- Fouda, M.M.G.; Abdelsalam, N.R.; Gohar, I.M.A.; Hanfy, A.E.M.; Othman, S.I.; Zaitoun, A.F.; Allam, A.A.; Morsy, O.M.; El-Naggar, M. Utilization of High throughput microcrystalline cellulose decorated silver nanoparticles as an eco-nematicide on root-knot nematodes. Colloids Surf. B Biointerfaces 2020, 188, 110805. [Google Scholar] [CrossRef]
- Dahlous, K.A.; Abd-Elkader, O.H.; Fouda, M.M.G.; Al Othman, Z.; El-Faham, A. Eco-friendly method for silver nanoparticles immobilized decorated silica: Synthesis & characterization and preliminary antibacterial activity. J. Taiwan Inst. Chem. Eng. 2019, 95, 324–331. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Chen, X.; Wan, J.; Qian, Y. A simple hydrothermal route to large-scale synthesis of uniform silver nanowires. Chemistry 2004, 11, 160–163. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of Silver Nanoparticles Using Aqueous Extract of Medicinal Plants’ (Impatiens balsamina and Lantana camara) Fresh Leaves and Analysis of Antimicrobial Activity. Int. J. Microbiol. 2019, 2019, 8642303. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.H.; Ismail, M.A.; Abdel-Mageed, W.M.; Mohamed Shoreit, A.A. Antimicrobial activity of green silver nanoparticles from endophytic fungi isolated from Calotropis procera (Ait) latex. Microbiology 2019, 165, 967–975. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Bastia, A.K.; Mohanta, T.K. Biosynthesis of Silver Nanoparticles from Protium serratum and Investigation of their Potential Impacts on Food Safety and Control. Front. Microbiol. 2017, 8, 626. [Google Scholar] [CrossRef] [Green Version]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouda, M.M.G.; Abdelsalam, N.R.; El-Naggar, M.E.; Zaitoun, A.F.; Salim, B.M.A.; Bin-Jumah, M.; Allam, A.A.; Abo-Marzoka, S.A.; Kandil, E.E. Impact of high throughput green synthesized silver nanoparticles on agronomic traits of onion. Int. J. Biol. Macromol. 2020, 149, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Hickel, R.; van Landuyt, K.L.; Reichl, F.X. Nanoparticles in dentistry. Dent. Mater. 2017, 33, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H. Stabilization and size control of gold nanoparticles during laser ablation in aqueous cyclodextrins. J. Am. Chem. Soc. 2004, 126, 7176–7177. [Google Scholar] [CrossRef]
- Takeshima, T.; Tada, Y.; Sakaguchi, N.; Watari, F.; Fugetsu, B. DNA/Ag Nanoparticles as Antibacterial Agents against Gram-Negative Bacteria. Nanomaterials 2015, 5, 284–297. [Google Scholar] [CrossRef]
- Syed, A.; Saraswati, S.; Kundu, G.C.; Ahmad, A. Biological synthesis of silver nanoparticles using the fungus Humicola sp. and evaluation of their cytoxicity using normal and cancer cell lines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, M.; El Roz, M.; Dong, B.; Retoux, R.; Aad, R.; Cardin, J.; Dufour, C.; Gourbilleau, F.; Gilson, J.P.; Mintova, S. Photochemical preparation of silver nanoparticles supported on zeolite crystals. Langmuir 2014, 30, 6250–6256. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Radtchenko, I.L.; Sukhorukov, G.B. Photoinduced reduction of silver inside microscale polyelectrolyte capsules. Chemphyschem 2003, 4, 1101–1103. [Google Scholar] [CrossRef]
- Jovanović, Ž.; Radosavljević, A.; Stojkovska, J.; Nikolić, B.; Obradovic, B.; Kačarević-Popović, Z.; Mišković-Stanković, V. Silver/poly(N-vinyl-2-pyrrolidone) hydrogel nanocomposites obtained by electrochemical synthesis of silver nanoparticles inside the polymer hydrogel aimed for biomedical applications. Polym. Compos. 2014, 35, 217–226. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, Y.; Li, Q.; Ma, X.; Quan, F.; Geng, C.; Han, Z. Microwave-assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 180–188. [Google Scholar] [CrossRef]
- Calderon-Jimenez, B.; Montoro Bustos, A.R.; Pereira Reyes, R.; Paniagua, S.A.; Vega-Baudrit, J.R. Novel pathway for the sonochemical synthesis of silver nanoparticles with near-spherical shape and high stability in aqueous media. Sci. Rep. 2022, 12, 882. [Google Scholar] [CrossRef]
- Liu, M.; Fang, F.; Song, X.; Yu, F.; Li, F.; Shi, X.; Xue, C.; Chen, T.; Wang, X. The first visually observable three-mode antibiotic switch and its relative 3D printing assisted applications. J. Mater. Chem. B. 2016, 4, 2544–2547. [Google Scholar] [CrossRef]
- Alves, M.F.; Murray, P.G. Biological Synthesis of Monodisperse Uniform-Size Silver Nanoparticles (AgNPs) by Fungal Cell-Free Extracts at Elevated Temperature and pH. J. Fungi 2022, 8, 439. [Google Scholar] [CrossRef]
- Alves, M.F.; Paschoal, A.C.C.; Klimeck, T.D.F.; Kuligovski, C.; Marcon, B.H.; de Aguiar, A.M.; Murray, P.G. Biological Synthesis of Low Cytotoxicity Silver Nanoparticles (AgNPs) by the Fungus Chaetomium thermophilum-Sustainable Nanotechnology. J. Fungi 2022, 8, 605. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci. Rep. 2021, 11, 770. [Google Scholar] [CrossRef]
- Khan, J.; Naseem, I.; Bibi, S.; Ahmad, S.; Altaf, F.; Hafeez, M.; Almoneef, M.M.; Ahmad, K. Green Synthesis of Silver Nanoparticles (Ag-NPs) Using Debregeasia Salicifolia for Biological Applications. Materials 2022, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Akbar, A.; Mussarat, S.; Murad, W.; Hameed, I.; Begum, S.; Nazir, R.; Ali, N.; Ali, E.A.; Bari, A.; et al. Phyto-Extract-Mediated Synthesis of Silver Nanoparticles (AgNPs) and Their Biological Activities. Bio. Res. Int. 2022, 2022, 9845022. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Shaiwale, N.S.; Deobagkar, D.N.; Deobagkar, D.D. Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant Deinococcus radiodurans, their characterization, and determination of bioactivity. Int. J. Nanomed. 2015, 10, 963–974. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kapoor, S.; Mukherjee, T. Preparation, characterization, and surface modification of silver nanoparticles in formamide. J. Phys. Chem. B 2005, 109, 7698–7704. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.K.; Sastry, T.P.; Sreedhar, B.; Mandal, A.B. One step synthesis of silver nanorods by autoreduction of aqueous silver ions with hydroxyapatite: An inorganic-inorganic hybrid nanocomposite. J. Biomed. Mater. Res. A 2007, 80, 391–398. [Google Scholar] [CrossRef]
- Fatema, U.K.; Rahman, M.M.; Islam, M.R.; Mollah, M.Y.A.; Susan, M. Silver/poly(vinyl alcohol) nanocomposite film prepared using water in oil microemulsion for antibacterial applications. J. Colloid. Interface Sci. 2018, 514, 648–655. [Google Scholar] [CrossRef]
- Nino-Martinez, N.; Martinez-Castanon, G.A.; Aragon-Pina, A.; Martinez-Gutierrez, F.; Martinez-Mendoza, J.R.; Ruiz, F. Characterization of silver nanoparticles synthesized on titanium dioxide fine particles. Nanotechnology 2008, 19, 065711. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liang, D.; Zhang, X.; Lin, Q.; Hao, L. Preparation and Antibacterial Performances of Electrocatalytic Zinc Oxide Nanoparticles with Diverse Morphologies. J. Biomed. Nanotechnol. 2021, 17, 1824–1829. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, C.; Li, C.; Lu, Z.; Ma, C.; Yan, Y.; Zhang, Y. Charge Transfer Tuned by the Surrounding Dielectrics in TiO(2)-Ag Composite Arrays. Nanomaterials 2018, 8, 1019. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wu, J.; Yang, H.; Liu, X.; Huang, Q.; Lu, Z. Antibacterial activity and mechanism of Ag/ZnO nanocomposite against anaerobic oral pathogen Streptococcus mutans. J. Mater. Sci. Mater. Med. 2017, 28, 23. [Google Scholar] [CrossRef]
- Cromwell, W.A.; Yang, J.; Starr, J.L.; Jo, Y.K. Nematicidal Effects of Silver Nanoparticles on Root-knot Nematode in Bermudagrass. J. Nematol. 2014, 46, 261–266. [Google Scholar]
- Baronia, R.; Kumar, P.; Singh, S.P.; Walia, R.K. Silver nanoparticles as a potential nematicide against Meloidogyne graminicola. J. Nematol. 2020, 52, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, K.F.; Abdelfattah, R.H.; El-Ansary, M.S.M. Green Nanoparticles Engineering on Root-knot Nematode Infecting Eggplants and Their Effect on Plant DNA Modification. Iran. J. Biotechnol. 2016, 14, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Goel, V.; Kaur, P.; Singla, L.D.; Choudhury, D. Biomedical Evaluation of Lansium parasiticum Extract-Protected Silver Nanoparticles Against Haemonchus contortus, a Parasitic Worm. Front. Mol. Biosci. 2020, 7, 595646. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S.; Tabassum, H.; Aladwan, N.S.M.; Naiman Ali, M.; Almaarik, B.; AlMahrouqi, S.; Abudawood, M.; Banu, N.; Alsubki, R. Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci. Rep. 2020, 10, 18564. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.; Nayak, P.; Singh, M.; Tambuwala, M.M.; Aljabali, A.A.; Chellappan, D.K.; Dua, K. Pharmaceutical Aspects of Green Synthesized Silver Nanoparticles: A Boon to Cancer Treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 1490–1509. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Al-Massarani, S.M.; Saquib, Q.; Wahab, R.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Anticancer Potential of Green Synthesized Silver Nanoparticles Using Extract of Nepeta deflersiana against Human Cervical Cancer Cells (HeLA). Bioinorg. Chem. Appl. 2018, 2018, 9390784. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.; Mehra, N.K.; Jain, N.K. Potentials and emerging trends in nanopharmacology. Curr. Opin. Pharmacol. 2014, 15, 97–106. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef]
- Lopes, L.C.S.; Brito, L.M.; Bezerra, T.T.; Gomes, K.N.; Carvalho, F.A.A.; Chaves, M.H.; Cantanhede, W. Silver and gold nanoparticles from tannic acid: Synthesis, characterization and evaluation of antileishmanial and cytotoxic activities. An. Acad. Bras. Cienc. 2018, 90, 2679–2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khadragy, M.; Alolayan, E.M.; Metwally, D.M.; El-Din, M.F.S.; Alobud, S.S.; Alsultan, N.I.; Alsaif, S.S.; Awad, M.A.; Abdel Moneim, A.E. Clinical Efficacy Associated with Enhanced Antioxidant Enzyme Activities of Silver Nanoparticles Biosynthesized Using Moringa oleifera Leaf Extract, Against Cutaneous Leishmaniasis in a Murine Model of Leishmania major. Int. J. Environ. Res. Public Health 2018, 15, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, M.; Zaki, L.; KarimiPourSaryazdi, A.; Tavakoli, P.; Tavajjohi, A.; Poursalehi, R.; Delavari, H.; Ghaffarifar, F. Efficacy of green synthesized silver nanoparticles via ginger rhizome extract against Leishmania major in vitro. PLoS ONE 2021, 16, e0255571. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Khan, W. Immunopathological response of leukocytes against microfilariae and adult worms in white rats infected with Setaria cervi. Vet. World 2017, 10, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Ullah, R.; Uddin, I.; Zia, I.; Rehman, L.; Abidi, S.M.A. In vitro anthelmintic effect of biologically synthesized silver nanoparticles on liver amphistome, Gigantocotyle explanatum. Exp. Parasitol. 2019, 198, 95–104. [Google Scholar] [CrossRef]
- Vidal, J.E. HIV-Related Cerebral Toxoplasmosis Revisited: Current Concepts and Controversies of an Old Disease. J. Int. Assoc. Provid. AIDS Care 2019, 18, 2325958219867315. [Google Scholar] [CrossRef] [Green Version]
- Venjakob, P.L.; Thiele, G.; Clausen, P.H.; Nijhof, A.M. Toxocara vitulorum infection in German beef cattle. Parasitol. Res. 2017, 116, 1085–1088. [Google Scholar] [CrossRef]
- Li, K.; Lan, Y.; Luo, H.; Zhang, H.; Liu, D.; Zhang, L.; Gui, R.; Wang, L.; Shahzad, M.; Sizhu, S.; et al. Prevalence, Associated Risk Factors, and Phylogenetic Analysis of Toxocara vitulorum Infection in Yaks on the Qinghai Tibetan Plateau, China. Korean J. Parasitol. 2016, 54, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Rostami, A.; Riahi, S.M.; Contopoulos-Ioannidis, D.G.; Gamble, H.R.; Fakhri, Y.; Shiadeh, M.N.; Foroutan, M.; Behniafar, H.; Taghipour, A.; Maldonado, Y.A.; et al. Acute Toxoplasma infection in pregnant women worldwide: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007807. [Google Scholar] [CrossRef] [Green Version]
- Schoener, E.; Wechner, F.; Ebmer, D.; Shahi-Barogh, B.; Harl, J.; Glawischnig, W.; Fuehrer, H.P. Toxocara vitulorum infection in a yak (Bos mutus grunniens) calf from Tyrol (Austria). Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100370. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Q.; Liu, G.H.; Zheng, W.B.; Hong, S.J.; Sugiyama, H.; Zhu, X.Q.; Elsheikha, H.M. Toxocariasis: A silent threat with a progressive public health impact. Infect. Dis. Poverty 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-Aziza, F.A.M.; Zaki, A.K.A.; Alajaji, A.I.; Al Barrak, S.M. Bone marrow mesenchymal stem cell co-adjuvant therapy with albendazole for managing Toxocara vitulorum-rat model. Vet. World 2021, 14, 347–363. [Google Scholar] [CrossRef]
- Zhu, W.; Li, J.; Pappoe, F.; Shen, J.; Yu, L. Strategies Developed by Toxoplasma gondii to Survive in the Host. Front. Microbiol. 2019, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C. Targeting DHFR in parasitic protozoa. Drug Discov. Today 2005, 10, 121–128. [Google Scholar] [CrossRef]
- Petersen, E. Toxoplasmosis. Semin. Fetal Neonatal Med. 2007, 12, 214–223. [Google Scholar] [CrossRef]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced Nanobiomaterials: Vaccines, Diagnosis and Treatment of Infectious Diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef] [Green Version]
- Machado, L.F.; Sanfelice, R.A.; Bosqui, L.R.; Assolini, J.P.; Scandorieiro, S.; Navarro, I.T.; Depieri Cataneo, A.H.; Wowk, P.F.; Nakazato, G.; Bordignon, J.; et al. Biogenic silver nanoparticles reduce adherence, infection, and proliferation of toxoplasma gondii RH strain in HeLa cells without inflammatory mediators induction. Exp. Parasitol. 2020, 211, 107853. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Molefe, N.I.; Awakan, O.J.; Nwonuma, C.O.; Alejolowo, O.O.; Olaolu, T.; Maimako, R.F.; Suganuma, K.; Han, Y.; Kato, K. Metal nanoparticles restrict the growth of protozoan parasites. Artif. Cells Nanomed. Biotechnol. 2018, 46, S86–S94. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Hunter, C.A. The role of macrophages in protective and pathological responses to Toxoplasma gondii. Parasite Immunol. 2020, 42, e12712. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Kato, K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017, 12, 1647–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahaaeldine, M.A.; El Garhy, M.; Fahmy, S.R.; Mohamed, A.S. In vitro anti-Toxocara vitulorum effect of silver nanoparticles. J. Parasit. Dis. 2022, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Arruda da Silva Sanfelice, R.; Silva, T.F.; Tomiotto-Pellissier, F.; Bortoleti, B.; Lazarin-Bidoia, D.; Scandorieiro, S.; Nakazato, G.; de Barros, L.D.; Garcia, J.L.; Verri, W.A.; et al. Biogenic silver nanoparticles reduce Toxoplasma gondii infection and proliferation in RAW 264.7 macrophages by inducing tumor necrosis factor-alpha and reactive oxygen species production in the cells. Microbes Infect. 2022, 24, 104971. [Google Scholar] [CrossRef]
- Innes, E.A.; Chalmers, R.M.; Wells, B.; Pawlowic, M.C. A One Health Approach to Tackle Cryptosporidiosis. Trends Parasitol. 2020, 36, 290–303. [Google Scholar] [CrossRef] [Green Version]
- Bones, A.J.; Josse, L.; More, C.; Miller, C.N.; Michaelis, M.; Tsaousis, A.D. Past and future trends of Cryptosporidium in vitro research. Exp. Parasitol. 2019, 196, 28–37. [Google Scholar] [CrossRef]
- Nassar, S.A.; Oyekale, T.O.; Oluremi, A.S. Prevalence of Cryptosporidium infection and related risk factors in children in Awo and Iragberi, Nigeria. J. Immunoass. Immunochem. 2017, 38, 2–9. [Google Scholar] [CrossRef]
- Berahmat, R.; Mahami-Oskouei, M.; Rezamand, A.; Spotin, A.; Aminisani, N.; Ghoyounchi, R.; Madadi, S. Cryptosporidium infection in children with cancer undergoing chemotherapy: How important is the prevention of opportunistic parasitic infections in patients with malignancies? Parasitol. Res. 2017, 116, 2507–2515. [Google Scholar] [CrossRef]
- Abou Elez, R.M.M.; Attia, A.S.A.; Tolba, H.M.N.; Anter, R.G.A.; Elsohaby, I. Molecular identification and antiprotozoal activity of silver nanoparticles on viability of Cryptosporidium parvum isolated from pigeons, pigeon fanciers and water. Sci. Rep. 2023, 13, 3109. [Google Scholar] [CrossRef]
- Carter, B.L.; Stiff, R.E.; Elwin, K.; Hutchings, H.A.; Mason, B.W.; Davies, A.P.; Chalmers, R.M. Health sequelae of human cryptosporidiosis-a 12-month prospective follow-up study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1709–1717. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, C. Porcine deltacoronavirus induces caspase-dependent apoptosis through activation of the cytochrome c-mediated intrinsic mitochondrial pathway. Virus Res. 2018, 253, 112–123. [Google Scholar] [CrossRef]
- Hassan, Z.R.; Salama, D.E.A.; Ibrahim, H.F. Apoptotic changes in the intestinal epithelium of Cryptosporidium-infected mice after silver nanoparticles treatment versus nitazoxanide. J. Parasit. Dis. 2022, 46, 1011–1020. [Google Scholar] [CrossRef]
- Knopp, S.; Steinmann, P.; Keiser, J.; Utzinger, J. Nematode infections: Soil-transmitted helminths and trichinella. Infect. Dis. Clin. N. Am. 2012, 26, 341–358. [Google Scholar] [CrossRef]
- WHO Expert Committee. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis: World Health Organization Technical Report Series; WHO Expert Committee: Geneva, Switzerland, 2002; Volume 912, pp. 1–57. [Google Scholar]
- Singh, R.; Bal, M.S.; Singla, L.D.; Kaur, P. Detection of anthelmintic resistance in sheep and goat against fenbendazole by faecal egg count reduction test. J. Parasit. Dis. 2017, 41, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Albonico, M.; Behnke, J.M.; Kotze, A.C.; Prichard, R.K.; McCarthy, J.S.; Montresor, A.; Levecke, B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int. J. Parasitol. Drugs Drug. Resist. 2011, 1, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullner, A.; Helfer, A.; Kotlyar, D.; Oswald, J.; Efferth, T. Chemistry and pharmacology of neglected helminthic diseases. Curr. Med. Chem. 2011, 18, 767–789. [Google Scholar] [CrossRef]
- Fernandes, B.; Matama, T.; Andreia, C.G.; Cavaco-Paulo, A. Cyclosporin A-loaded poly(d,l-lactide) nanoparticles: A promising tool for treating alopecia. Nanomedicine 2020, 15, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Rajamanikam, A.; Kumar, S.; Samudi, C.; Kudva, M. Exacerbated symptoms in Blastocystis sp.-infected patients treated with metronidazole: Two case studies. Parasitol. Res. 2018, 117, 2585–2590. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Younis, M.S.; Abououf, E.; Ali, A.E.S.; Abd Elhady, S.M.; Wassef, R.M. In vitro Effect of Silver Nanoparticles on Blastocystis hominis. Int. J. Nanomed. 2020, 15, 8167–8173. [Google Scholar] [CrossRef]
- Lim, D.; Roh, J.Y.; Eom, H.J.; Choi, J.Y.; Hyun, J.; Choi, J. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2012, 31, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.M.; Silva, L.P.C.; de Freitas Soares, F.E.; Souza, R.L.O.; Tobias, F.L.; de Araujo, J.V.; Veloso, F.B.R.; Laviola, F.P.; Endringer, D.C.; de Gives, P.M.; et al. Effect of silver nanoparticles (AgNP’s) from Duddingtonia flagrans on cyathostomins larvae (subfamily: Cyathostominae). J. Invertebr. Pathol. 2020, 174, 107395. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraishy, S.; Murshed, M.; Delic, D.; Al-Shaebi, E.M.; Qasem, M.A.A.; Mares, M.M.; Dkhil, M.A. Plasmodium chabaudi-infected mice spleen response to synthesized silver nanoparticles from Indigofera oblongifolia extract. Lett. Appl. Microbiol. 2020, 71, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Roy, P.; Saini, P.; Mondal, M.K.; Chowdhury, P.; Sinha Babu, S.P. Carbohydrate polymer inspired silver nanoparticles for filaricidal and mosquitocidal activities: A comprehensive view. Carbohydr. Polym. 2016, 137, 390–401. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, H.; Hakim; Yang, X.; Zhang, X. A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol. Plant. 2018, 162, 439–454. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- Flores-Lopez, L.Z.; Espinoza-Gomez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, M.; Lin, Z.; Zhao, M.; Xiao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Polyethylenimine-functionalized silver nanoparticle-based co-delivery of paclitaxel to induce HepG2 cell apoptosis. Int. J. Nanomed. 2016, 11, 6693–6702. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Li, P.; Lou, J.; Zhang, H. Surface coating-modulated toxic responses to silver nanoparticles in Wolffia globosa. Aquat. Toxicol. 2017, 189, 150–158. [Google Scholar] [CrossRef]
- Ghorbani, M.; Fekrvand, S.; Shahkarami, S.; Yazdani, R.; Sohani, M.; Shaghaghi, M.; Hassanpour, G.; Mohammadi, J.; Negahdari, B.; Abolhassani, H.; et al. The evaluation of neutropenia in common variable immune deficiency patients. Expert. Rev. Clin. Immunol. 2019, 15, 1225–1233. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Gong, J.; Jiang, Y.; Long, Y.; Lei, W.; Gao, X.; Guo, D. Application of Silver Nanoparticles in Parasite Treatment. Pharmaceutics 2023, 15, 1783. https://doi.org/10.3390/pharmaceutics15071783
Zhang P, Gong J, Jiang Y, Long Y, Lei W, Gao X, Guo D. Application of Silver Nanoparticles in Parasite Treatment. Pharmaceutics. 2023; 15(7):1783. https://doi.org/10.3390/pharmaceutics15071783
Chicago/Turabian StyleZhang, Ping, Jiahao Gong, Yan Jiang, Yunfeng Long, Weiqiang Lei, Xiuge Gao, and Dawei Guo. 2023. "Application of Silver Nanoparticles in Parasite Treatment" Pharmaceutics 15, no. 7: 1783. https://doi.org/10.3390/pharmaceutics15071783



