Useful Role of a New Generation of Dexamethasone, Vitamin E and Human Serum Albumin Microparticles in the Prevention of Excitotoxicity Injury in Retinal Ocular Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microspheres
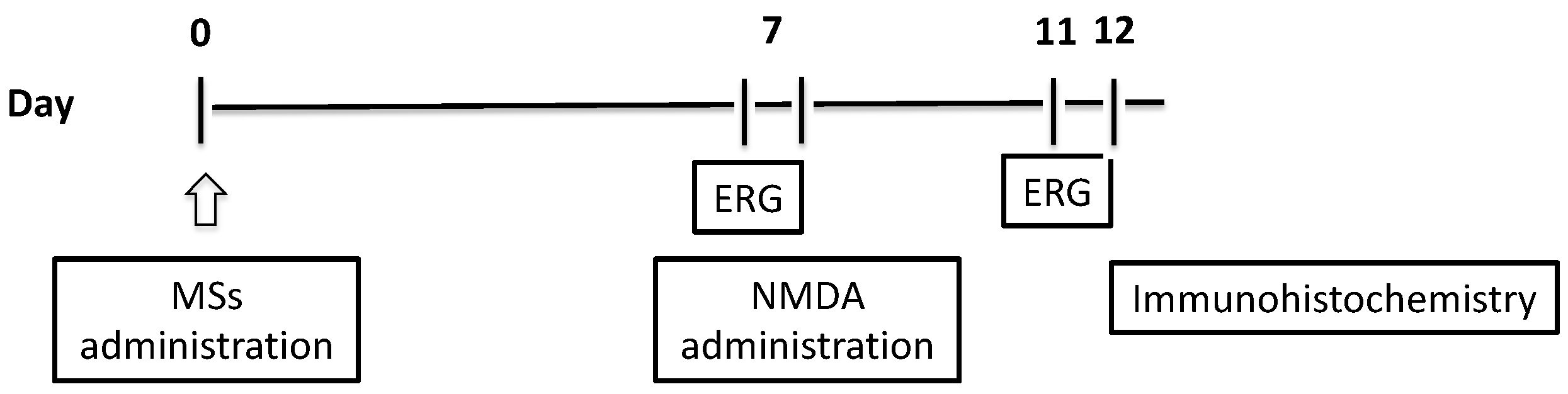
2.2. Animals and Treatments
2.2.1. Microsphere Assessment and Intravitreal Injection
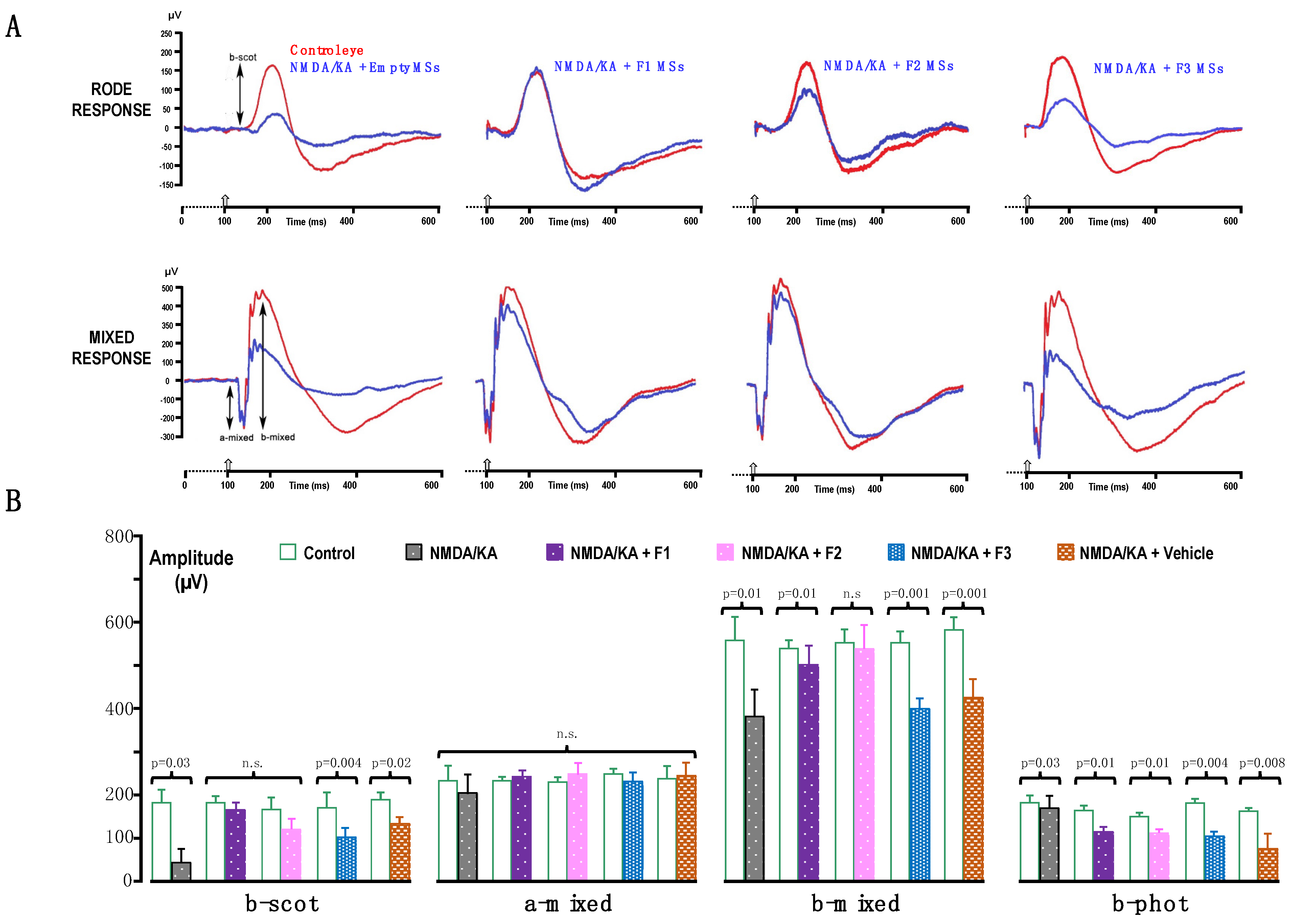
2.2.2. Electroretinogram (ERG) Recording
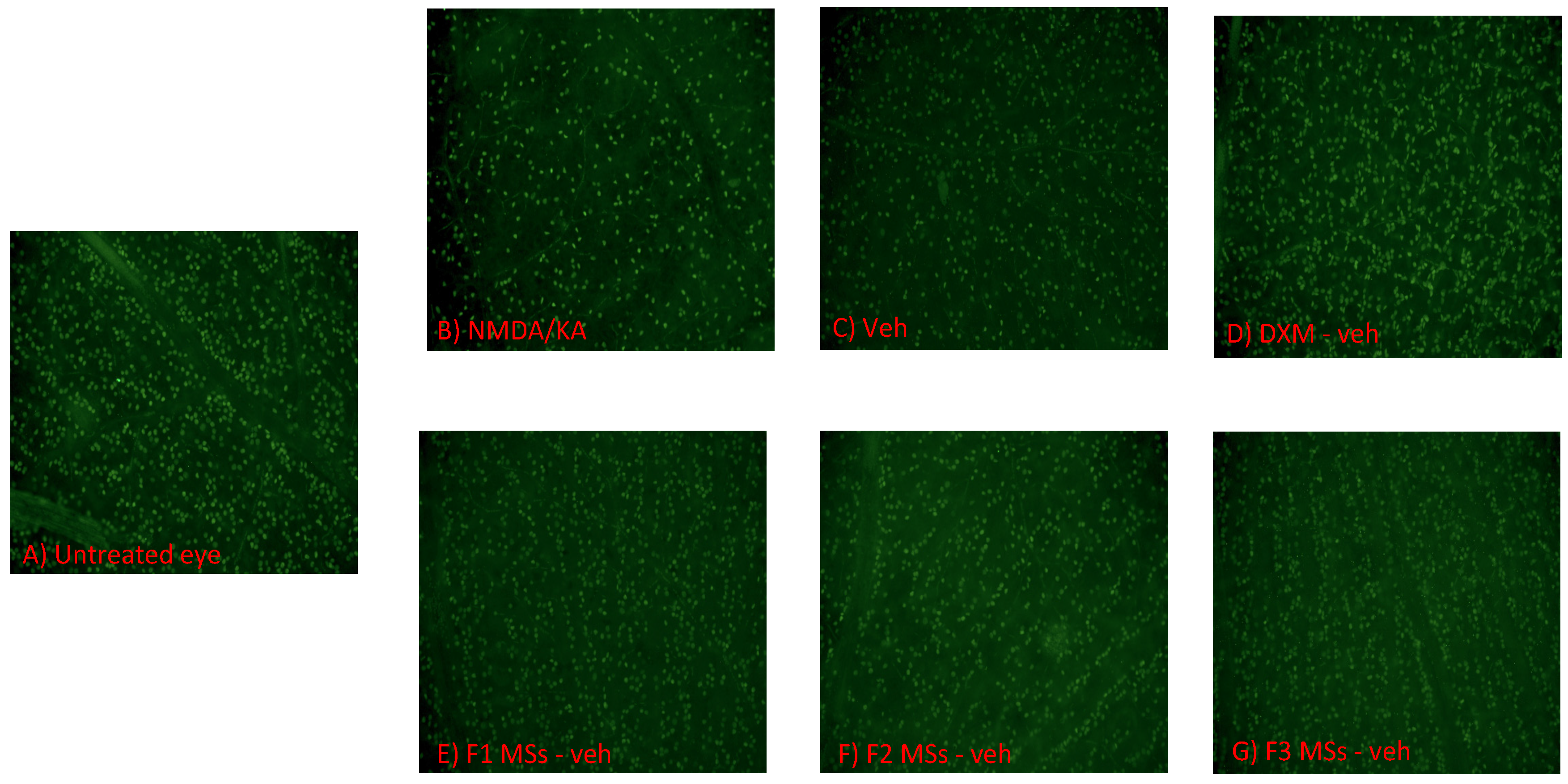
2.2.3. Immunohistochemistry
2.2.4. Confocal Microscopy and Quantification of Surviving RGCs
2.3. Statistical Analysis
2.4. Limitations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, R.; Cavaliere, F.; Varano, G.P.; Milanese, M.; Adornetto, A.; Nucci, C.; Bonanno, G.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G. Impairment of neuronal glutamate uptake and modulation of the glutamate transporter GLT-1 induced by retinal ischemia. PLoS ONE 2013, 8, e69250. [Google Scholar] [CrossRef]
- Rejdak, R.; Szkaradek, M.; Czepita, M.; Taslaq, W.; Lewicka-Chomont, A.; Grieb, P. Retinal degenerative diseases-mechanisms and perspectives of treatment. Klin. Oczna 2012, 114, 301–307. [Google Scholar]
- Galvao, J.; Elvas, F.; Martins, T.; Cordeiro, M.F.; Ambrósio, A.F.; Santiago, A.R. Adenosine A3 receptor activation is neuroprotective against retinal neurodegeneration. Exp. Eye Res. 2015, 140, 65–74. [Google Scholar] [CrossRef]
- Rodríguez Villanueva, J.; Martín Esteban, J.; Rodríguez Villanueva, L.J. Retinal Cell Protection in Ocular Excitotoxicity Diseases. Possible Alternatives Offered by Microparticulate Drug Delivery Systems and Future Prospects. Pharmaceutics 2020, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Chen, Q.; Olney, J.W. The intact isolated (ex vivo) retina as a model system for the study of excitotoxicity. Prog. Retin. Eye Res. 1998, 17, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Iwao, K.; Hayashi, H.; Kirihara, T.; Kawaji, T.; Inoue, T.; Hino, S.; Nakao, M.; Tanihara, H. Potential Neuroprotective Effects of an LSD1 Inhibitor in Retinal Ganglion Cells via p38 MAPK Activity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6461–6473. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.C.; Sande, P.; Marcos, H.A.; de Zavalía, N.; Keller Sarmiento, M.I.; Rosenstein, R.E. Effect of glaucoma on the retinal glutamate/glutamine cycle activity. FASEB J. 2005, 19, 1161–1162. [Google Scholar] [CrossRef]
- Fischer, A.J.; Seltner, R.L.; Poon, J.; Stell, W.K. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J. Comp. Neurol. 1998, 393, 1–15. [Google Scholar] [CrossRef]
- Calvo, E.; Milla-Navarro, S.; Ortuño-Lizarán, I.; Gómez-Vicente, V.; Cuenca, N.; De la Villa, P.; Germain, F. Deleterious Effect of NMDA Plus Kainate on the Inner Retinal Cells and Ganglion Cell Projection of the Mouse. Int. J. Mol. Sci. 2020, 21, 1570. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vicente, V.; Lax, P.; Fernández-Sánchez, L.; Rondón, N.; Esquiva, G.; Germain, F.; de la Villa, P.; Cuenca, N. Neuroprotective Effect of Tauroursodeoxycholic Acid on N-Methyl-D-Aspartate-Induced Retinal Ganglion Cell Degeneration. PLoS ONE 2015, 10, e0137826. [Google Scholar] [CrossRef]
- Baxter, P.S.; Bell, K.F.; Hasel, P.; Kaindl, A.M.; Fricker, M.; Thomson, D.; Cregan, S.P.; Gillingwater, T.H.; Hardingham, G.E. Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat. Commun. 2015, 6, 6761. [Google Scholar] [CrossRef]
- Vohra, R.; Tsai, J.C.; Kolko, M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013, 58, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Suzuki, Y.; Kurauchi, Y.; Mori, A.; Nakahara, T.; Ishii, K. Hydrogen sulfide attenuates NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp. Eye Res. 2014, 120, 90–96. [Google Scholar] [CrossRef]
- Maekawa, S.; Sato, K.; Kokubun, T.; Himori, N.; Yabana, T.; Ohno-Oishi, M.; Shi, G.; Omodaka, K.; Nakazawa, T. A Plant-Derived Antioxidant Supplement Prevents the Loss of Retinal Ganglion Cells in the Retinas of NMDA-Injured Mice. Clin. Ophthalmol. 2022, 16, 823–832. [Google Scholar] [CrossRef]
- Qiu, J.; Tan, Y.W.; Hagenston, A.M.; Martel, M.A.; Kneisel, N.; Skehel, P.A.; Wyllie, D.J.; Bading, H.; Hardingham, G.E. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat. Commun. 2013, 4, 2034. [Google Scholar] [CrossRef]
- Calvo, M.; Sanz-Blasco, S.; Caballero, E.; Villalobos, C.; Núñez, L. Susceptibility to excitotoxicity in aged hippocampal cultures and neuroprotection by non-steroidal anti-inflammatory drugs: Role of mitochondrial calcium. J. Neurochem. 2015, 132, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Villanueva, J.; Rodríguez Villanueva, L.; Guzmán Navarro, M. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int. J. Pharm. 2017, 516, 342–351. [Google Scholar] [CrossRef]
- Cáceres-del-Carpio, J.; Costa, R.D.; Haider, A.; Narayanan, R.; Kuppermann, B.D. Corticosteroids: Triamcinolone, Dexamethasone and Fluocinolone. Dev. Ophthalmol. 2016, 55, 221–231. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Skorik, Y.A. Dexamethasone Conjugates: Synthetic Approaches and Medical Prospects. Biomedicines 2021, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Villanueva, J.; Bravo-Osuna, I.; Herrero-Vanrell, R.; Molina Martínez, I.T.; Guzmán Navarro, M. Optimising the controlled release of dexamethasone from a new generation of PLGA-based microspheres intended for intravitreal administration. Eur. J. Pharm. Sci. 2016, 92, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Gavini, E.; Bonferoni, M.C.; Rassu, G.; Obinu, A.; Ferrari, F.; Giunchedi, P. Biodegradable Microspheres as Intravitreal Delivery Systems for Prolonged Drug Release. What is their Eminence in the Nanoparticle Era? Curr. Drug Deliv. 2018, 15, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Checa-Casalengua, P.; Jiang, C.; Bravo-Osuna, I.; Tucker, B.A.; Molina-Martínez, I.T.; Young, M.J.; Herrero-Vanrell, R. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J. Control. Release 2011, 156, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Chang-Lin, J.E.; Burke, J.A.; Peng, Q.; Lin, T.; Orilla, W.C.; Ghosn, C.R.; Zhang, K.M.; Kuppermann, B.D.; Robinson, M.R.; Whitcup, S.M.; et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4605–4609. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; Bravo-Osuna, I.; Andrés-Guerrero, V.; Vicario-de-la-Torre, M.; Molina-Martínez, I.T. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog. Retin. Eye Res. 2014, 42, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Sobrado-Calvo, P.; Nieto-López, L.; Cánovas-Martínez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Navarro, M.; Jiménez-López, M.; Valiente-Soriano, F.J.; Alarcón-Martínez, L.; Avilés-Trigueros, M.; Mayor, S.; Holmes, T.; Lund, R.D.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Retinal ganglion cell population in adult albino and pigmented mice: A computerized analysis of the entire population and its spatial distribution. Vis. Res. 2009, 49, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M. Abnormalities in glutamate metabolism and excitotoxicity in the retinal diseases. Scientifica 2013, 2013, 528940. [Google Scholar] [CrossRef]
- Schuettauf, F.; Naskar, R.; Vorwerk, C.K.; Zurakowski, D.; Dreyer, E.B. Ganglion cell loss after optic nerve crush mediated through AMPA-kainate and NMDA receptors. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4313–4316. [Google Scholar]
- Fletcher, E.L.; Hack, I.; Brandstätter, J.H.; Wässle, H. Synaptic localization of NMDA receptor subunits in the rat retina. J. Comp. Neurol. 2000, 420, 98–112. [Google Scholar] [CrossRef]
- Talas, D.U.; Nayci, A.; Polat, G.; Atis, S.; Comelekoglu, U.; Bagdatoglu, O.T.; Bagdatoglu, C. The effects of dexamethasone on lipid peroxidation and nitric oxide levels on the healing of tracheal anastomoses: An experimental study in rats. Pharmacol. Res. 2002, 46, 265–271. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Chen, H.; Zhang, J.; Xu, C.; Li, J.; Li, M. Enhancement of ICAM-1 via the JAK2/STAT3 signaling pathway in a rat model of severe acute pancreatitis-associated lung injury. Exp. Ther. Med. 2016, 11, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bui, N.T.; Ho, M.T.; Kim, Y.M.; Cho, M.; Shin, D.B. Dexamethasone Inhibits TGF-beta1-Induced Cell Migration by Regulating the ERK and AKT Pathways in Human Colon Cancer Cells Via CYR61. Cancer Res. Treat. 2016, 48, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Osuna, I.; Andrés-Guerrero, V.; Pastoriza Abal, P.; Molina-Martínez, I.T.; Herrero-Vanrell, R. Pharmaceutical microscale and nanoscale approaches for efficient treatment of ocular diseases. Drug Deliv. Transl. Res. 2016, 6, 686–707. [Google Scholar] [CrossRef]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G.; Walters, J.W. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 514–522. [Google Scholar]
- Saszik, S.M.; Robson, J.G.; Frishman, L.J. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J. Physiol. 2002, 543, 899–916. [Google Scholar] [CrossRef]
- Seeliger, M.W.; Kretschmann, U.H.; Apfelstedt-Sylla, E.; Zrenner, E. Implicit time topography of multifocal electroretinograms. Investig. Ophthalmol. Vis. Sci. 1998, 39, 718–723. [Google Scholar]
- Wachtmeister, L. Oscillatory potentials in the retina: What do they reveal. Prog. Retin. Eye Res. 1998, 17, 485–521. [Google Scholar] [CrossRef] [PubMed]
- Prenek, L.; Boldizsar, F.; Kugyelka, R.; Ugor, E.; Berta, G.; Nemeth, P.; Berki, T. The regulation of the mitochondrial apoptotic pathway by glucocorticoid receptor in collaboration with Bcl-2 family proteins in developing T cells. Apoptosis 2016, 22, 239–253. [Google Scholar] [CrossRef]
- Russo, R.; Rotiroti, D.; Tassorelli, C.; Nucci, C.; Bagetta, G.; Bucci, M.G.; Corasaniti, M.T.; Morrone, L.A. Identification of novel pharmacological targets to minimize excitotoxic retinal damage. Int. Rev. Neurobiol. 2009, 85, 407–423. [Google Scholar] [CrossRef]
- Gallina, D.; Zelinka, C.P.; Cebulla, C.M.; Fischer, A.J. Activation of glucocorticoid receptors in Müller glia is protective to retinal neurons and suppresses microglial reactivity. Exp. Neurol. 2015, 273, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Germain, F.; Calvo, M.; de la Villa, P. Rabbit retinal ganglion cell survival after optic nerve section and its effect on the inner plexiform layer. Exp. Eye Res. 2004, 78, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, G.; Gong, P.; Wang, Z. The effects of dexamethasone on 17β-HSD1 levels at the rat optic nerve. Technol. Health Care 2019, 27, 357–365. [Google Scholar] [CrossRef] [PubMed]



| MSs | Composition | Loading (μg/mg MSs) (a) Dxm (b) HSA | Dxm Burst Release after 4 h (%) | Dxm Released after 7 Days (μg/mg MSs) | |||
|---|---|---|---|---|---|---|---|
| PLGA | Dxm | Vit. E | HSA | ||||
| Blank | 200 mg | - | - | - | - | - | - |
| F1 | 200 mg | 20 mg | - | - | (a) 90.5 ± 11.3 | 3.50 ± 0.79 | 36.26 ± 3.27 |
| F2 | 200 mg | 20 mg | 20 μg | - | (a) 80.9 ± 14.1 | 4.60 ± 1.64 | 28.31 ± 2.83 |
| F3 | 200 mg | 20 mg | 20 μg | 20 μg | (a) 84.8 ± 12.7 (b) 0.113 ± 0.001 | 1.36 ± 0.25 | 12.72 ± 1.31 |
| Group | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| Treatment | No treatment (NMDA/KA) | Hyaluronic acid (0.1% w/v) (vehicle) | Dxm and hyaluronic acid (0.1% w/v) | F1-MSs in hyaluronic acid (0.1% w/v) | F2-MSs in hyaluronic acid (0.1% w/v) | F3-MSs in hyaluronic acid (0.1% w/v) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Villanueva, J.; de la Villa, P.; Herrero-Vanrell, R.; Bravo-Osuna, I.; Guzmán-Navarro, M. Useful Role of a New Generation of Dexamethasone, Vitamin E and Human Serum Albumin Microparticles in the Prevention of Excitotoxicity Injury in Retinal Ocular Diseases. Pharmaceutics 2024, 16, 406. https://doi.org/10.3390/pharmaceutics16030406
Rodríguez Villanueva J, de la Villa P, Herrero-Vanrell R, Bravo-Osuna I, Guzmán-Navarro M. Useful Role of a New Generation of Dexamethasone, Vitamin E and Human Serum Albumin Microparticles in the Prevention of Excitotoxicity Injury in Retinal Ocular Diseases. Pharmaceutics. 2024; 16(3):406. https://doi.org/10.3390/pharmaceutics16030406
Chicago/Turabian StyleRodríguez Villanueva, Javier, Pedro de la Villa, Rocío Herrero-Vanrell, Irene Bravo-Osuna, and Manuel Guzmán-Navarro. 2024. "Useful Role of a New Generation of Dexamethasone, Vitamin E and Human Serum Albumin Microparticles in the Prevention of Excitotoxicity Injury in Retinal Ocular Diseases" Pharmaceutics 16, no. 3: 406. https://doi.org/10.3390/pharmaceutics16030406





