The Development and Characterization of Novel Ionic Liquids Based on Mono- and Dicarboxylates with Meglumine for Drug Solubilizers and Skin Permeation Enhancers
Abstract
:1. Introduction
2. Materials and Methods
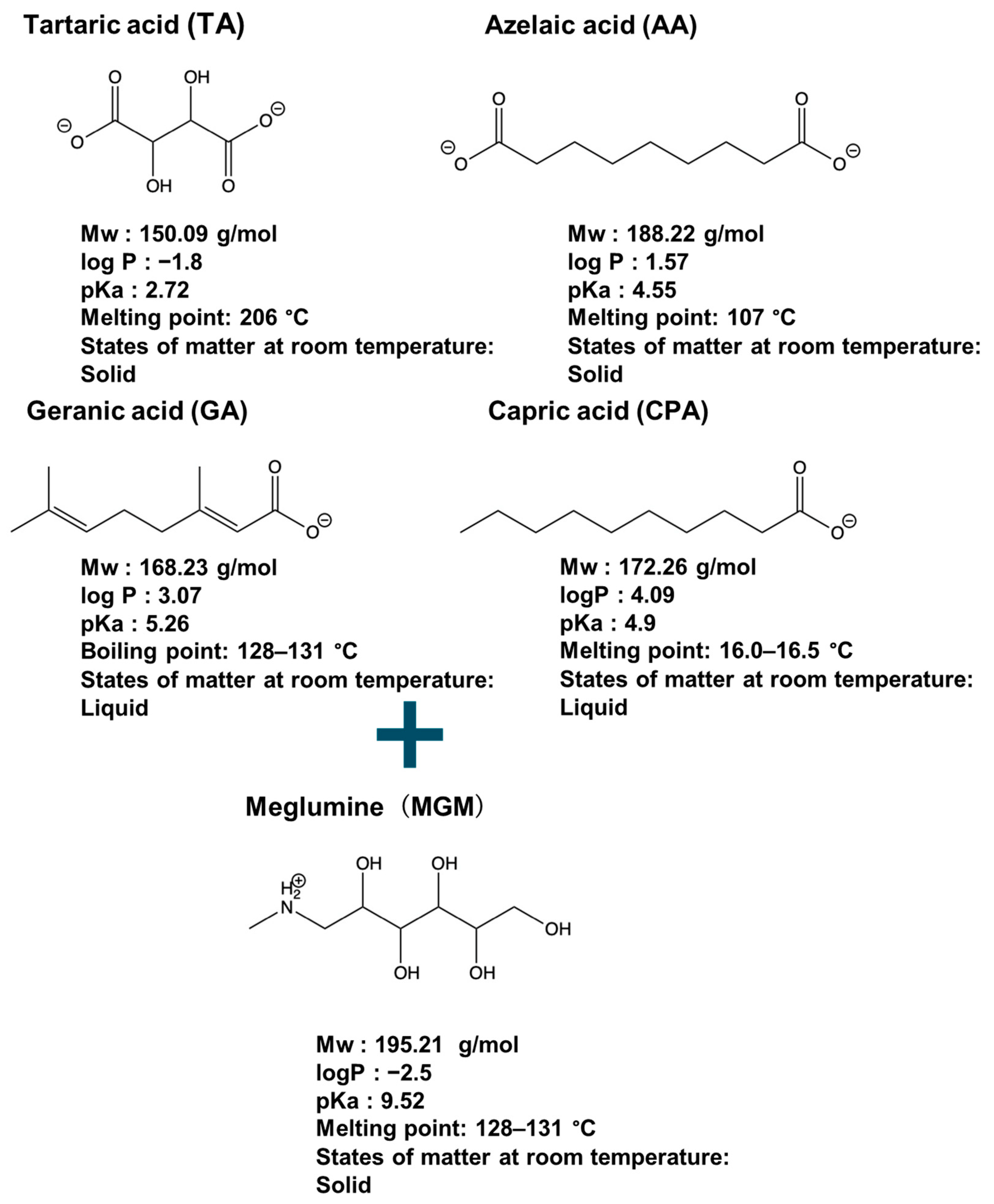
2.1. Materials
2.2. Preparation of ILs between Organic Acids and MGM
2.3. Characterization of ILs between Organic Acids and MGM
2.3.1. Attenuated Total Reflection (ATR)–Fourier Transform Infrared (ATR-FTIR) Measurements
2.3.2. DSC Measurements
2.4. Solubility Capability Examination of Drug-In ILs between Organic Acids and MGM
2.5. Skin Permeation Studies
2.6. Analytical Method
2.7. ATR-FTIR Assessment of the SC Samples
2.8. Statical Analysis
3. Results and Discussion
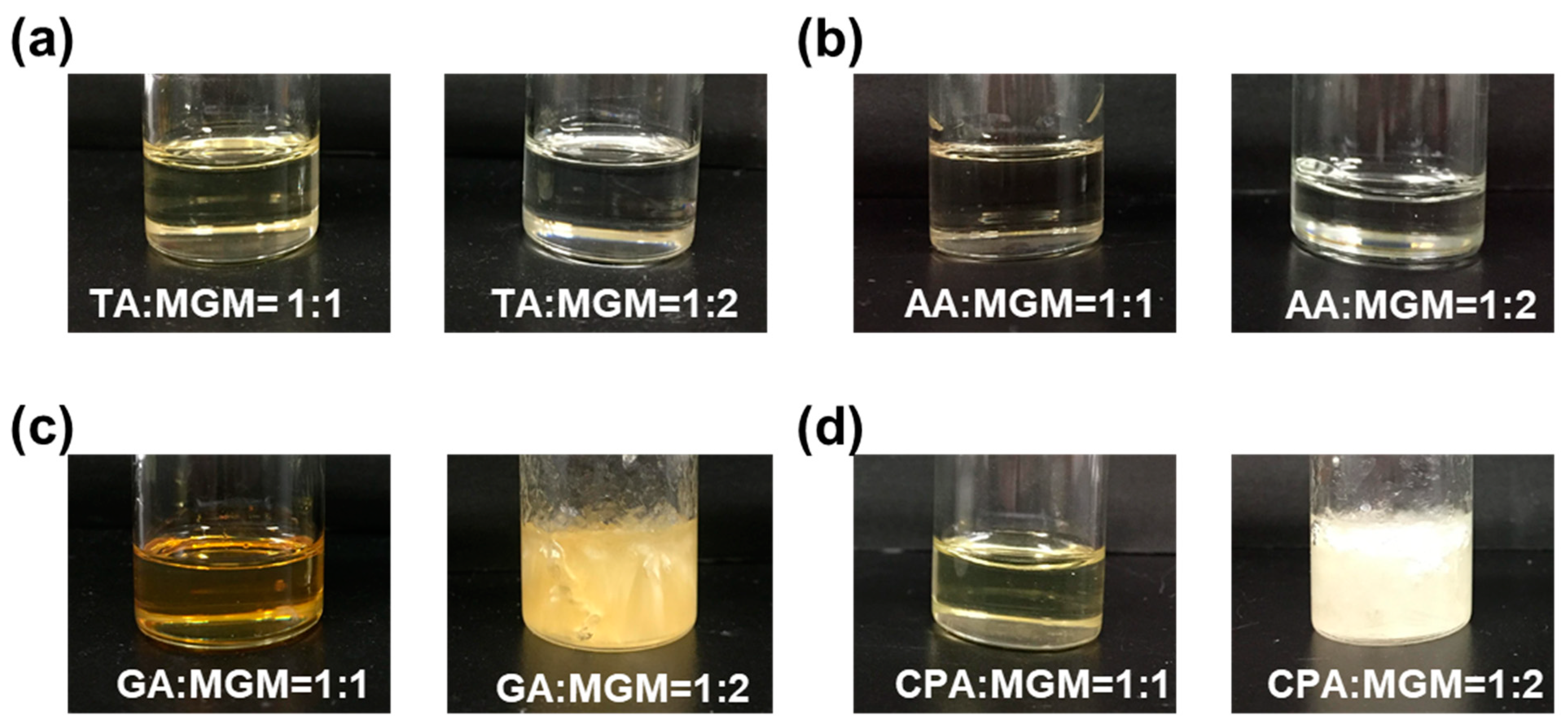
3.1. Preparation of MGM-ILs
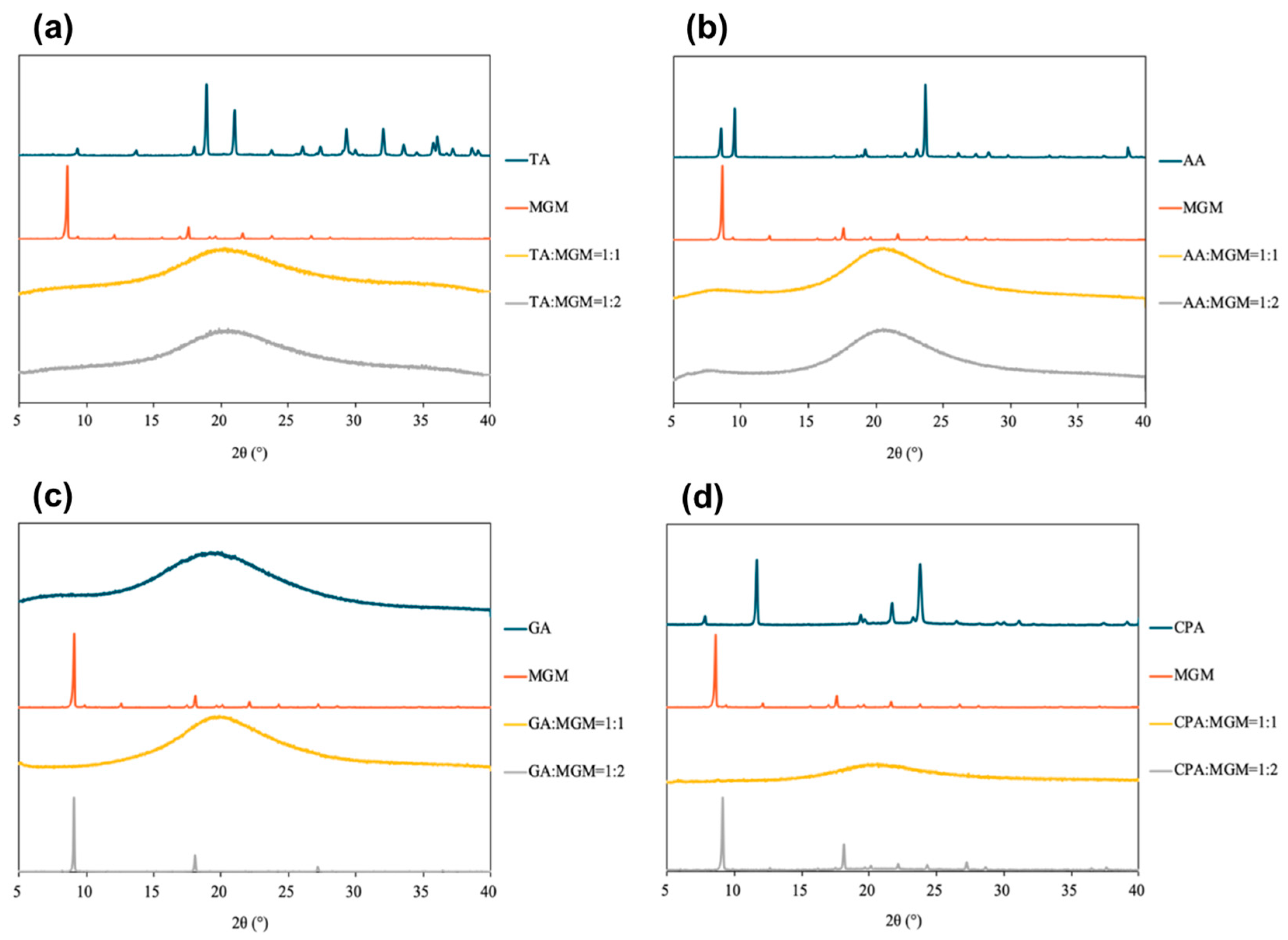
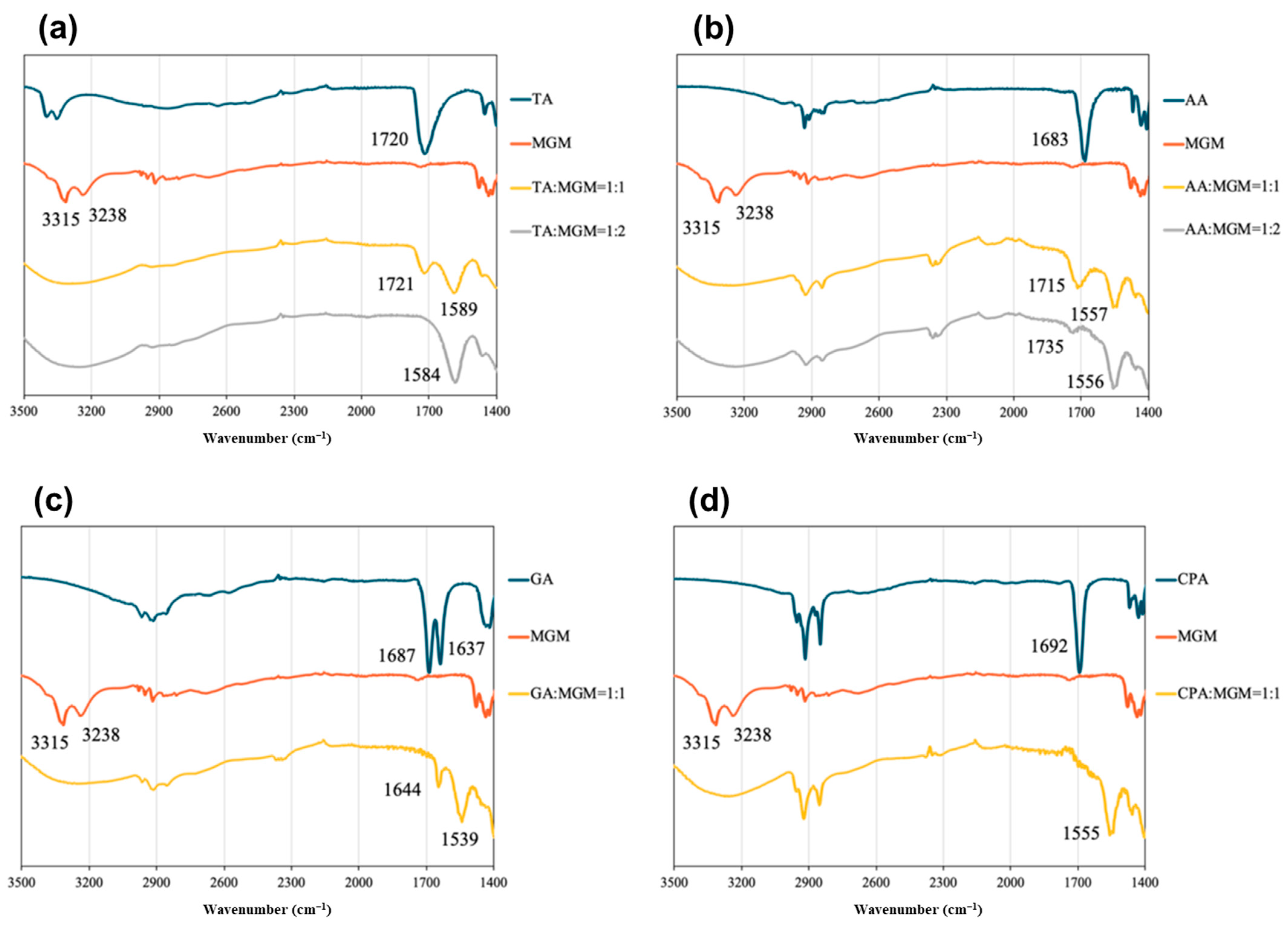
3.2. Characterization of MGM-ILs
3.3. MGM-ILs’ Solubility
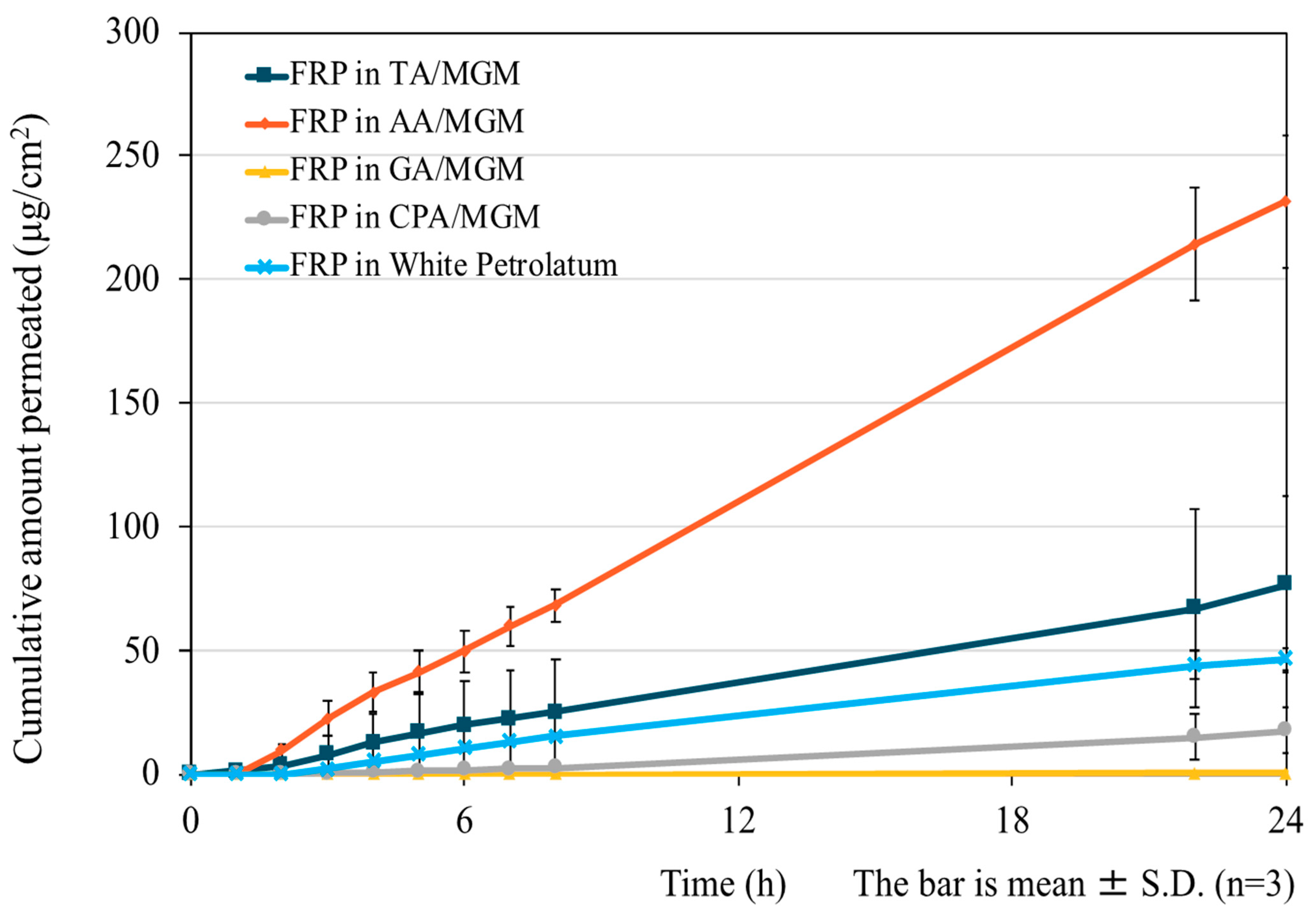
3.4. In Vitro Skin Permeation Test
3.5. ATR-FTIR Assessment of the MGM-IL-Treated SC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Tao, D.-J.; Cheng, Z.; Chen, F.-F.; Li, Z.-M.; Hu, N.; Chen, X.-S. Synthesis and Thermophysical Properties of Biocompatible Cholinium-Based Amino Acid Ionic Liquids. J. Chem. Eng. Data 2013, 58, 1542–1548. [Google Scholar] [CrossRef]
- Ossowicz, P.; Klebeko, J.; Roman, B.; Janus, E.; Rozwadowski, Z. The Relationship between the Structure and Properties of Amino Acid Ionic Liquids. Molecules 2019, 24, 3252. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Basak, D.; Hopefl, R.; Minofar, B. Potential Application of Ionic Liquids in Pharmaceutical Dosage Forms for Small Molecule Drug and Vaccine Delivery System. J. Pharm. Pharm. Sci. 2020, 23, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Schmidts, T.; Zinecker, C.; Schlupp, P.; Schafer, J.; Runkel, F. Hydrophilic Ionic Liquids as Ingredients of Gel-Based Dermal Formulations. AAPS PharmSciTech 2016, 17, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R. Applicability of Ionic Liquids in Topical Drug Delivery Systems: A Mini Review. J. Pharmco Clin. Res. 2017, 4, 555649. [Google Scholar] [CrossRef]
- Petrovic, Z.D.; Markovic, S.; Petrovic, V.P.; Simijonovic, D. Triethanolammonium acetate as a multifunctional ionic liquid in the palladium-catalyzed green Heck reaction. J. Mol. Model. 2012, 18, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.M.; Charnay, C.; De Angelis, F.; Lamaty, F.; Martinez, J.; Colacino, E. Poly(ethylene glycol)-based ionic liquids: Properties and uses as alternative solvents in organic synthesis and catalysis. ChemSusChem 2014, 7, 45–65. [Google Scholar] [CrossRef]
- Kumar, R.; Saima; Shard, A.; Andhare, N.H.; Richa; Sinha, A.K. Thiol-ene “click” reaction triggered by neutral ionic liquid: The “ambiphilic” character of [hmim]Br in the regioselective nucleophilic hydrothiolation. Angew. Chem. Int. Ed. Engl. 2015, 54, 828–832. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; FA, E.S.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, G.; Li, N. Ionic Liquid Solutions as a Green Tool for the Extraction and Isolation of Natural Products. Molecules 2018, 23, 1765. [Google Scholar] [CrossRef]
- Sas, O.G.; Dominguez, I.; Gonzalez, B.; Dominguez, A. Liquid-liquid extraction of phenolic compounds from water using ionic liquids: Literature review and new experimental data using [C(2)mim]FSI. J. Environ. Manag. 2018, 228, 475–482. [Google Scholar] [CrossRef]
- Dai, Z.; Xiao, Y.; Yu, X.; Mai, Z.; Zhao, X.; Zou, X. Direct electrochemistry of myoglobin based on ionic liquid-clay composite films. Biosens. Bioelectron. 2009, 24, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Z.; Fu, Y.C.; Wei, Y.M.; Yan, J.W.; Mao, B.W. The electrode/ionic liquid interface: Electric double layer and metal electrodeposition. Chemphyschem 2010, 11, 2764–2778. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.; Bolzinger, M.A.; Fessi, H.; Briancon, S. Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur. J. Dermatol. EJD 2009, 19, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. Ionic Liquids in Drug Delivery. Encyclopedia 2021, 1, 324–339. [Google Scholar] [CrossRef]
- Beaven, E.; Kumar, R.; An, J.M.; Mendoza, H.; Sutradhar, S.C.; Choi, W.; Narayan, M.; Lee, Y.K.; Nurunnabi, M. Potentials of ionic liquids to overcome physical and biological barriers. Adv. Drug Deliv. Rev. 2024, 204, 115157. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Goto, M. Ionic Liquids: Future Solvents and Reagents for Pharmaceuticals. J. Chem. Eng. Jpn. 2011, 44, 370–381. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Pedro, S.N.; CS, R.F.; Silvestre, A.J.D.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Lu, B.; Liu, T.; Wang, H.; Wu, C.; Chen, H.; Liu, Z.; Zhang, J. Ionic liquid transdermal delivery system: Progress, prospects, and challenges. J. Mol. Liq. 2022, 351, 118643. [Google Scholar] [CrossRef]
- Dobler, D.; Schmidts, T.; Klingenhofer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, Z.; Li, Y.; Yu, Q.; Lu, Y.; Zhu, Q.; Li, Y.; An, D.; Qi, J.; Wu, W. Improving dermal delivery of hydrophilic macromolecules by biocompatible ionic liquid based on choline and malic acid. Int. J. Pharm. 2019, 558, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Aguiar, L.; Ferraz, R.; Teixeira, C.; Gomes, P. The Emerging Role of Ionic Liquid-Based Approaches for Enhanced Skin Permeation of Bioactive Molecules: A Snapshot of the Past Couple of Years. Int. J. Mol. Sci. 2021, 22, 11991. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Chiappe, C.; Mele, A. Sugar-derived ionic liquids. Chimia 2011, 65, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zullo, V.; Iuliano, A.; Guazzelli, L. Sugar-Based Ionic Liquids: Multifaceted Challenges and Intriguing Potential. Molecules 2021, 26, 2052. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Valeeva, F.G.; Syakaev, V.V.; Lukashenko, S.S.; Zakharov, S.V.; Kuryashov, D.A.; Bashkirtseva, N.Y.; Zakharova, L.Y.; Latypov, S.K.; Sinyashin, O.G. Novel supramolecular system based on a cationic amphiphile bearing glucamine fragment: Structural behavior and hydrophobic probe binding. Mendeleev Commun. 2015, 25, 174–176. [Google Scholar] [CrossRef]
- Joshi, M.D.; Chalumot, G.; Kim, Y.-W.; Anderson, J.L. Synthesis of glucaminium-based ionic liquids and their application in the removal of boron from water. Chem. Commun. 2012, 48, 1410–1412. [Google Scholar] [CrossRef]
- Li, T.; Joshi, M.D.; Ronning, D.R.; Anderson, J.L. Ionic liquids as solvents for in situ dispersive liquid-liquid microextraction of DNA. J. Chromatogr. A 2013, 1272, 8–14. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, S.; Li, H.; Shan, Y.; Dou, A.; Shi, X.; Xu, G. A novel surface-confined glucaminium-based ionic liquid stationary phase for hydrophilic interaction/anion-exchange mixed-mode chromatography. J. Chromatogr. A 2014, 1360, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.D.; Li, T.; Zhong, Q.; Anderson, J.L. Using glucaminium-based ionic liquids for improving the separation of 2-aminopyrimidine-5-ylboronic acid and its pinacol ester by high performance liquid chromatography. J. Chromatogr. A 2013, 1308, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Miao, H.; Shi, J.; Hu, Z.; Li, G.; Ding, Y. The synthesis of carbon/cerium oxide composites clusters with the assistance of the glucaminium-based surfactant and their electrochemical performance in the glucose monitoring. J. Alloys Compd. 2017, 713, 125–131. [Google Scholar] [CrossRef]
- Zhao, M.; Li, T.; Jia, L.; Li, H.; Yuan, W.; Li, C.M. Pristine-Graphene-Supported Nitrogen-Doped Carbon Self-Assembled from Glucaminium-Based Ionic Liquids as Metal-Free Catalyst for Oxygen Evolution. ChemSusChem 2019, 12, 5041–5050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, M.; Wang, X.; Guo, Y.; Qiu, H.; Zhang, S. Glucaminium ionic liquid-functionalized stationary phase for the separation of nucleosides in hydrophilic interaction chromatography. Anal. Bioanal. Chem. 2015, 407, 7667–7672. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.D.; Steyer, D.J.; Anderson, J.L. Evaluating the complexation behavior and regeneration of boron selective glucaminium-based ionic liquids when used as extraction solvents. Anal. Chim. Acta 2012, 740, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.D.; Joshi, M.D.; Silver, M.A.; Anderson, J.L. Selective extraction of genotoxic impurities and structurally alerting compounds using polymeric ionic liquid sorbent coatings in solid-phase microextraction: Alkyl halides and aromatics. J. Chromatogr. A 2012, 1240, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, E.; Souza-Silva, É.A.; Ho, T.D.; Anderson, J.L.; Pawliszyn, J. Exploiting the tunable selectivity features of polymeric ionic liquid-based SPME sorbents in food analysis. Talanta 2018, 188, 522–530. [Google Scholar] [CrossRef]
- Dunn, P.J. The importance of green chemistry in process research and development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef]
- Keramatnia, F.; Jouyban, A.; Valizadeh, H.; Delazar, A.; Shayanfar, A. Ketoconazole ionic liquids with citric and tartaric acid: Synthesis, characterization and solubility study. Fluid. Phase Equilibria 2016, 425, 108–113. [Google Scholar] [CrossRef]
- Zotova, J.; Wojnarowska, Z.; Twamley, B.; Tajber, L. Formation of stoichiometric and non-stoichiometric ionic liquid and cocrystal multicomponent phases of lidocaine with azelaic acid by changing counterion ratios. J. Mol. Liq. 2021, 344, 117737. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control Release 2018, 286, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Guazzelli, L.; Burgalassi, S.; Carpi, S.; Chetoni, P.; Mezzetta, A.; Nieri, P.; Polini, B.; Pomelli, C.S.; Terreni, E.; et al. pH-Responsive Nanostructures Based on Surface Active Fatty Acid-Protic Ionic Liquids for Imiquimod Delivery in Skin Cancer Topical Therapy. Pharmaceutics 2020, 12, 1078. [Google Scholar] [CrossRef]
- Moumene, T.; Belarbi, E.H.; Haddad, B.; Villemin, D.; Abbas, O.; Khelifa, B.; Bresson, S. Study of imidazolium dicationic ionic liquids by Raman and FTIR spectroscopies: The effect of the nature of the anion. J. Mol. Struct. 2015, 1083, 179–186. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Zha, M.; Zhang, J.; Wang, S.-S. Synthesis, characterization and properties of geminal imidazolium ionic liquids. Colloids Surf. A Physicochem. Eng. 2007, 298, 201–205. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.Q.N.; Ferreira, R.; Leitão, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatible cholinium-based ionic liquids—Toxicity and biodegradability. Green. Chem. 2010, 12, 643. [Google Scholar] [CrossRef]
- Marsh, K.N.; Deev, A.; Wu, A.C.T.; Tran, E.; Klamt, A. Room temperature ionic liquids as replacements for conventional solvents—A review. Korean J. Chem. Eng. 2002, 19, 357–362. [Google Scholar] [CrossRef]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Synthesis and characterization of choline-fatty-acid-based ionic liquids: A new biocompatible surfactant. J. Colloid Interface Sci. 2019, 551, 72–80. [Google Scholar] [CrossRef]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Biocompatible Ionic Liquid Surfactant-Based Microemulsion as a Potential Carrier for Sparingly Soluble Drugs. ACS Sustain. Chem. Eng. 2020, 8, 6263–6272. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Rao, K.S.; Singh, T.; Mandal, S.K.; Sutradhar, N.; Panda, A.B.; Kumar, A. Task-specific, biodegradable amino acid ionic liquid surfactants. ChemSusChem 2011, 4, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic-Liquid-Based Paclitaxel Preparation: A New Potential Formulation for Cancer Treatment. Mol. Pharm. 2018, 15, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Chowdhury, M.R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic Liquid-In-Oil Microemulsions Prepared with Biocompatible Choline Carboxylic Acids for Improving the Transdermal Delivery of a Sparingly Soluble Drug. Pharmaceutics 2020, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, K.; Anbu, J.; Ravichandiran, V.; Venkateswarlu, V.; Rao, Y.M. Lipid nanoparticles for transdermal delivery of flurbiprofen: Formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis. 2009, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Quan, P.; Liu, X.; Guo, W.; Song, W.; Cun, D.; Wang, Z.; Fang, L. Enhancement of skin permeation of flurbiprofen via its transdermal patches using isopulegol decanoate (ISO-C10) as an absorption enhancer: Pharmacokinetic and pharmacodynamic evaluation. J. Pharm. Pharmacol. 2015, 67, 1232–1239. [Google Scholar] [CrossRef]
- Xu, Q.; Furuishi, T.; Fukuzawa, K.; Yonemochi, E. Physicochemical Properties and Transdermal Absorption of a Flurbiprofen and Lidocaine Complex in the Non-Crystalline Form. Pharmaceutics 2023, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Egiziano, E.; Burgalassi, S.; Chetoni, P.; Chiappe, C.; Sanzone, A.; Tampucci, S. Ionic liquids as potential enhancers for transdermal drug delivery. Int. J. Pharm. 2017, 516, 45–51. [Google Scholar] [CrossRef]
- Ali, M.K.; Moshikur, R.M.; Goto, M.; Moniruzzaman, M. Recent Developments in Ionic Liquid-Assisted Topical and Transdermal Drug Delivery. Pharm. Res. 2022, 39, 2335–2351. [Google Scholar] [CrossRef]
- Kovacik, A.; Kopecna, M.; Vavrova, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert. Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Boncheva, M.; Damien, F.; Normand, V. Molecular organization of the lipid matrix in intact Stratum corneum using ATR-FTIR spectroscopy. Biochim. Biophys. Acta 2008, 1778, 1344–1355. [Google Scholar] [CrossRef]
- Kumar, S.; Zakrewsky, M.; Chen, M.; Menegatti, S.; Muraski, J.A.; Mitragotri, S. Peptides as skin penetration enhancers: Mechanisms of action. J. Control Release 2015, 199, 168–178. [Google Scholar] [CrossRef]
- Schwarz, J.C.; Pagitsch, E.; Valenta, C. Comparison of ATR-FTIR spectra of porcine vaginal and buccal mucosa with ear skin and penetration analysis of drug and vehicle components into pig ear. Eur. J. Pharm. Sci. 2013, 50, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, J.; Zhang, D.; Yang, Y.; Zheng, L.; Qu, Y.; Yang, X.; Cui, X. Ionic liquid-microemulsions assisting in the transdermal delivery of Dencichine: Preparation, in-vitro and in-vivo evaluations, and investigation of the permeation mechanism. Int. J. Pharm. 2018, 535, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Chowdhury, M.R.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Choline and amino acid based biocompatible ionic liquid mediated transdermal delivery of the sparingly soluble drug acyclovir. Int. J. Pharm. 2020, 582, 119335. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, J.; Yin, T. Solubility and permeation enhancement of poor soluble drug by cholinium-amino acid based ionic liquids. J. Drug Deliv. Sci. Tech. 2020, 60, 102037. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]





| Drug | Structure | Log P | pKa | m.p. (°C) | Mw (g/mol) | CAS Number |
|---|---|---|---|---|---|---|
| Rutin | 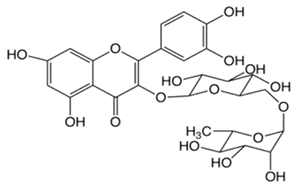 | −1.33 | 6.43 | 214–215 | 611 | 207671-50-9 |
| Ofloxacin (OFLX) | 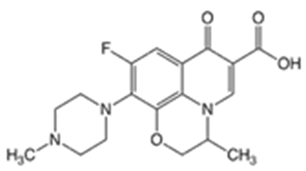 | −0.48 | pKa1 = 5.97 pKa2 = 9.28 | 270–275 | 361 | 82419-36-1 |
| Captopril (CPP) | 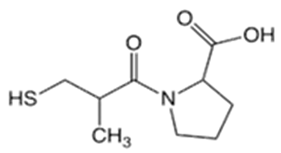 | 0.34 | 3.64 | 105–110 | 217 | 62571-86-2 |
| Minoxidil (MXD) |  | 1.24 | 4.61 | 272–274 | 209 | 38304-91-5 |
| Ferulic acid (FA) |  | 1.51 | 4.58 | 173–176 | 194 | 1135-24-6 |
| Flurbiprofen (FRP) | 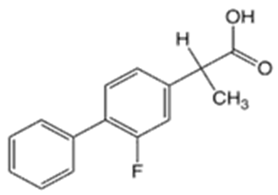 | 4.16 | 3.78 | 110–112 | 244 | 5104-49-4 |
| Isosorbide mononitrate (ISMN) |  | −0.15 | -- | 88–93 | 191 | 16051-77-7 |
| Allopurinol (ALP) |  | −1.80 | 9.34 | >300 | 136 | 315-30-0 |
| Atenolol (ATL) |  | 0.16 | 9.6 | 152–156 | 266 | 29122-68-7 |
| Ethenzamide (ETZ) |  | 1.02 | 13.7 | 270–273 | 165 | 938-73-8 |
| Carbamazepine (CBZ) |  | 2.54 | 7.00 | 189–193 | 236 | 298-46-4 |
| Disopyramide (DPA) |  | 2.58 | 8.36 | 85–87 | 339 | 3737-09-5 |
| Curcumin (CCM) |  | 3.29 | 8.09 | 179–185 | 368 | 458-37-7 |
| Carvedilol (CVD) |  | 4.19 | 7.8 | 114–119 | 406 | 72956-09-3 |
| Coenzyme Q10 (CoQ10) | 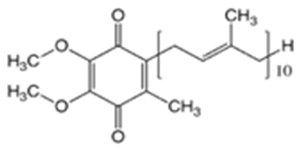 | 9.94 | 10.9 | 47–52 | 863 | 303-98-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuishi, T.; Taguchi, S.; Wang, S.; Fukuzawa, K.; Yonemochi, E. The Development and Characterization of Novel Ionic Liquids Based on Mono- and Dicarboxylates with Meglumine for Drug Solubilizers and Skin Permeation Enhancers. Pharmaceutics 2024, 16, 322. https://doi.org/10.3390/pharmaceutics16030322
Furuishi T, Taguchi S, Wang S, Fukuzawa K, Yonemochi E. The Development and Characterization of Novel Ionic Liquids Based on Mono- and Dicarboxylates with Meglumine for Drug Solubilizers and Skin Permeation Enhancers. Pharmaceutics. 2024; 16(3):322. https://doi.org/10.3390/pharmaceutics16030322
Chicago/Turabian StyleFuruishi, Takayuki, Sara Taguchi, Siran Wang, Kaori Fukuzawa, and Etsuo Yonemochi. 2024. "The Development and Characterization of Novel Ionic Liquids Based on Mono- and Dicarboxylates with Meglumine for Drug Solubilizers and Skin Permeation Enhancers" Pharmaceutics 16, no. 3: 322. https://doi.org/10.3390/pharmaceutics16030322







