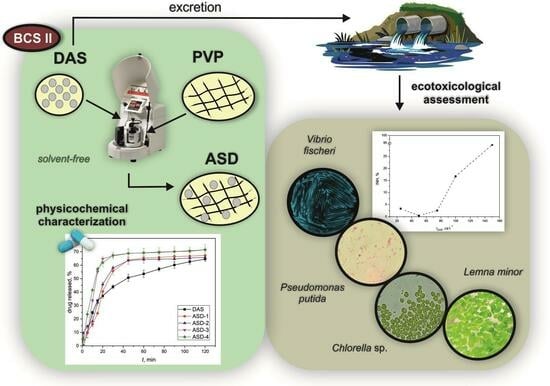
Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Amorphous Solid Dispersions
2.2.2. Differential Scanning Calorimetry
2.2.3. Fourier Transform Infrared Spectroscopy
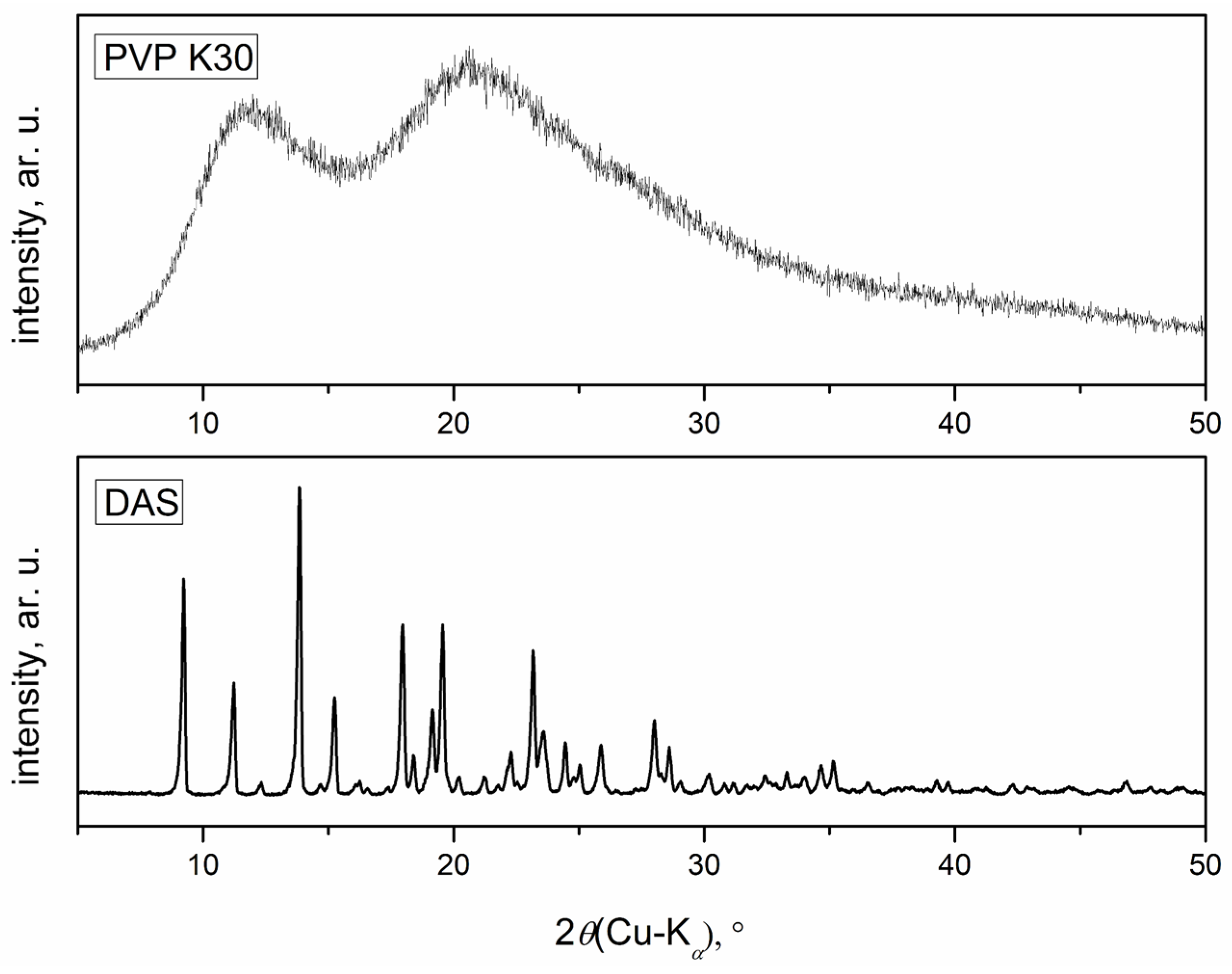
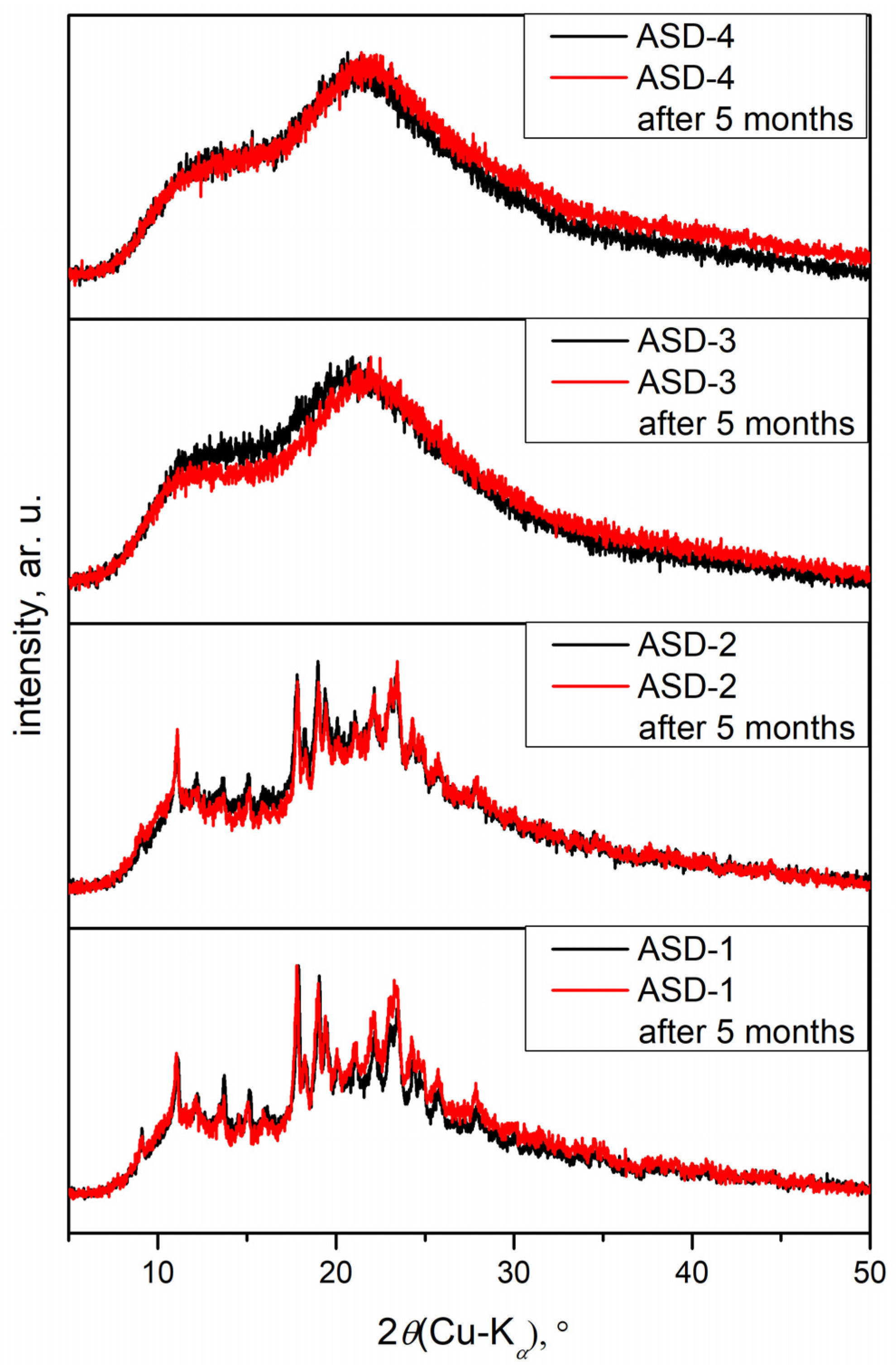
2.2.4. X-ray Powder Diffraction
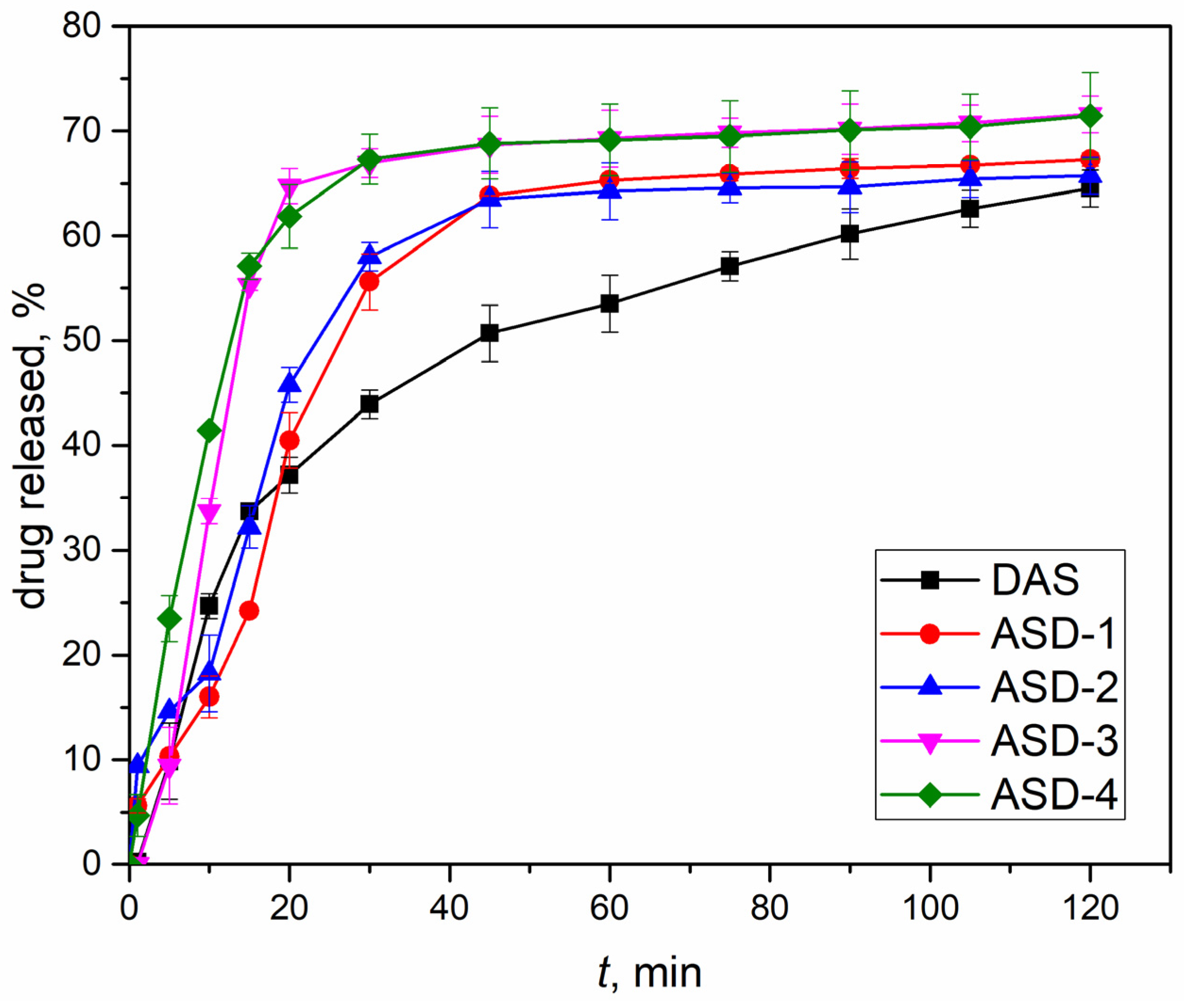
2.2.5. Drug Release
2.2.6. Ecotoxicity Tests with Bacterium Vibrio fischeri
2.2.7. Ecotoxicity Tests with Bacterium Pseudomonas putida
2.2.8. Ecotoxicity Tests with Chlorella sp.
2.2.9. Ecotoxicity Tests with Lemna minor
3. Results and Discussion
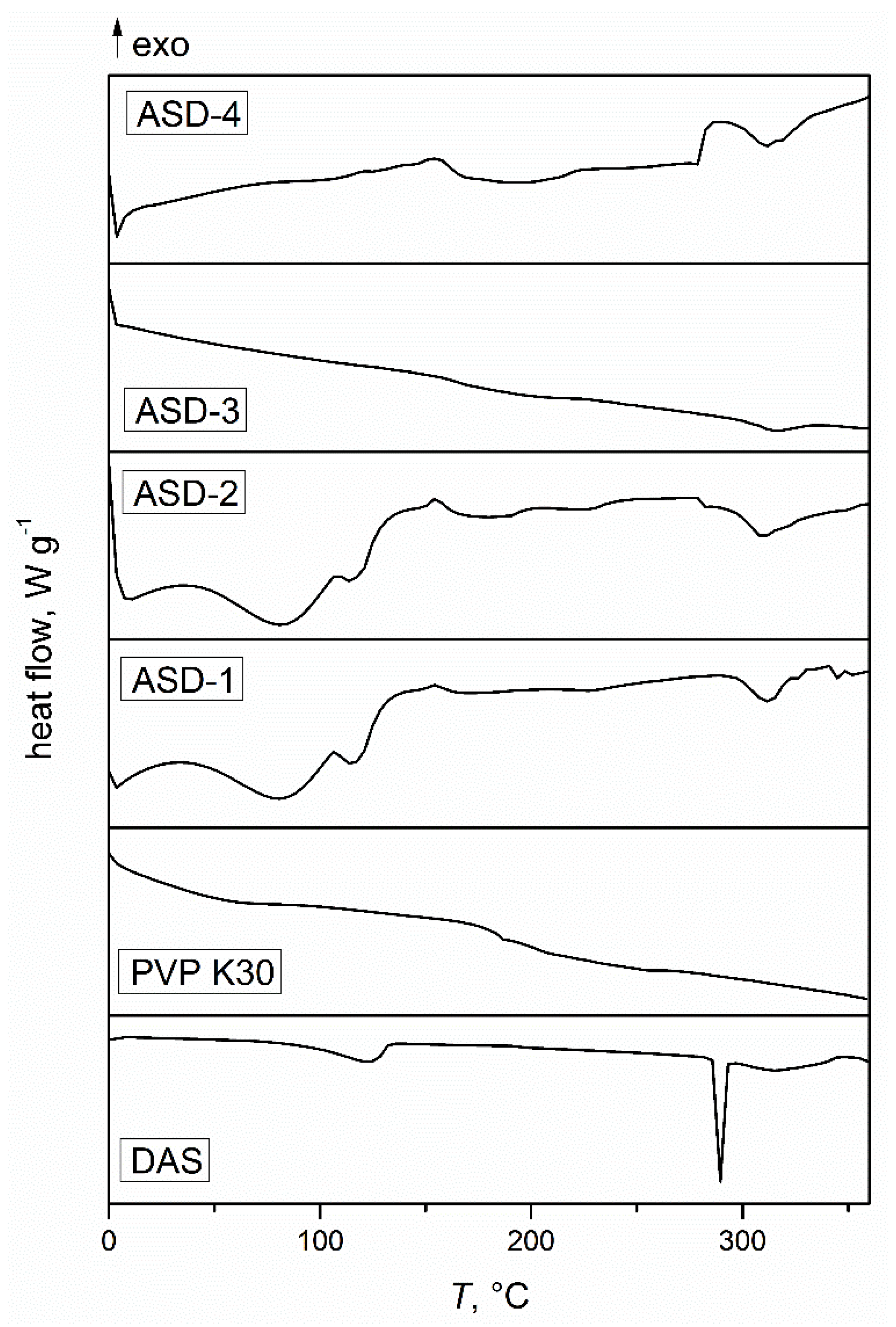
3.1. Differential Scanning Calorimetry
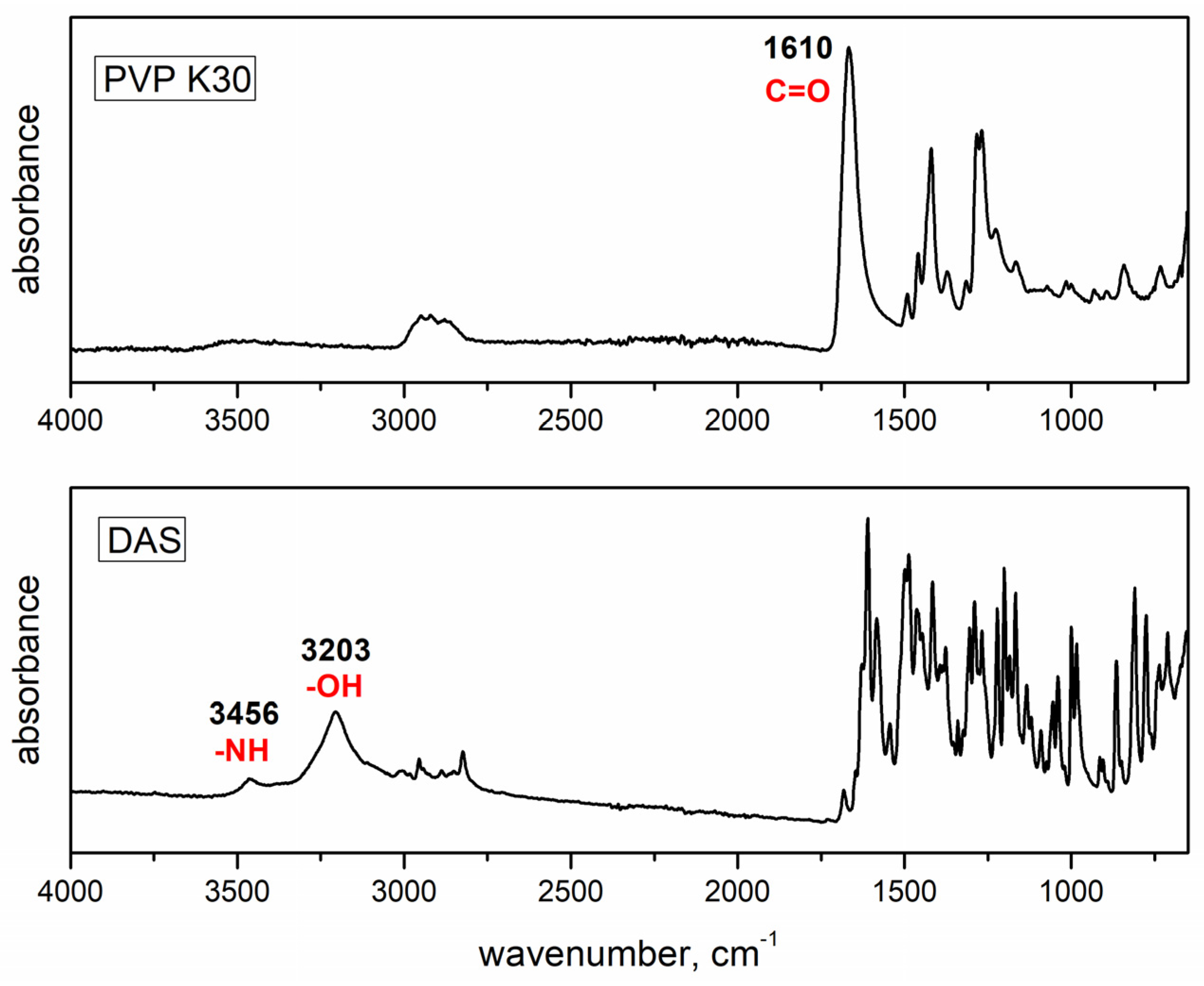
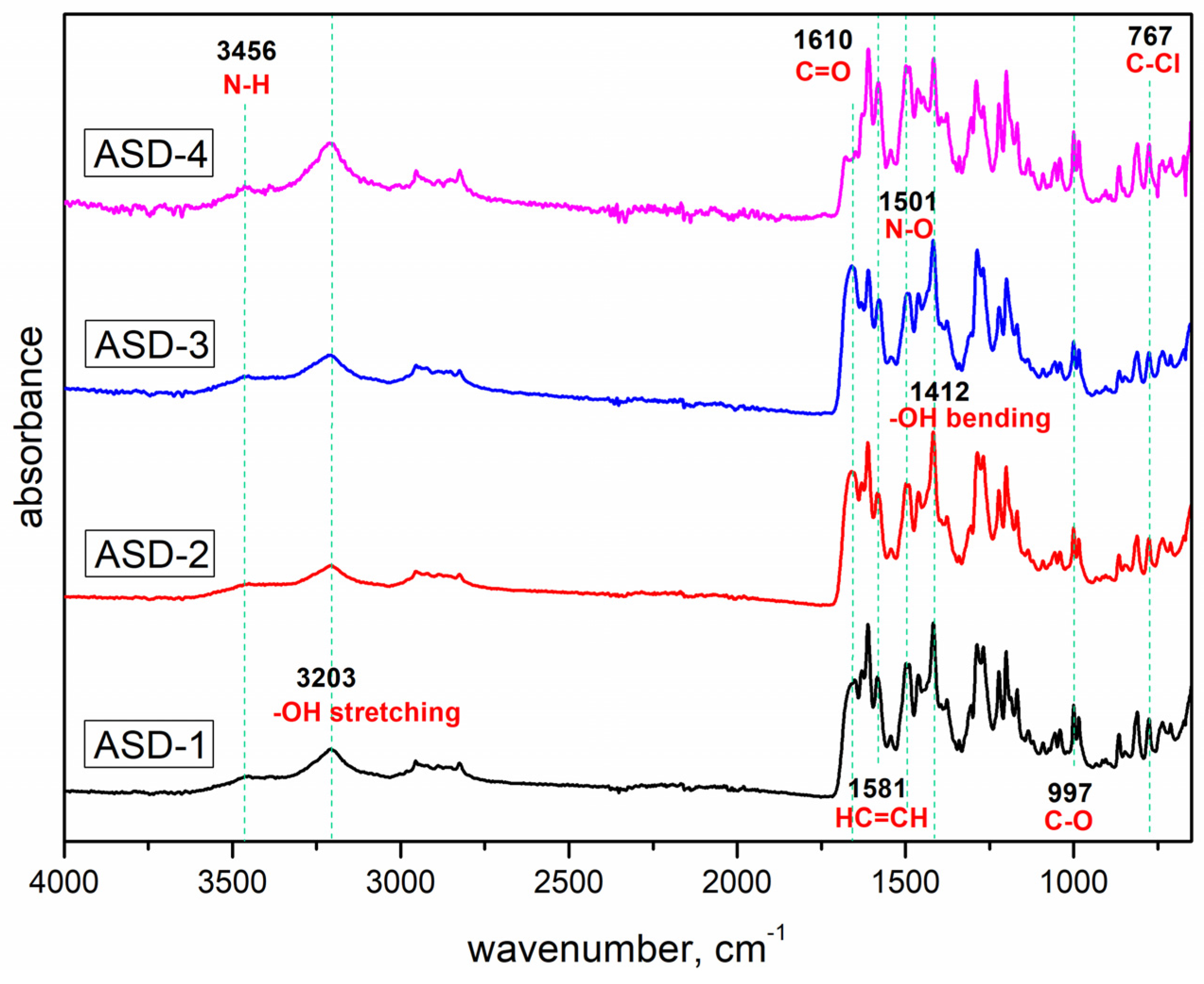
3.2. Fourier Transform Infrared Spectroscopy
3.3. X-ray Powder Diffraction
3.4. Drug Release
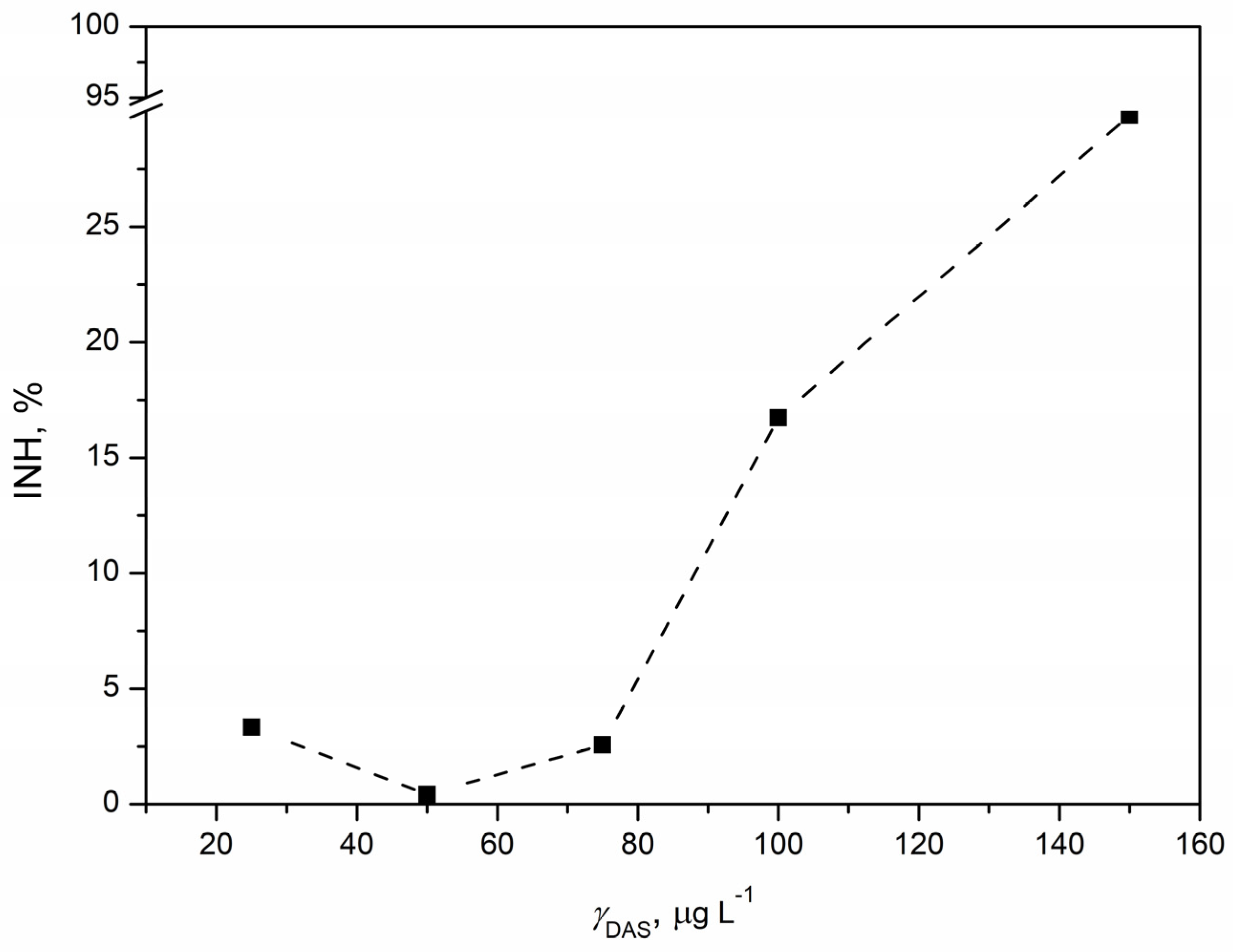
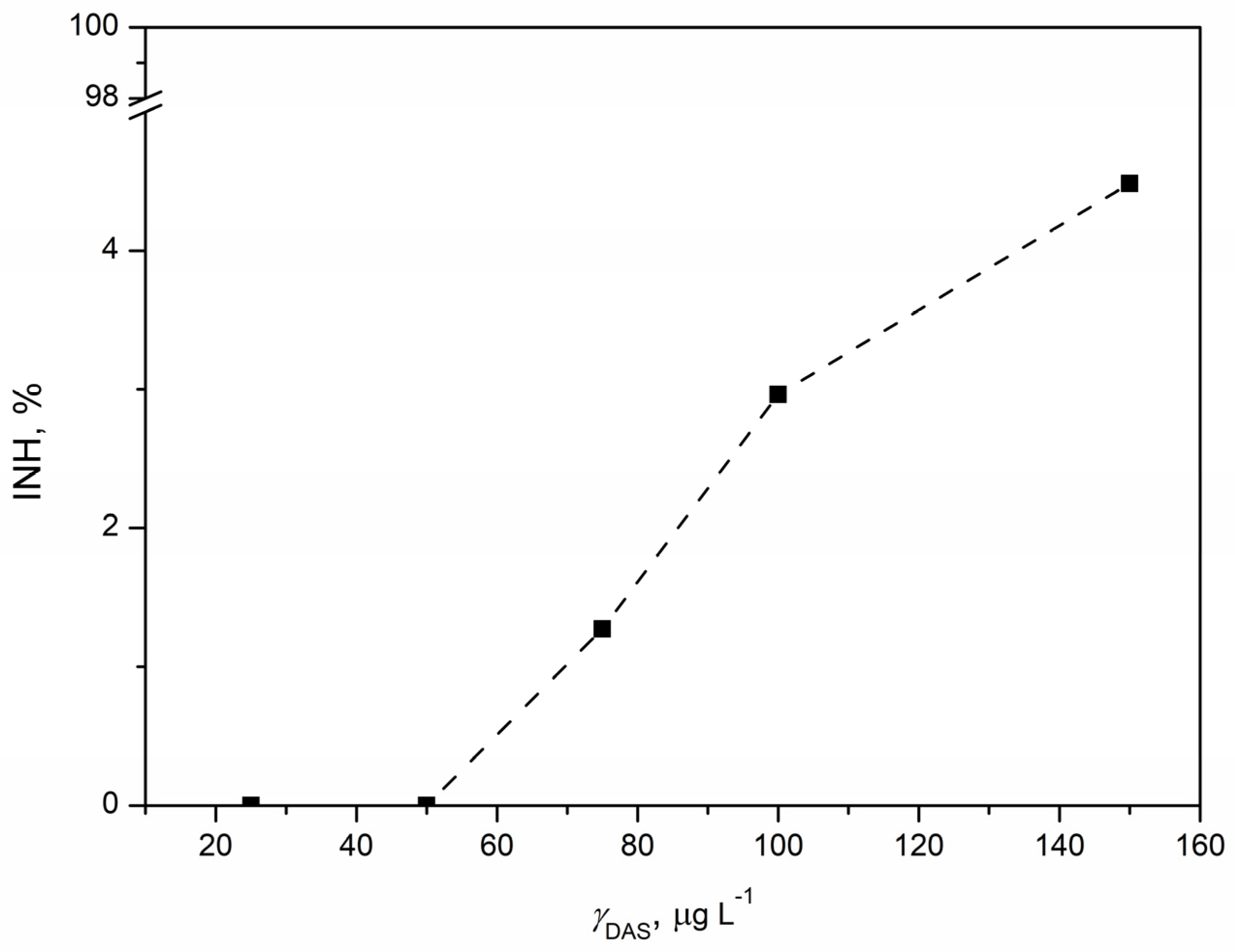
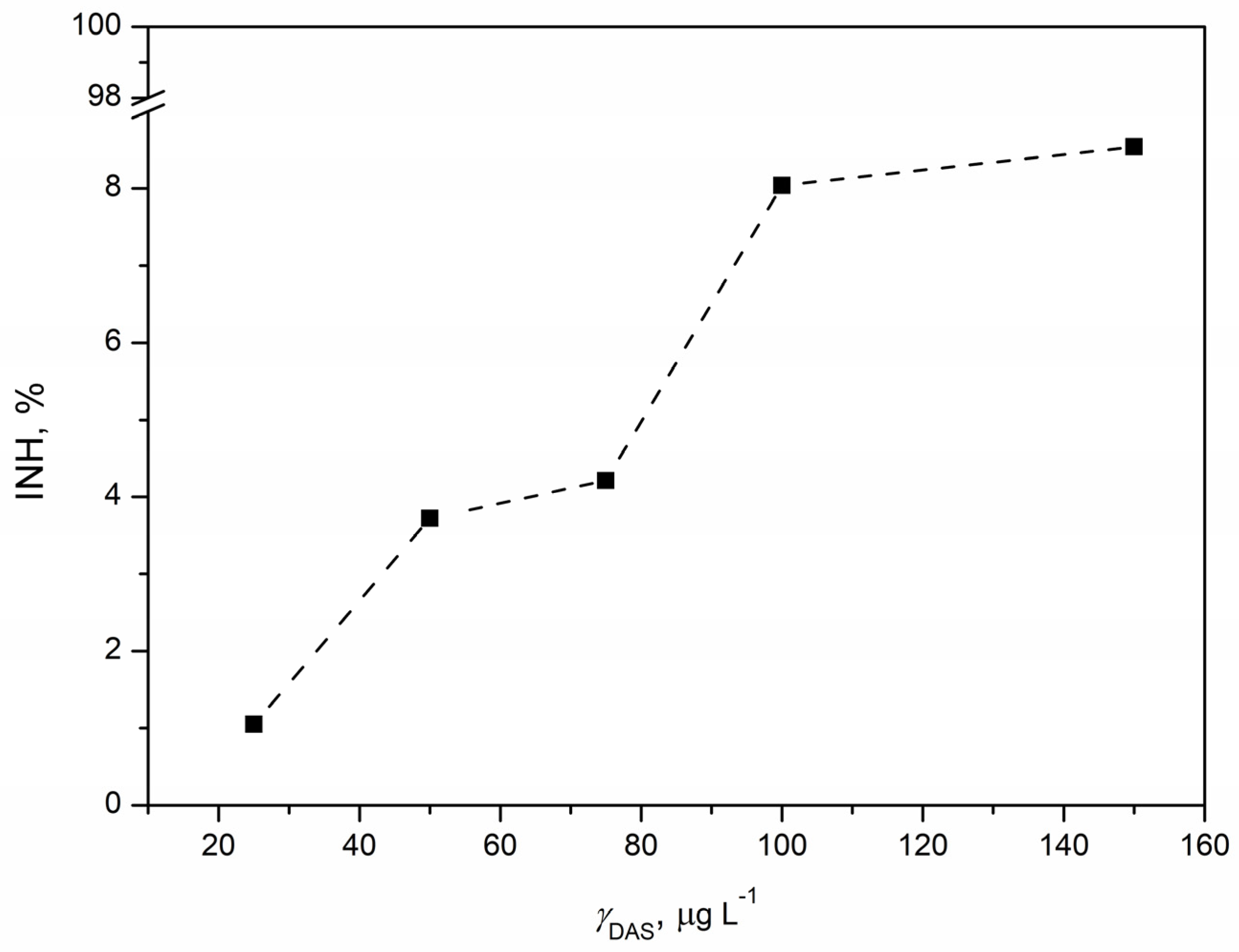
3.5. Ecotoxicological Evaluation
3.5.1. Ecotoxicity Tests with Vibrio fischeri
3.5.2. Ecotoxicity Tests with Pseudomonas putida
3.5.3. Ecotoxicity Tests with Chlorella sp.
3.5.4. Ecotoxicity Tests with Lemna minor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keskin, D.; Sadri, S.; Eskazan, A.E. Dasatinib for the Treatment of Chronic Myeloid Leukemia: Patient Selection and Special Considerations. Drug Des. Dev. Ther. 2016, 10, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; Rousselot, P.; Schiffer, C.; Rea, D.; Cortes, J.E.; Milone, J.; Mohamed, H.; Healey, D.; Kantarjian, H.; Hochhaus, A.; et al. Dasatinib in Imatinib-Resistant or -Intolerant Chronic-Phase, Chronic Myeloid Leukemia Patients: 7-Year Follow-up of Study CA180-034. Am. J. Hematol. 2016, 91, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Dasatinib: A Review in Chronic Myeloid Leukaemia and Ph+ Acute Lymphoblastic Leukaemia. Drugs 2017, 77, 85–96. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L.; Keam, S.J. Dasatinib: A Review of Its Use in the Treatment of Chronic Myeloid Leukaemia and Philadelphia Chromosome-Positive Acute Lymphoblastic Leukaemia. Drugs 2011, 71, 1771–1795. [Google Scholar] [CrossRef] [PubMed]
- Dharani, S.; Mohamed, E.M.; Khuroo, T.; Rahman, Z.; Khan, M.A. Formulation Characterization and Pharmacokinetic Evaluation of Amorphous Solid Dispersions of Dasatinib. Pharmaceutics 2022, 14, 2450. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.Y.; Gudipati, P.R. Formulation and Evaluation of Dasatinib Loaded Solid Lipid Nanoparticles. Int. J. Pharm. Pharm. Sci. 2018, 10, 14. [Google Scholar] [CrossRef]
- Martínez, L.M.; Cruz-Angeles, J.; Vázquez-Dávila, M.; Mártinez, E.; Cabada, P.; Navarrete-Bernal, C.; Cortez, F. Mechanical Activation by Ball Milling as a Strategy to Prepare Highly Soluble Pharmaceutical Formulations in the Form of Co-Amorphous, Co-Crystals, or Polymorphs. Pharmaceutics 2022, 14, 2003. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- S’ari, M.; Blade, H.; Cosgrove, S.; Drummond-Brydson, R.; Hondow, N.; Hughes, L.P.; Brown, A. Characterization of Amorphous Solid Dispersions and Identification of Low Levels of Crystallinity by Transmission Electron Microscopy. Mol. Pharm. 2021, 18, 1905–1919. [Google Scholar] [CrossRef]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef]
- Gala, U.H.; Miller, D.A.; Williams, R.O. Harnessing the Therapeutic Potential of Anticancer Drugs through Amorphous Solid Dispersions. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188319. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, H.; Wang, Y.; Wang, R.; Xu, J.; Zhang, C. Amorphous Solid Dispersions: Role of the Polymer and Its Importance in Physical Stability and In Vitro Performance. Pharmaceutics 2022, 14, 1747. [Google Scholar] [CrossRef] [PubMed]
- Bolourchian, N.; Talamkhani, Z.; Nokhodchi, A. Preparation and Physicochemical Characterization of Binary and Ternary Ground Mixtures of Carvedilol with PVP and SLS Aimed to Improve the Drug Dissolution. Pharm. Dev. Technol. 2019, 24, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Ben Osman, Y.; Liavitskaya, T.; Vyazovkin, S. Polyvinylpyrrolidone Affects Thermal Stability of Drugs in Solid Dispersions. Int. J. Pharm. 2018, 551, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Prosapio, V.; Reverchon, E.; De Marco, I. Formation of PVP/Nimesulide Microspheres by Supercritical Antisolvent Coprecipitation. J. Supercrit. Fluids 2016, 118, 19–26. [Google Scholar] [CrossRef]
- Sui, X.; Wei, W.; Yang, L.; Zu, Y.; Zhao, C.; Zhang, L.; Yang, F.; Zhang, Z. Preparation, Characterization and In Vivo Assessment of the Bioavailability of Glycyrrhizic Acid Microparticles by Supercritical Anti-Solvent Process. Int. J. Pharm. 2021, 423, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sanganwar, G.P.; Sathigari, S.; Babu, R.J.; Gupta, R.B. Simultaneous Production and Co-Mixing of Microparticles of Nevirapine with Excipients by Supercritical Antisolvent Method for Dissolution Enhancement. Eur. J. Pharm. Sci. 2010, 39, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, P.; Moser, J.; Williams, L.; Chiang, T.; Riordan, C.; Metzger, M.; Zhang-Plasket, F.; Wang, F.; Collins, J.; Williams, J. Driving Spray Drying towards Better Yield: Tackling a Problem That Sticks Around. Pharmaceutics 2023, 15, 2137. [Google Scholar] [CrossRef]
- Li, N.; Cape, J.L.; Mankani, B.R.; Zemlyanov, D.Y.; Shepard, K.B.; Morgen, M.M.; Taylor, L.S. Water-Induced Phase Separation of Spray-Dried Amorphous Solid Dispersions. Mol. Pharm. 2020, 17, 4004–4017. [Google Scholar] [CrossRef]
- Mustafa, W.W.; Fletcher, J.; Khoder, M.; Alany, R.G. Solid Dispersion of Gefitinib Prepared by Spray Drying with Improved Mucoadhesive and Drug Dissolution Properties. AAPS PharmSciTech 2022, 23, 48. [Google Scholar] [CrossRef]
- Van Le, H.; Dulong, V.; Picton, L.; Le Cerf, D. Lyophilization for Formulation Optimization of Drug-Loaded Thermoresponsive Polyelectrolyte Complex Nanogels from Functionalized Hyaluronic Acid. Pharmaceutics 2023, 15, 929. [Google Scholar] [CrossRef]
- Jakubowska, E.; Lulek, J. The Application of Freeze-Drying as a Production Method of Drug Nanocrystals and Solid Dispersions—A review. J. Drug Deliv. Sci. Technol. 2021, 62, 102357. [Google Scholar] [CrossRef]
- Al-Japairai, K.A.S.; Alkhalidi, H.M.; Mahmood, S.; Almurisi, S.H.; Doolaanea, A.A.; Al-Sindi, T.A.; Chatterjee, B. Lyophilized Amorphous Dispersion of Telmisartan in a Combined Carrier-Alkalizer System: Formulation Development and In Vivo Study. ACS Omega 2020, 5, 32466–32480. [Google Scholar] [CrossRef]
- Torres-Martínez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drug Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Penton, K.E.; Kinler, Z.; Davis, A.; Spiva, J.A.; Hamilton, S.K. Electrospinning Drug-Loaded Alginate-Based Nanofibers towards Developing a Drug Release Rate Catalog. Polymers 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun Nanofibers for Drug Delivery and Biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef]
- Simo, O.; Filipcik, J.; Martaus, A.; Jegorov, A.; Gavenda, A.; Aronhime, J.; Vraspír, P.; Koltai, T.; Faustmann, J.; Gabriel, R. Polymorphs of Dasatinib and Process for Preparation Thereof. U.S. Patent No. US20100256158A1, 14 June 2010. [Google Scholar]
- Garcia Jimenez, S.; Alvarez Fernandez, L.; Velada Calzada, J. Pharmaceutical Composition Comprising Amorphous Dasatinib. PCT No. WO2017108605, 16 December 2017. [Google Scholar]
- Jug, M.; Mura, P. Grinding as Solvent-Free Green Chemistry Approach for Cyclodextrin Inclusion Complex Preparation in the Solid State. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.D.; Singh, K.K.; Singh, N.A.; Patil, A.K. Process for Preparation of Amorphous Form of Dasatinib. U.S. Patent No. US9249134B2, 24 March 2014. [Google Scholar]
- Li, D.; Chen, H.; Liu, H.; Schlenk, D.; Mu, J.; Lacorte, S.; Ying, G.-G.; Xie, L. Anticancer Drugs in the Aquatic Ecosystem: Environmental Occurrence, Ecotoxicological Effect and Risk Assessment. Environ. Int. 2021, 153, 106543. [Google Scholar] [CrossRef]
- Mohan, H.; Rajput, S.S.; Jadhav, E.B.; Singh Sankhla, M.; Sonone, S.; Jadhav, S.; Kumar, R. Ecotoxicity, Occurrence, and Removal of Pharmaceuticals and Illicit Drugs from Aquatic Systems. Biointerface Res. Appl. Chem. 2021, 11, 12530–12546. [Google Scholar]
- Nassour, C.; Nabhani-Gebara, S.; Barton, S.J.; Barker, J. Aquatic Ecotoxicology of Anticancer Drugs: A Systematic Review. Sci. Total Environ. 2021, 800, 149598. [Google Scholar] [CrossRef]
- Khodayari, P.; Jalilian, N.; Ebrahimzadeh, H.; Amini, S. Trace-Level Monitoring of Anti-Cancer Drug Residues in Wastewater and Biological Samples by Thin-Film Solid-Phase Micro-Extraction Using Electrospun Polyfam/Co-MOF-74 Composite Nanofibers Prior to Liquid Chromatography Analysis. J. Chromatogr. A 2021, 1655, 462484. [Google Scholar] [CrossRef]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. The Role of Polymer Concentration on the Molecular Mobility and Physical Stability of Nifedipine Solid Dispersions. Mol. Pharm. 2015, 12, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Dissolution Methods Database. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/dissolution-methods-database (accessed on 20 March 2024).
- Shiyu, H.; Jialu, B.; Qianhang, S.; Ying, Z.; Xu, H.; Xingxian, L.; Yufei, F.; Lin, H. Therapeutic Drug Monitoring and Individualized Medicine of Dasatinib: Focus on Clinical Pharmacokinetics and Pharmacodynamics. Front. Pharmacol. 2021, 12, 797881. [Google Scholar]
- Tsume, Y.; Takeuchi, S.; Matsui, K.; Amidon, G.E.; Amidon, G.L. In Vitro Dissolution Methodology, mini-Gastrointestinal Simulator (mGIS), Predicts Better In Vivo Dissolution of a Weak Base Drug, Dasatinib. Eur. J. Pharm. Sci. 2015, 76, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Awadallah, A.; Sunoqrot, S.; Tarawneh, O.; Nazzal, S.; AlBaraghthi, T.; Al Sayyad, J.; Abbas, A. pH-Dependent Solubility and Dissolution of Carvedilol—Case Example of a Weakly Basic BCS Class II Drug. AAPS PharmSciTech 2016, 17, 418–426. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- ISO 11348-3:2007/Amd 1:2018; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria—Amendment 1. ISO Publishing: Geneva, Switzerland, 2018.
- ISO 10712; Water Quality—Pseudomonas putida Growth Inhibition Test, Pseudomonas Cell Multiplication Inhibition Test. International Organization for Standardization: Geneva, Switzerland, 1995.
- Hafner, C. Pseudomonas putida Growth Inhibition Test. In Ecotoxicological Characterization of Waste; Moser, H., Römbke, J., Eds.; Springer: New York, NY, USA, 2009; pp. 153–159. [Google Scholar]
- OECD. Test No. 201: Alga, Growth Inhibition Test. In OECD Guidelines for Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 1984. [Google Scholar]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar]
- OECD. Test No. 221: Lemna sp. Growth Inhibition Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006. [Google Scholar]
- Kalčíková, G.; Skalar, T.; Marolt, G.; Jemec Kokalj, A. An Environmental Concentration of Aged Microplastics with Adsorbed Silver Significantly Affects Aquatic Organisms. Water Res. 2020, 175, 115644. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Sahoo, A.; Suryanarayanan, R.; Siegel, R.A. Stabilization of Amorphous Drugs by Polymers: The Role of Overlap Concentration (C*). Mol. Pharm. 2020, 17, 4401–4406. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.; Tin, Y.-Y.; Soe, M.-T.-P.; Ko, B.; Park, S.; Lee, J. Recent Technologies for Amorphization of Poorly Water-Soluble Drugs. Pharmaceutics 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous Solid Dispersions: An Update for Preparation, Characterization, Mechanism on Bioavailability, Stability, Regulatory Considerations and Marketed Products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef]
- Mucsi, G. A Review on Mechanical Activation and Mechanical Alloying in Stirred Media Mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Deac, A.; Qi, Q.; Indulkar, A.S.; Purohit, H.S.; Gao, Y.; Zhang, G.G.Z.; Taylor, L.S. Dissolution Mechanisms of Amorphous Solid Dispersions: Role of Drug Load and Molecular Interactions. Mol. Pharm. 2023, 20, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.P.; Silva, J.D.; Marques, P.S.; Gonçalves-de-Albuquerque, C.F.; Santos, H.L.; Vascocellos, A.P.; Takiya, C.M.; Morales, M.M.; Pelosi, P.; Mócsai, A.; et al. The Effects of Dasatinib in Experimental Acute Respiratory Distress Syndrome Depend on Dose and Etiology. Cell Physiol. Biochem. 2015, 36, 1644–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tian, J.; Wang, R.; Song, M.; Zhao, R.; Chen, H.; Liu, K.; Shim, J.-H.; Zhu, F.; Dong, Z.; et al. Dasatinib Inhibits Lung Cancer Cell Growth and Patient Derived Tumor Growth in Mice by Targeting LIMK1. Front. Cell Dev. Biol. 2020, 8, 556532. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, L.; Pankov, D.; Brea, E.J.; Dao, T.; Scott, A.; Yan, S.; O’Reilly, R.J.; Liu, C.; Scheinberg, D.A. A TCR-Mimic Antibody to WT1 Bypasses Tyrosine Kinase Inhibitor Resistance in Human BCR-ABL+ Leukemias. Blood 2014, 123, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb Company. Safety Data Sheet; Bristol-Myers Squibb Company: New Brunswick, NJ, USA, 2013; pp. 1–17. [Google Scholar]
- Waters, C.M.; Bassler, B.L. Quorum Sensing: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Białk-Bielińska, A.; Mulkiewicz, E.; Stokowski, M.; Stolte, S.; Stepnowski, P. Acute Aquatic Toxicity Assessment of Six Anti-Cancer Drugs and One Metabolite Using Biotest Battery—Biological Effects and Stability under Test Conditions. Chemosphere 2017, 189, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Dams, R.I.; Biswas, A.; Olesiejuk, A.; Fernandes, T.; Christofi, N. Silver Nanotoxicity Using a Light-Emitting Biosensor Pseudomonas Putida Isolated from a Wastewater Treatment Plant. J. Hazard. Mater. 2011, 195, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, É.P.; Ribeiro, F.; Costa-Lotufo, L.V.; Soares, A.M.V.M.; Pavlaki, M.D.; Loureiro, S. Assessing the Impact of Antineoplastic Drugs in the Aquatic Environment: State of the Art and Future Perspective for Freshwater Organisms. Environ. Toxicol. Pharmacol. 2023, 99, 104109. [Google Scholar] [CrossRef]
- Weimer, A.; Kohlstedt, M.; Volke, D.C.; Nikel, P.I.; Wittmann, C. Industrial Biotechnology of Pseudomonas putida: Advances and Prospects. Appl. Microbiol. Biotechnol. 2020, 104, 7745–7766. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Balraj, S.; Kanagaraj, S.; Sundaravadivelu, S. Microbial Degradation of Pharmaceuticals. Preprints 2022. [Google Scholar] [CrossRef]
- Bygd, M.D.; Aukema, K.G.; Richman, J.E.; Wackett, L.P. Unexpected Mechanism of Biodegradation and Defluorination of 2,2-Difluoro-1,3-Benzodioxole by Pseudomonas putida F1. mBio 2021, 12, 3001–3021. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Gu, Y.-J. Biodegradation Kinetic Studies of Phenol and P-Cresol in a Batch and Continuous Stirred-Tank Bioreactor with Pseudomonas putida ATCC 17484 Cells. Processes 2021, 9, 133. [Google Scholar] [CrossRef]
- Geed, S.R.; Kureel, M.K.; Shukla, A.K.; Singh, R.S.; Rai, B.N. Biodegradation of Malathion and Evaluation of Kinetic Parameters Using Three Bacterial Species. Resour.-Effic. Technol. 2016, 2, 3–11. [Google Scholar] [CrossRef]
- Ni, Y.; Schwaneberg, U.; Sun, Z.-H. Arginine Deiminase, a Potential Anti-Tumor Drug. Cancer Lett. 2008, 261, 1–11. [Google Scholar] [CrossRef]
- Miazek, K.; Brozek-Pluska, B. Effect of PHRs and PCPs on Microalgal Growth, Metabolism and Microalgae-Based Bioremediation Processes: A Review. Int. J. Mol. Sci. 2019, 20, 2492. [Google Scholar] [CrossRef]

| Sample | Jar and Ball Material | Number of Grinding Balls | Ball Mass to the Sample Weight Ratio | Revolutions Speed (Operational), rpm |
|---|---|---|---|---|
| ASD-1 | agate | 2 | 5:1 | 500 |
| ASD-2 | agate | 2 | 5:1 | 600 |
| ASD-3 | zirconium oxide | 10 | 20:1 | 500 |
| ASD-4 | zirconium oxide | 10 | 20:1 | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokač, K.; Miloloža, M.; Kučić Grgić, D.; Žižek, K. Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment. Pharmaceutics 2024, 16, 551. https://doi.org/10.3390/pharmaceutics16040551
Sokač K, Miloloža M, Kučić Grgić D, Žižek K. Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment. Pharmaceutics. 2024; 16(4):551. https://doi.org/10.3390/pharmaceutics16040551
Chicago/Turabian StyleSokač, Katarina, Martina Miloloža, Dajana Kučić Grgić, and Krunoslav Žižek. 2024. "Polymeric Amorphous Solid Dispersions of Dasatinib: Formulation and Ecotoxicological Assessment" Pharmaceutics 16, no. 4: 551. https://doi.org/10.3390/pharmaceutics16040551