Host–Guest Complexation of Itraconazole with Cyclodextrins for Bioavailability Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Modelling
2.2. Samples and Preparation
2.3. ATR–FTIR Analysis
2.4. Thermal Analysis
2.5. Antifungal Activity
2.5.1. Fungal Strains
2.5.2. The Antifungal Activity
2.5.3. Limitations of the Study
3. Results and Discussion
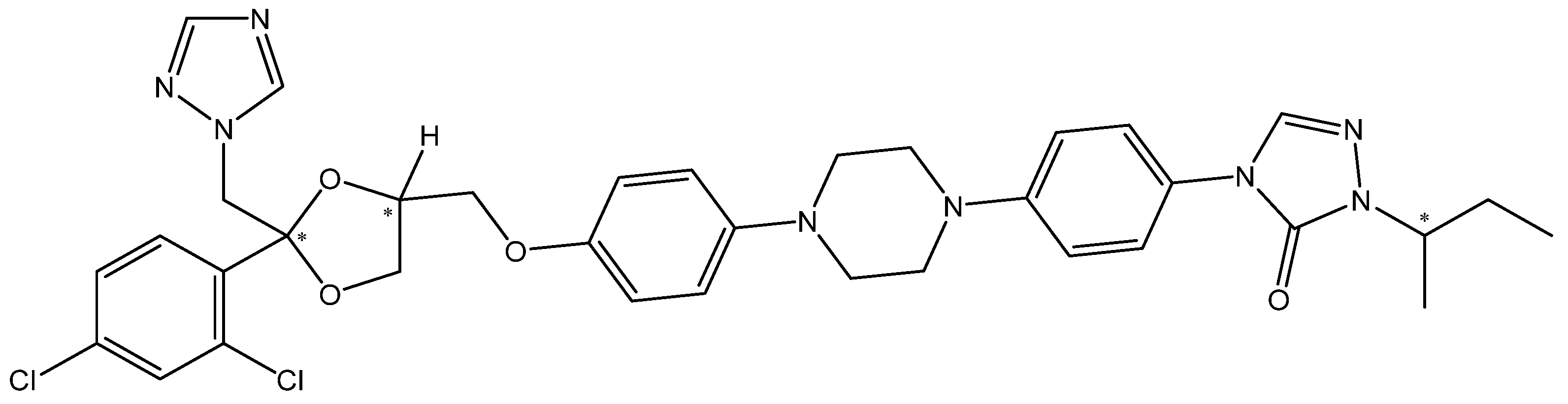
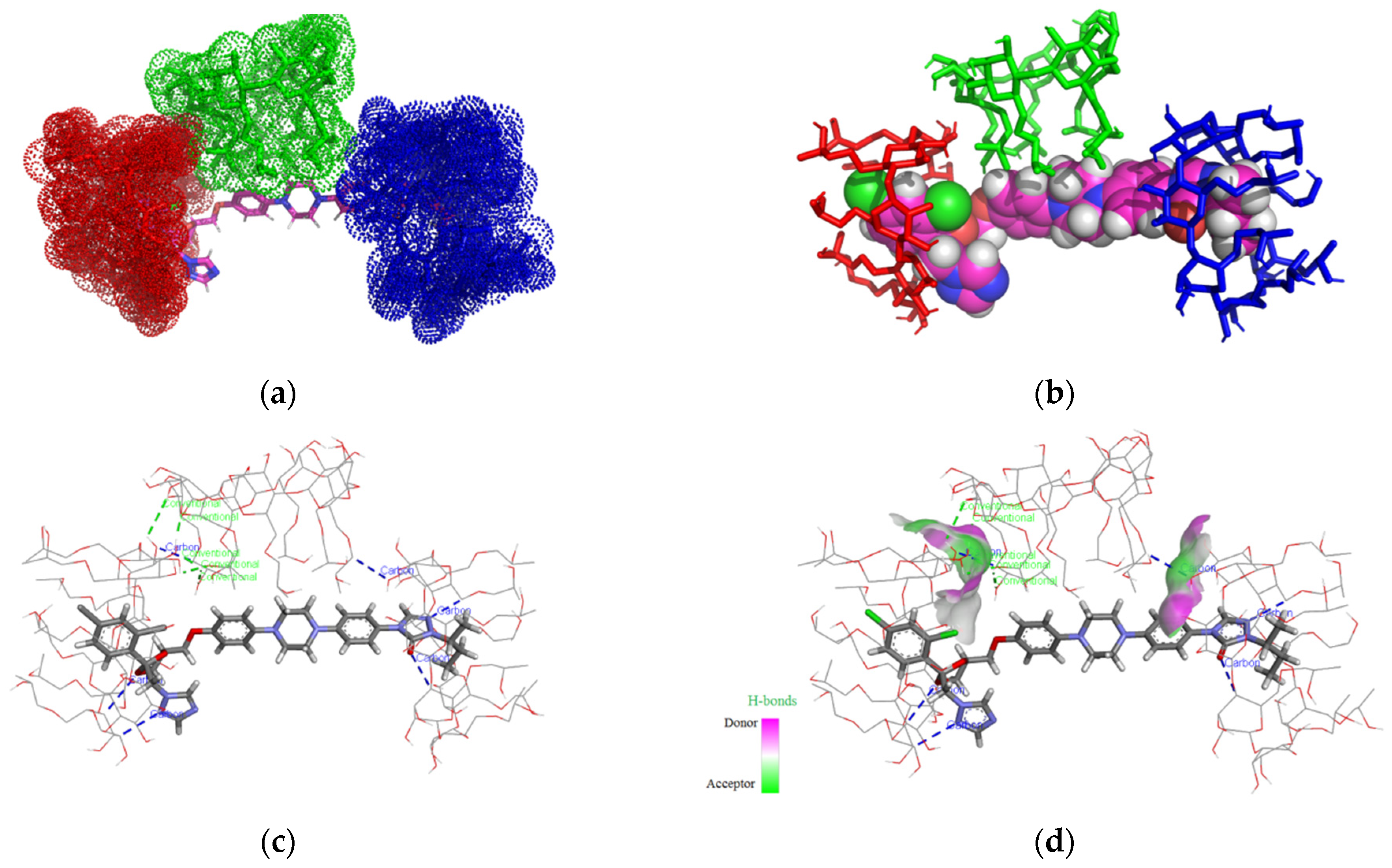
3.1. Molecular Modeling
3.2. ATR–FTIR Analysis
- SBECD: 2926 and 2874 cm−1;
- RAMEB: 2927 and 2838 cm−1;
- 2HPGCD: 2928 cm−1;
- 2HPBCD: 2973; 2929 and 2887 cm−1;
- BCD: 2970; 2927 and 2882 cm−1.
- SBECD: 1368 cm−1 (symmetrical) and 1458 cm−1 (asymmetrical);
- RAMEB: 1365 cm−1 (symmetrical) and 1459 cm−1 (asymmetrical);
- 2HPGCD: 1365 cm−1 (symmetrical) and 1459 cm−1 (asymmetrical);
- 2HPBCD: 1365 cm−1 (symmetrical) and 1459 cm−1 (asymmetrical);
- BCD: 1365 cm−1 (symmetrical) and 1457 cm−1 (asymmetrical).
3.3. Thermal Analysis
3.4. Antifungal Activity
- The MIC in blue is unchanged compared to the MIC from unassociated ITZ;
- MIC with green is higher but remains sensitive;
- The MIC in yellow is higher than the MIC from unassociated ITZ and is interpreted as dose-dependent sensitivity;
- MIC with pink indicates a lower MIC (better antifungal activity).
- Sensitive: MIC ≤ 0.125;
- Sensitivity dependent on dose (low): 0.25 ≤ MIC ≤ 0.5;
- Resistant: MIC ≥ 1.
- The inclusion complex of ITZ+SBECD exhibited an increased value of the MIC, without generally influencing the antifungal activity; most of the tested strains were sensitive to this association, and only four strains exhibited dose-dependent sensitivity.
- The inclusion complex of ITZ+2HPBCD showed an increase in the MIC value, without influencing the antifungal activity; most of the tested strains were sensitive to this complex, and only three strains presented dose-dependent sensitivity.
- For the complex of ITZ+RAMEB, although the MIC increased, it did not influence the antifungal activity, with only one strain exhibiting a dose-dependent sensitivity and the other ones remaining sensitive.
- In the case of the inclusion complex of ITZ+2HPGCD, the MIC value remained mostly unchanged, and in one case (CP2 strain), even a lower value for MIC was observed.
- Regarding the inclusion complex of ITZ+BCD, the results predominantly show a constant value of the MIC, with one exception for the CP2 strain, where a lower MIC value was observed.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Center for Biotechnology Information. PubChem Compound Summary for CID 55283, Itraconazole. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Itraconazole (accessed on 24 January 2024).
- Kunze, K.L.; Nelson, W.L.; Kharasch, E.D.; Thummel, K.E.; Isoherranen, N. Stereochemical aspects of itraconazole metabolism in vitro and in vivo. Drug Metab. Dispos. 2006, 34, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Burapapadh, K. Characterization of recrystallized itraconazole prepared by cooling and anti-solvent crystallization. Asian J. Pharm. Sci. 2015, 10, 230–238. [Google Scholar] [CrossRef]
- Kumar, N.; Shishu; Bansal, G.; Kumar, S.; Jana, A.K. Ditosylate salt of itraconazole and dissolution enhancement using cyclodextrins. AAPS PharmSciTech 2012, 13, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.M.; Boleslavská, T.; Beránek, J.; Tieger, E.; Twamley, B.; Santos-Martinez, M.J.; Dammer, O.; Tajber, L. Identification and pharmaceutical characterization of a new itraconazole terephthalic acid cocrystal. Pharmaceutics 2020, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- ChemicalBook. CAS DataBase 84625-61-6. Itraconazole. Available online: https://www.scbt.com/p/itraconazole-84625-61-6 (accessed on 24 January 2024).
- DrugBank. Itraconazole. Available online: https://go.drugbank.com/articles/A263227 (accessed on 25 January 2024).
- Abuhelwa, A.Y.; Foster, D.J.R.; Mudge, S.; Hayes, D.; Upton, R.N. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob. Agents Chemother. 2015, 59, 5681–5696. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, N.A.; Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Perlovich, G.L. Novel cocrystals of itraconazole: Insights from phase diagrams, formation thermodynamics and solubility. Int. J. Pharm. 2021, 599, 120441. [Google Scholar] [CrossRef] [PubMed]
- Thiry, J.; Broze, G.; Pestieau, A.; Tatton, A.S.; Baumans, F.; Damblon, C.; Krier, F.; Evrard, B. Investigation of a suitable in vitro dissolution test for itraconazole-based solid dispersions. Eur. J. Pharm. Sci. 2016, 85, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Mellaerts, R.; Mols, R.; Jammaer, J.A.G.; Aerts, C.A.; Annaert, P.; Van Humbeeck, J.; Van den Mooter, G.; Augustijns, P.; Martens, J.A. Increasing the oral bioavailability of the poorly water soluble drug itraconazole with ordered mesoporous silica. Eur. J. Pharm. Biopharm. 2008, 69, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, H.S.; Dyas, A.M.; Ford, J.L.; Hutcheon, G.A. In vitro evaluation of the dissolution behaviour of itraconazole in bio-relevant media. Int. J. Pharm. 2009, 366, 117–123. [Google Scholar] [CrossRef]
- Borbás, E.; Kádár, S.; Tsinman, K.; Tsinman, O.; Csicsák, D.; Takács-Novák, K.; Völgyi, G.; Sinkó, B.; Pataki, H. Prediction of Bioequivalence and Food Effect Using Flux- and Solubility-Based Methods. Mol. Pharm. 2019, 16, 4121–4130. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Wassvik, C.M.; Johansson, K.; Hubatsch, I. Poorly soluble marketed drugs display solvation limited solubility. J. Med. Chem. 2007, 50, 5858–5862. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goindi, S.; Saini, B.; Bansal, G. Thermal characterization and compatibility studies of itraconazole and excipients for development of solid lipid nanoparticles. J. Therm. Anal. Calorim. 2014, 115, 2375–2383. [Google Scholar] [CrossRef]
- Berben, P.; Mols, R.; Brouwers, J.; Tack, J.; Augustijns, P. Gastrointestinal behavior of itraconazole in humans—Part 2: The effect of intraluminal dilution on the performance of a cyclodextrin-based solution. Int. J. Pharm. 2017, 526, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.K.; Gayen, S.; Ghosh, M. Investigation of the Detailed Internal Structure and Dynamics of Itraconazole by Solid-State NMR Measurements. ACS Omega 2019, 4, 21627–21635. [Google Scholar] [CrossRef] [PubMed]
- Taupitz, T.; Dressman, J.B.; Buchanan, C.M.; Klein, S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example: Itraconazole. Eur. J. Pharm. Biopharm. 2013, 83, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Erum, R.; Samad, F.; Khan, A.; Kazmi, S.U. A comparative study on production of extracellular hydrolytic enzymes of Candida species isolated from patients with surgical site infection and from healthy individuals and their co-relation with antifungal drug resistance. BMC Microbiol. 2020, 20, 368. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Gastine, S. Therapeutic drug monitoring for antifungal triazoles: Pharmacologic background and current status. In Handbook of Analytical Separations; Elsevier Science B.V.: Amsterdam, The Netherlands, 2020; Volume 7, pp. 185–224. ISBN 9780444640666. [Google Scholar]
- Dhiman, P.; Bhatia, M. Pharmaceutical applications of cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 171–186. [Google Scholar] [CrossRef]
- Vaduva, D.M.B.; Velimirovici, D.E.; Vaduva, M.M.B.; Stanga, L.; Petrescu, H.; Rada, M.; Cipu, D.; Vaduva, B.M.B.; Radulescu, M. Phenotypic study and sensitivity to anti-infective chemotherapy of bacterial strains isolated from cutaneous-mucosal infections. Mater. Plast. 2018, 55, 372–375. [Google Scholar] [CrossRef]
- Chioibas, R.; Susan, R.; Susan, M.; Mederle, O.; Vaduva, D.B.; Radulescu, M.; Berceanu, M.; Danciu, C.; Khaled, Z.; Draghici, G.; et al. Antimicrobial Activity Exerted by Total Extracts of Germander. Rev. Chim. 2019, 70, 3242–3244. [Google Scholar] [CrossRef]
- Jianu, C.; Lukinich-Gruia, A.T.; Radulescu, M.; Mioc, M.; Mioc, A.; Soica, C.; Constantin, A.T.; David, I.; Bujanca, G.; Radu, R.G. Essential Oil of Origanum vulgare var. aureum L. from Western Romania: Chemical Analysis, In Vitro and In Silico Screening of Its Antioxidant Activity. Appl. Sci. 2023, 13, 5076. [Google Scholar] [CrossRef]
- El Achkar, T.; Moufawad, T.; Ruellan, S.; Landy, D.; Greige-Gerges, H.; Fourmentin, S. Cyclodextrins: From solute to solvent. Chem. Commun. 2020, 56, 3385–3388. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; Lucas-Abellán, C.; Pellicer, J.A.; Pérez-Garrido, A.; Pérez-Sánchez, H.; Yáñez-Gascón, M.J.; Gabaldón, J.A.; Núñez-Delicado, E. Comprehensive Characterization of Linalool-HP-β-Cyclodextrin Inclusion Complexes. Molecules 2020, 25, 5069. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Domingues, C.; Mascarenhas-Melo, F.; Silva, I.; Jarak, I.; Veiga, F.; Figueiras, A. The Role of Cyclodextrins in COVID-19 Therapy—A Literature Review. Int. J. Mol. Sci. 2023, 24, 2974. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Jin, Y.; Xiao, H. Polycyclodextrins: Synthesis, functionalization, and applications. Carbohydr. Polym. 2020, 242, 116277. [Google Scholar] [CrossRef]
- Matencio, A.; Caldera, F.; Cecone, C.; López-nicolás, J.M.; Trotta, F. Cyclic oligosaccharides as active drugs, an updated review. Pharmaceuticals 2020, 13, 281. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Kumar, N.; Shishu; Bansal, G.; Kumar, S.; Jana, A.K. Preparation and cyclodextrin assisted dissolution rate enhancement of itraconazolium dinitrate salt. Drug Dev. Ind. Pharm. 2013, 39, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Peeters, J.; Neeskens, P.; Tollenaere, J.P.; Van Remoortere, P.; Brewster, M.E. Characterization of the interaction of 2-hydroxypropyl-β-cyclodextrin With itraconazole at pH 2, 4, and 7. J. Pharm. Sci. 2002, 91, 1414–1422. [Google Scholar] [CrossRef]
- Al-Marzouqi, A.H.; Shehatta, I.; Jobe, B.; Dowaidar, A. Phase solubility and inclusion complex of itraconazole with β-cyclodextrin using supercritical carbon dioxide. J. Pharm. Sci. 2006, 95, 292–304. [Google Scholar] [CrossRef]
- Cevher, E.; Açma, A.; Sinani, G.; Aksu, B.; Zloh, M.; Mülazi Moǧlu, L. Bioadhesive tablets containing cyclodextrin complex of itraconazole for the treatment of vaginal candidiasis. Int. J. Biol. Macromol. 2014, 69, 124–136. [Google Scholar] [CrossRef]
- Alsarra, I.A.; Alanazi, F.K.; Ahmed, S.M.; Bosela, A.A.; Alhamed, S.S.; Mowafy, H.A.; Neau, S.H. Comparative study of itraconazole-cyclodextrin inclusion complex and its commercial product. Arch. Pharm. Res. 2010, 33, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Stappaerts, J.; Augustijns, P. Displacement of itraconazole from cyclodextrin complexes in biorelevant media: In vitro evaluation of supersaturation and precipitation behavior. Int. J. Pharm. 2016, 511, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, C.M.; Buchanan, N.L.; Edgar, K.J.; Klein, S.; Little, J.L.; Ramsey, M.G.; Ruble, K.M.; Wacher, V.J.; Wempe, M.F. Pharmacokinetics of itraconazole after intravenous and oral dosing of itraconazole-cyclodextrin formulations. J. Pharm. Sci. 2007, 96, 3100–3116. [Google Scholar] [CrossRef] [PubMed]
- Berben, P.; Stappaerts, J.; Vink, M.J.A.; Domínguez-Vega, E.; Somsen, G.W.; Brouwers, J.; Augustijns, P. Linking the concentrations of itraconazole and 2-hydroxypropyl-β-cyclodextrin in human intestinal fluids after oral intake of Sporanox®. Eur. J. Pharm. Biopharm. 2018, 132, 231–236. [Google Scholar] [CrossRef] [PubMed]
- SID, D.; BAITICHE, M.; ELBAHRI, Z.; DJERBOUA, F.; MESSALTI, S.; BOUTAHALA, M. Efficient Enhancement in Itraconazole Solubility through its Cyclodextrin-Water Soluble Polymer Ternary Inclusion Complexes. J. Drug Deliv. Ther. 2020, 10, 11–14. [Google Scholar] [CrossRef]
- Cuoco, A.; Eriksen, J.B.; Luppi, B.; Brandl, M.; Bauer-Brandl, A. When Interactions Between Bile Salts and Cyclodextrin Cause a Negative Food Effect: Dynamic Dissolution/Permeation Studies with Itraconazole (Sporanox®) and Biomimetic Media. J. Pharm. Sci. 2023, 112, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J.; the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. EUCAST Definitive Document E.DEF 7.3.2. April 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf (accessed on 19 March 2024).
- Pfaller, M.A.; Haturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A.; Gosey, L.L.; Odds, F.C.; Rex, J.H.; Rinaldi, M.G.; Sheehan, D.J.; Walsh, T.J.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. In Approved Standard—Second Edition Serving the World’ s Medical Science Community Through Voluntary Consensus; NCCLS: Wayne, PA, USA, 2002; Volume 22, ISBN 1562384694. [Google Scholar]
- Sălăgeanu, L.; Muntean, D.; George, H.F.; Lascu, A.; Anghel, D.; Bagiu, I.C.; Fagadar-Cosma, E. Antimicrobial activity of different substituted meso-porphyrin derivatives. Rev. Rom. Med. Lab. 2020, 28, 205–216. [Google Scholar] [CrossRef]
- Fecker, R.; Buda, V.; Alexa, E.; Avram, S.; Pavel, I.Z.; Muntean, D.; Cocan, I.; Watz, C.; Minda, D.; Dehelean, C.A.; et al. Phytochemical and biological screening of Oenothera biennis L. Hydroalcoholic extract. Biomolecules 2020, 10, 818. [Google Scholar] [CrossRef]
- Singh, R.; Bharti, N.; Madan, J.R.; Hiremath, S.N. Characterization of Cyclodextrin Inclusion Complexes—A Review. J. Pharm. Sci. Technol. 2010, 2, 171–183. [Google Scholar]









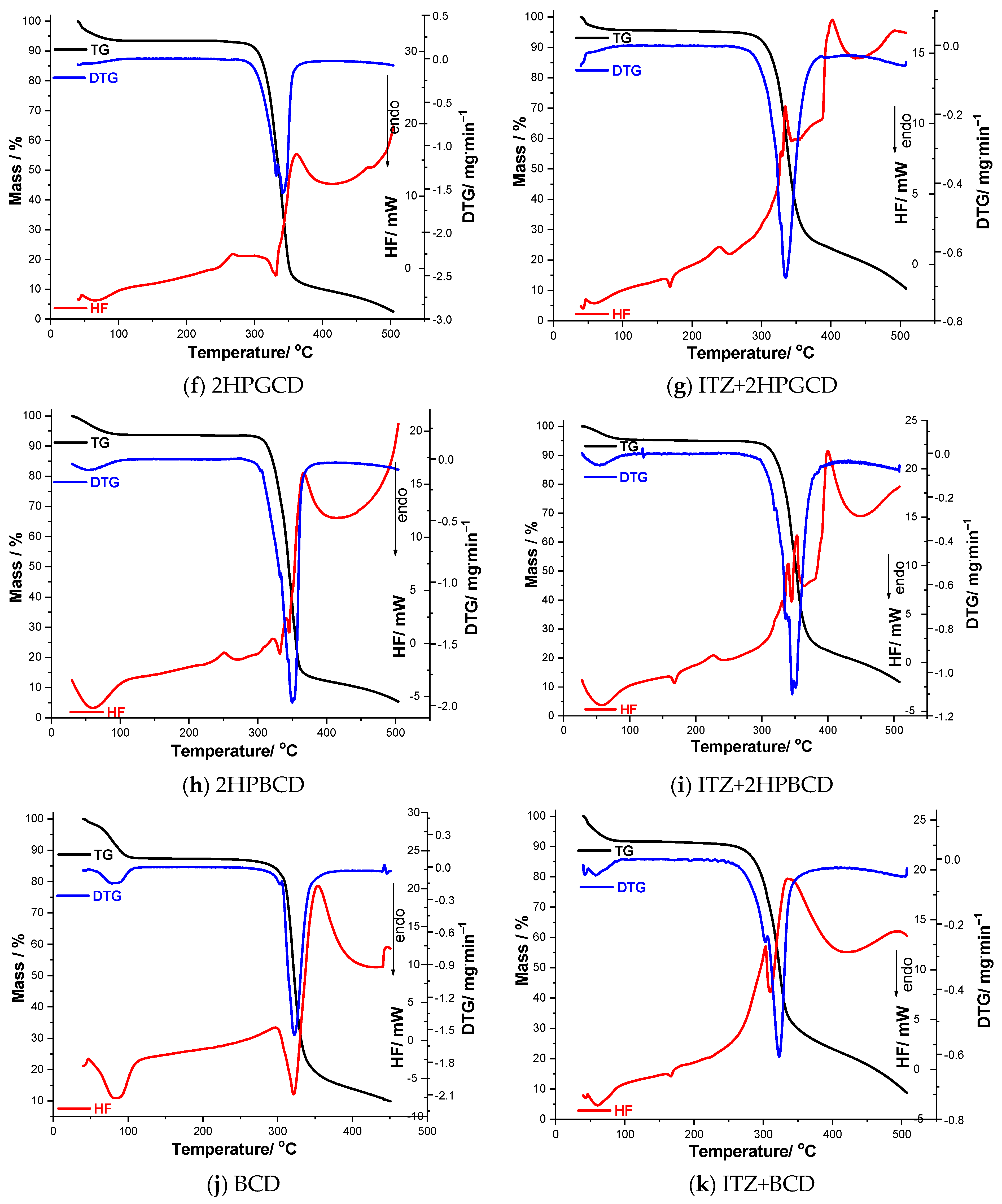
| Sample | TG | Δm/% | DTG | HF | |||
|---|---|---|---|---|---|---|---|
| Tonset/°C | Toffset/°C | Tonset/°C | Tpeak/°C | Tonset/°C | Tpeak/°C | ||
| ITZ | 305 | 403 | 58.09 | 314 | 373 | 160 | 170 |
| 403 | 505 | 10.53 | – | – | 309 | 375 | |
| SBECD | 39 | 126 | 8.04 | – | – | – | – |
| 244 | 318 | 24.55 | 243 | 274 | 249 | 263 | |
| 318 | 415 | 20.67 | 333 | 352 | 293; 341; 388 | 323; 351; 422 | |
| ITZ+SBECD | 40 | 125 | 6.52 | 40 | 44 | 40 | 43 |
| 240 | 320 | 21.25 | 242 | 278 | 160; 244; 275 | 168; 264; 281; 288; 299 | |
| 320 | 390 | 16.17 | 330 | 356 | 335 | 359 | |
| 390 | 505 | 7.29 | – | – | 391 | 408 | |
| RAMEB | 34 | 78 | 4.7 | 34 | 48 | 39 | 51 |
| 296 | 498 | 85.9 | 359 | 310; 395 | 362; 408 | ||
| ITZ+RAMEB | 40 | 70 | 2.17 | 40 | 41 | 40; 152 | 42; 160 |
| 270 | 402 | 72.08 | 274 | 356 | 317; 367 | 358; 373 | |
| 402 | 505 | 17.27 | – | – | 406 | 477 | |
| 2HPGCD | 44 | 85 | 4.9 | – | – | 47 | 67 |
| 288 | 455 | 85.4 | 288 | 332; 342 | 237; 331 | 267; 362 | |
| ITZ+2HPGCD | 40 | 90 | 4.20 | 46 | 54 | 40 | 58 |
| 90 | 280 | 1.31 | – | – | 160; 213 | 168; 239 | |
| 280 | 440 | 74.47 | 254 | 334 | 304; 387 | 328; 334; 402 | |
| 2HPBCD | 35 | 86 | 5.3 | 39 | 55; 352 | 35 | 59 |
| 298 | 485 | 85.8 | 221; 297 | 251; 367 | |||
| ITZ+2HPBCD | 30 | 100 | 4.56 | 30 | 54 | 30 | 57 |
| 100 | 280 | 0.70 | – | – | 160; 203 | 168; 226 | |
| 280 | 450 | 76.3 | 277 | 317; 335; 345; 350 | 289; 381 | 330; 340; 354; 400 | |
| BCD | 42 | 104 | 12.1 | 60 | 83 | 48 | 85 |
| 279 | 434 | 74.8 | 260 | 322 | 220; 321 | 297; 354 | |
| ITZ+BCD | 40 | 88 | 8.06 | 47 | 58 | 40; 156 | 60; 167 |
| 250 | 415 | 68.74 | 254 | 302; 323 | 225; 309 | 311; 419 | |
| Strains | Antifungal Agent | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITZ+RAMEB | ITZ+2HPGCD | ITZ+SBECD | ITZ+2HPBCD | ITZ+BCD | ITZ | ||||||||
| Study Test | Sensititre | ||||||||||||
| MIC μg/mL | MFC μg/mL | MIC μg/mL | MFC μg/mL | MIC μg/mL | MFC μg/mL | MIC μg/mL | MFC μg/mL | MIC μg/mL | MFC μg/mL | MIC μg/mL | MFC μg/mL | MIC μg/mL | |
| CP ATCC 22019 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.25 | 0.12 | 0.25 | 0.06 | 0.12 | 0.06 | 0.12 | 0.06 |
| CK ATCC 6258 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.25 | 0.12 | 0.25 | 0.06 | 0.25 | 0.06 | 0.12 | 0.06 |
| CA1 | 0.06 | 0.25 | 0.06 | 0.12 | 0.12 | 0.25 | 0.12 | 0.25 | 0.06 | 0.12 | 0.03 | 0.12 | 0.03 |
| CA2 | 0.12 | 0.5 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.12 | 0.25 | 0.12 | 0.25 | 0.12 |
| CA3 | 0.12 | 0.5 | 0.06 | 0.25 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | 0.12 | 0.06 |
| CA4 | 0.12 | 0.5 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.25 | 0.06 | 0.12 | 0.06 |
| CA5 | 0.12 | 0.5 | 0.06 | 0.25 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.25 | 0.06 | 0.25 | 0.06 |
| CA6 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.25 | 0.12 | 0.5 | 0.06 | 0.25 | 0.06 | 0.12 | 0.06 |
| CA7 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 | 0.12 | 0.25 | 0.12 | 0.25 | 0.06 |
| CA8 | 0.12 | 0.25 | 0.06 | 0.25 | 0.12 | 0.25 | 0.12 | 0.5 | 0.06 | 0.25 | 0.06 | 0.25 | 0.06 |
| CA9 | 0.12 | 0.25 | 0.03 | 0.12 | 0.12 | 0.25 | 0.12 | 0.25 | 0.03 | 0.12 | 0.03 | 0.12 | 0.03 |
| CA10 | 0.12 | 0.5 | 0.06 | 0.12 | 0.12 | 0.25 | 0.12 | 0.25 | 0.03 | 0.12 | 0.03 | 0.06 | 0.03 |
| CP1 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.25 | 0.12 | 0.25 | 0.03 | 0.06 | 0.03 | 0.06 | 0.03 |
| CP2 | 0.25 | 0.5 | 0.06 | 0.12 | 0.25 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.12 | 0.25 | 0.12 |
| CP3 | 0.12 | 0.5 | 0.06 | 0.25 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | 0.12 | 0.06 |
| CP4 | 0.12 | 0.5 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | 0.25 | 0.06 |
| CP5 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | 0.12 | 0.06 |
| CP6 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | 0.12 | 0.06 |
| CP7 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | 0.12 | 0.06 |
| CP8 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.12 | 0.5 | 0.12 | 0.5 | 0.12 | 0.25 | 0.12 |
| CP9 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.25 | 0.06 | 0.12 | 0.03 |
| CP10 | 0.12 | 0.25 | 0.12 | 0.25 | 0.12 | 0.5 | 0.12 | 0.5 | 0.12 | 0.25 | 0.12 | 0.5 | 0.12 |
| CP11 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.25 | 0.06 | 0.12 | 0.03 |
| CP12 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | 0.12 | 0.06 |
| CP13 | 0.12 | 0.25 | 0.06 | 0.12 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.25 | 0.06 | 0.12 | 0.06 |
| CP14 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 | 0.12 | 0.25 | 0.12 | 0.25 | 0.12 |
| CK | 0.12 | 0.25 | 0.06 | 0.25 | 0.12 | 0.5 | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | 0.12 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şuta, L.-M.; Ridichie, A.; Ledeţi, A.; Temereancă, C.; Ledeţi, I.; Muntean, D.; Rădulescu, M.; Văruţ, R.-M.; Watz, C.; Crăineanu, F.; et al. Host–Guest Complexation of Itraconazole with Cyclodextrins for Bioavailability Enhancement. Pharmaceutics 2024, 16, 560. https://doi.org/10.3390/pharmaceutics16040560
Şuta L-M, Ridichie A, Ledeţi A, Temereancă C, Ledeţi I, Muntean D, Rădulescu M, Văruţ R-M, Watz C, Crăineanu F, et al. Host–Guest Complexation of Itraconazole with Cyclodextrins for Bioavailability Enhancement. Pharmaceutics. 2024; 16(4):560. https://doi.org/10.3390/pharmaceutics16040560
Chicago/Turabian StyleŞuta, Lenuţa-Maria, Amalia Ridichie, Adriana Ledeţi, Claudia Temereancă, Ionuţ Ledeţi, Delia Muntean, Matilda Rădulescu, Renata-Maria Văruţ, Claudia Watz, Florentin Crăineanu, and et al. 2024. "Host–Guest Complexation of Itraconazole with Cyclodextrins for Bioavailability Enhancement" Pharmaceutics 16, no. 4: 560. https://doi.org/10.3390/pharmaceutics16040560










