Assessing the Occurrence and Influence of Cancer Chemotherapy-Related Pharmacogenetic Alleles in the Chilean Population
Abstract
:1. Introduction
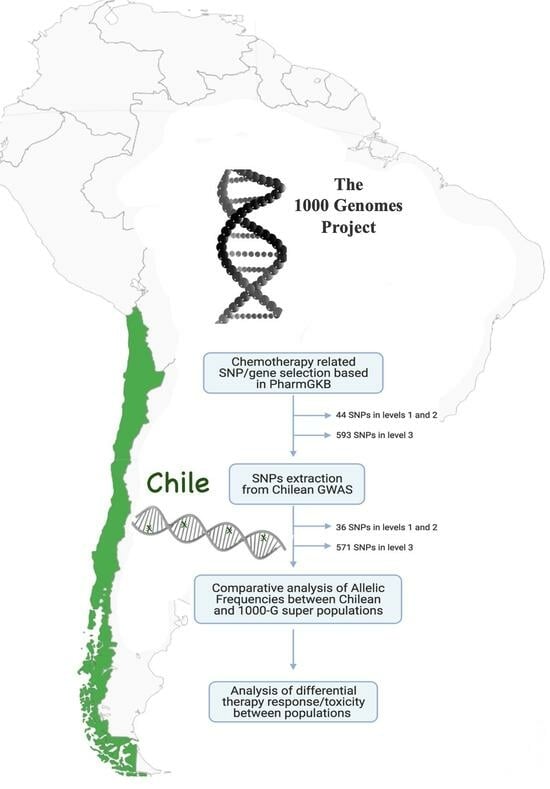
2. Methods
2.1. Study Population
2.2. PharmacoSNPs Selection
2.3. Comparative Analysis of Variants and Haplotypes Frequencies across Different Ethnicities
2.4. Ancestry-Based Association Analysis
3. Results
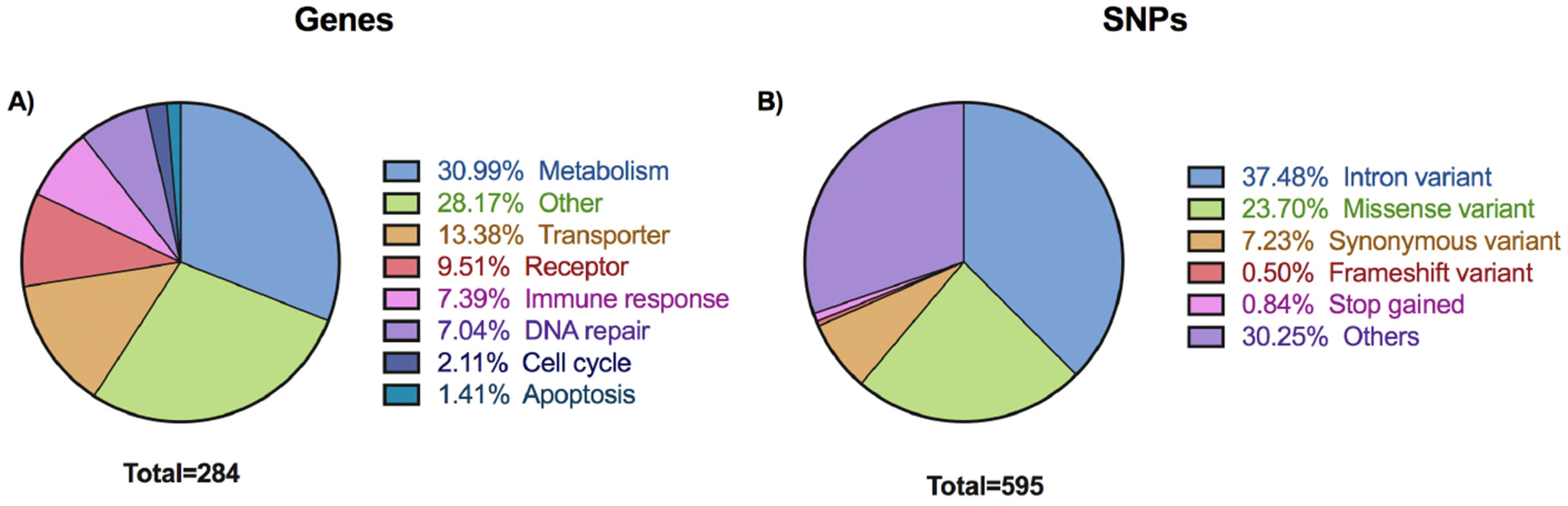
3.1. Overview of Selected Variants
3.2. Pharmacogene SNPs Frequency Differences among Chilean Population and 1000 Genomes Superpopulations
3.3. Very Important Pharmacogenes (VIP) SNPs
3.4. Haplotype Analysis of DPYD, ABCB1, and TPMT Pharmacogenes
3.5. Ancestry-Related Pharmacogenes SNPs in the Chilean Cohort
4. Discussion
4.1. DPYD Gene
4.2. UGT1A1 Gene
4.3. NUDT15 and TPMT Genes
4.4. ABCB1 Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SNP | Single nucleotide polymorphisms |
| GWAS | Genome-wide association studies (GWAS) |
| MAF | Minor allele frequency |
| DPYD | Dihydropyrimidine dehydrogenase gene |
| DPD | Dihydropyrimidine dehydrogenase enzyme |
| NUDT15 | Nudix hydrolase 15 |
| GSTP1 | Glutathione S-transferase P |
| TPMT | Thiopurine methyltransferase |
| UGT1A1 | UDP-glucuronosyltransferase 1-1 |
| NQO1 | NAD(P)H dehydrogenase [quinone] 1 |
| ABCB1 | ATP-binding cassette sub-family B member 1 gene |
| XRCC1 | X-ray repair cross-complementing protein 1 |
| CPNDS | Canadian Pharmacogenomics Network for Drug Safety |
| CPIC | The Clinical Pharmacogenomic International Consortium |
| AMR | Admixed American |
| EUR | European |
| SAS | South Asian |
| EAS | East Asian |
| AFR | African |
| Hap | Haplotype |
| VIP | Very important pharmacogene |
| LoE | Level of evidence |
References
- Popejoy, A.B. Diversity In Precision Medicine And Pharmacogenetics: Methodological And Conceptual Considerations For Broadening Participation. Pharmacogenomics Pers. Med. 2019, 12, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Salas-Hernández, A.; Galleguillos, M.; Carrasco, M.; López-Cortés, A.; Redal, M.A.; Fonseca-Mendoza, D.; Esperón, P.; González-Martínez, F.; Lares-Asseff, I.; Lazarowski, A.; et al. An updated examination of the perception of barriers for pharmacogenomics implementation and the usefulness of drug/gene pairs in Latin America and the Caribbean. Front. Pharmacol. 2023, 14, 1175737. [Google Scholar] [CrossRef] [PubMed]
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The Missing Diversity in Human Genetic Studies. Cell 2019, 177, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Swen, J.J.; Nijenhuis, M.; de Boer, A.; Grandia, L.; Maitland-van der Zee, A.H.; Mulder, H.; Rongen, G.A.P.J.M.; van Schaik, R.H.N.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From bench to byte--an update of guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Carleton, B.; Poole, R.; Smith, M.; Leeder, J.; Ghannadan, R.; Ross, C.; Phillips, M.; Hayden, M. Adverse drug reaction active surveillance: Developing a national network in Canada’s children’s hospitals. Pharmacoepidemiol. Drug Saf. 2009, 18, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Klein, T.E. CPIC: Clinical pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011, 89, 464–467. [Google Scholar] [CrossRef]
- Amstutz, U.; Henricks, L.M.; Offer, S.M.; Barbarino, J.; Schellens, J.H.M.; Swen, J.J.; Klein, T.E.; McLeod, H.L.; Caudle, K.E.; Diasio, R.B.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Cecchin, E.; Gasparini, G.; D’Andrea, M.; Azzarello, G.; Basso, U.; Mini, E.; Pessa, S.; De Mattia, E.; Lo Re, G.; et al. Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Onoue, M.; Terada, T.; Kobayashi, M.; Katsura, T.; Matsumoto, S.; Yanagihara, K.; Nishimura, T.; Kanai, M.; Teramukai, S.; Shimizu, A.; et al. UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients. Int. J. Clin. Oncol. 2009, 14, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Schwab, M.; Whirl-Carrillo, M.; Suarez-Kurtz, G.; Pui, C.-H.; Stein, C.M.; Moyer, A.M.; Evans, W.E.; Klein, T.E.; Antillon-Klussmann, F.G.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 2019, 105, 1095–1105. [Google Scholar] [CrossRef]
- Diouf, B.; Crews, K.R.; Lew, G.; Pei, D.; Cheng, C.; Bao, J.; Zheng, J.J.; Yang, W.; Fan, Y.; Wheeler, H.E.; et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 2015, 313, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Cura, Y.; Pérez Ramírez, C.; Sánchez Martín, A.; Martínez Martínez, F.; Calleja Hernández, M.Á.; Ramírez Tortosa, M.D.C.; Jiménez Morales, A. Genetic polymorphisms on the effectiveness or safety of breast cancer treatment: Clinical relevance and future perspectives. Mutat. Res. Rev. Mutat. Res. 2021, 788, 108391. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.S.; Keat, K.; Li, B.; Hoffecker, G.; Risman, M.; Sangkuhl, K.; Whirl-Carrillo, M.; Dudek, S.; Verma, A.; Klein, T.E.; et al. Evaluating the frequency and the impact of pharmacogenetic alleles in an ancestrally diverse Biobank population. J. Transl. Med. 2022, 20, 550. [Google Scholar] [CrossRef] [PubMed]
- Silgado-Guzmán, D.F.; Angulo-Aguado, M.; Morel, A.; Niño-Orrego, M.J.; Ruiz-Torres, D.-A.; Contreras Bravo, N.C.; Restrepo, C.M.; Ortega-Recalde, O.; Fonseca-Mendoza, D.J. Characterization of ADME Gene Variation in Colombian Population by Exome Sequencing. Front. Pharmacol. 2022, 13, 931531. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Kurtz, G. Population impact of pharmacogenetic tests in admixed populations across the Americas. Pharmacogenomics J. 2021, 21, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Crow, J. The Mapuche in Modern Chile; University Press of Florida: Gainesville, FL, USA, 2013; ISBN 9780813044286. [Google Scholar]
- Bustos, B.I.; Pérez-Palma, E.; Buch, S.; Azócar, L.; Riveras, E.; Ugarte, G.D.; Toliat, M.; Nürnberg, P.; Lieb, W.; Franke, A.; et al. Variants in ABCG8 and TRAF3 genes confer risk for gallstone disease in admixed Latinos with Mapuche Native American ancestry. Sci. Rep. 2019, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.A.; Moyano, T.C.; Bustos, B.I.; Pérez-Palma, E.; Moraga, C.; Riveras, E.; Montecinos, A.; Azócar, L.; Soto, D.C.; Vidal, M.; et al. Whole Genome Sequence, Variant Discovery and Annotation in Mapuche-Huilliche Native South Americans. Sci. Rep. 2019, 9, 2132. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Dudbridge, F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum. Hered. 2008, 66, 87–98. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Hamzic, S.; Schärer, D.; Offer, S.M.; Meulendijks, D.; Nakas, C.; Diasio, R.B.; Fontana, S.; Wehrli, M.; Schürch, S.; Amstutz, U.; et al. Haplotype structure defines effects of common DPYD variants c.85T>C (rs1801265) and c.496A>G (rs2297595) on dihydropyrimidine dehydrogenase activity: Implication for 5-fluorouracil toxicity. Br. J. Clin. Pharmacol. 2021, 87, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Kurtz, G.; de Araújo, G.S. Pharmacogenetic differentiation across Latin America. Pharmacogenomics 2022, 23, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Koenigstein, F.; Boekstegers, F.; Wilson, J.F.; Fuentes-Guajardo, M.; Gonzalez-Jose, R.; Bedoya, G.; Bortolini, M.C.; Acuña-Alonzo, V.; Gallo, C.; Ruiz Linares, A.; et al. Inbreeding, Native American ancestry and child mortality: Linking human selection and paediatric medicine. Hum. Mol. Genet. 2022, 31, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.; Pulgar, I.; Gallo, C.; Bortolini, M.-C.; Canizales-Quinteros, S.; Bedoya, G.; González-José, R.; Ruiz-Linares, A.; Rothhammer, F. Gene geography of Chile: Regional distribution of American, European and African genetic contributions. Rev. Med. Chil. 2014, 142, 281–289. [Google Scholar] [CrossRef]
- Cordova-Delgado, M.; Bravo, M.L.; Cumsille, E.; Hill, C.N.; Muñoz-Medel, M.; Pinto, M.P.; Retamal, I.N.; Lavanderos, M.A.; Miquel, J.F.; Rodriguez-Fernandez, M.; et al. A case-control study of a combination of single nucleotide polymorphisms and clinical parameters to predict clinically relevant toxicity associated with fluoropyrimidine and platinum-based chemotherapy in gastric cancer. BMC Cancer 2021, 21, 1030. [Google Scholar] [CrossRef]
- Gonzalez-Covarrubias, V.; Morales-Franco, M.; Cruz-Correa, O.F.; Martínez-Hernández, A.; García-Ortíz, H.; Barajas-Olmos, F.; Genis-Mendoza, A.D.; Martínez-Magaña, J.J.; Nicolini, H.; Orozco, L.; et al. Variation in Actionable Pharmacogenetic Markers in Natives and Mestizos From Mexico. Front. Pharmacol. 2019, 10, 1169. [Google Scholar] [CrossRef]
- Suarez-Kurtz, G.; Fernandes, V.C.; Elias, A.B.R. Implementation of DPYD Genotyping in Admixed American Populations: Brazil as a Model Case. Clin. Pharmacol. Ther. 2023, 114, 23–24. [Google Scholar] [CrossRef]
- Botton, M.R.; Hentschke-Lopes, M.; Matte, U. Frequency of DPYD gene variants and phenotype inference in a Southern Brazilian population. Ann. Hum. Genet. 2022, 86, 102–107. [Google Scholar] [CrossRef]
- Naslavsky, M.S.; Scliar, M.O.; Yamamoto, G.L.; Wang, J.Y.T.; Zverinova, S.; Karp, T.; Nunes, K.; Ceroni, J.R.M.; de Carvalho, D.L.; da Silva Simões, C.E.; et al. Whole-genome sequencing of 1,171 elderly admixed individuals from Brazil. Nat. Commun. 2022, 13, 1004. [Google Scholar] [CrossRef] [PubMed]
- Medwid, S.; Wigle, T.J.; Kim, R.B. Fluoropyrimidine-associated toxicity and DPYD variants c.85T>C, c.496A>G, and c.1236G>A: Impact of haplotype. Cancer Chemother. Pharmacol. 2023, 91, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Kawaguchi, T.; Kotaka, M.; Manaka, D.; Hasegawa, J.; Takagane, A.; Munemoto, Y.; Kato, T.; Eto, T.; Touyama, T.; et al. Poor association between dihydropyrimidine dehydrogenase (DPYD) genotype and fluoropyrimidine-induced toxicity in an Asian population. Cancer Med. 2023, 12, 7808–7814. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Cunningham, D.; Roth, A.D.; Navarro, M.; James, R.D.; Karasek, P.; Jandik, P.; Iveson, T.; Carmichael, J.; Alakl, M.; et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000, 355, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Sparreboom, A. Pharmacogenetics of irinotecan disposition and toxicity: A review. Curr. Clin. Pharmacol. 2010, 5, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Karas, S.; Innocenti, F. All You Need to Know About UGT1A1 Genetic Testing for Patients Treated With Irinotecan: A Practitioner-Friendly Guide. JCO Oncol. Pract. 2022, 18, 270–277. [Google Scholar] [CrossRef]
- Moriyama, T.; Yang, W.; Smith, C.; Pui, C.-H.; Evans, W.E.; Relling, M.V.; Bhatia, S.; Yang, J.J. Comprehensive characterization of pharmacogenetic variants in TPMT and NUDT15 in children with acute lymphoblastic leukemia. Pharmacogenet. Genom. 2022, 32, 60–66. [Google Scholar] [CrossRef]
- Suarez-Kurtz, G.; Araújo, G.S.d.; de Sousa, S.J. Pharmacogeomic implications of population diversity in Latin America: TPMT and NUDT15 polymorphisms and thiopurine dosing. Pharmacogenet. Genom. 2020, 30, 1–4. [Google Scholar] [CrossRef]
- Suarez-Kurtz, G.; Brisson, G.D.; Hutz, M.H.; Petzl-Erler, M.L.; Salzano, F.M. NUDT15 Polymorphism in Native American Populations of Brazil. Clin. Pharmacol. Ther. 2019, 105, 1321–1322. [Google Scholar] [CrossRef]
- Von Muhlenbrock, C.; Estay, C.; Covarrubias, N.; Miranda, J.; Venegas, M. The c.415C>T polymorphism in NUDT15 is more frequent than the polymorphisms in TPMT in Chilean patients who use thiopurine drugs. Pharmacogenet. Genom. 2023, 33, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, Y.; Kinouchi, Y.; Shimosegawa, T. Pharmacogenetics of thiopurines for inflammatory bowel disease in East Asia: Prospects for clinical application of NUDT15 genotyping. J. Gastroenterol. 2018, 53, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Elion, G.B. The purine path to chemotherapy. Science 1989, 244, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Farfan, M.J.; Salas, C.; Canales, C.; Silva, F.; Villarroel, M.; Kopp, K.; Torres, J.P.; Santolaya, M.E.; Morales, J. Prevalence of TPMT and ITPA gene polymorphisms and effect on mercaptopurine dosage in Chilean children with acute lymphoblastic leukemia. BMC Cancer 2014, 14, 299. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Cóndor, J.; Naranjo, P.; Atarihuana, S.; Coello, D.; Guevara-Ramírez, P.; Flores-Espinoza, R.; Burgos, G.; López-Cortés, A.; Cabrera-Andrade, A. Population-Specific Distribution of TPMT Deficiency Variants and Ancestry Proportions in Ecuadorian Ethnic Groups: Towards Personalized Medicine. Ther. Clin. Risk Manag. 2023, 19, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, D.C.; Wanderley, A.V.; Dos Santos, A.M.R.; Moreira, F.C.; de Sá, R.B.A.; Fernandes, M.R.; Modesto, A.A.C.; de Souza, T.P.; Cohen-Paes, A.; Leitão, L.P.C.; et al. Characterization of pharmacogenetic markers related to Acute Lymphoblastic Leukemia toxicity in Amazonian native Americans population. Sci. Rep. 2020, 10, 10292. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pelleymounter, L.; Weinshilboum, R.; Johnson, J.A.; Hebert, J.M.; Altman, R.B.; Klein, T.E. Very important pharmacogene summary: Thiopurine S-methyltransferase. Pharmacogenet. Genom. 2010, 20, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Mrozikiewicz-Rakowska, B.; Malinowski, M.; Nehring, P.; Bartkowiak-Wieczorek, J.; Bogacz, A.; Żurawińska-Grzelka, E.; Krasnodębski, P.; Muszyński, J.; Grzela, T.; Przybyłkowski, A.; et al. The MDR1/ABCB1 gene rs 1045642 polymorphism in colorectal cancer. Arch. Med. Sci. 2020, 16, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Yunis, L.K.; Linares-Ballesteros, A.; Aponte, N.; Barros, G.; García, J.; Niño, L.; Uribe, G.; Quintero, E.; Yunis, J.J. Pharmacogenetics of ABCB1, CDA, DCK, GSTT1, GSTM1 and outcomes in a cohort of pediatric acute myeloid leukemia patients from Colombia. Cancer Rep. 2023, 6, e1744. [Google Scholar] [CrossRef]
- Megías-Vericat, J.E.; Montesinos, P.; Herrero, M.J.; Moscardó, F.; Bosó, V.; Rojas, L.; Martínez-Cuadrón, D.; Hervás, D.; Boluda, B.; García-Robles, A.; et al. Impact of ABC single nucleotide polymorphisms upon the efficacy and toxicity of induction chemotherapy in acute myeloid leukemia. Leuk. Lymphoma 2017, 58, 1197–1206. [Google Scholar] [CrossRef]
- Zhou, H.; Wan, H.; Zhu, L.; Mi, Y. Research on the effects of rs1800566 C/T polymorphism of NAD(P)H quinone oxidoreductase 1 gene on cancer risk involves analysis of 43,736 cancer cases and 56,173 controls. Front. Oncol. 2022, 12, 980897. [Google Scholar] [CrossRef] [PubMed]
- Das, A.P.; Saini, S.; Tyagi, S.; Chaudhary, N.; Agarwal, S.M. Elucidation of Increased Cervical Cancer Risk Due to Polymorphisms in XRCC1 (R399Q and R194W), ERCC5 (D1104H), and NQO1 (P187S). Reprod. Sci. 2023, 30, 1118–1132. [Google Scholar] [CrossRef] [PubMed]
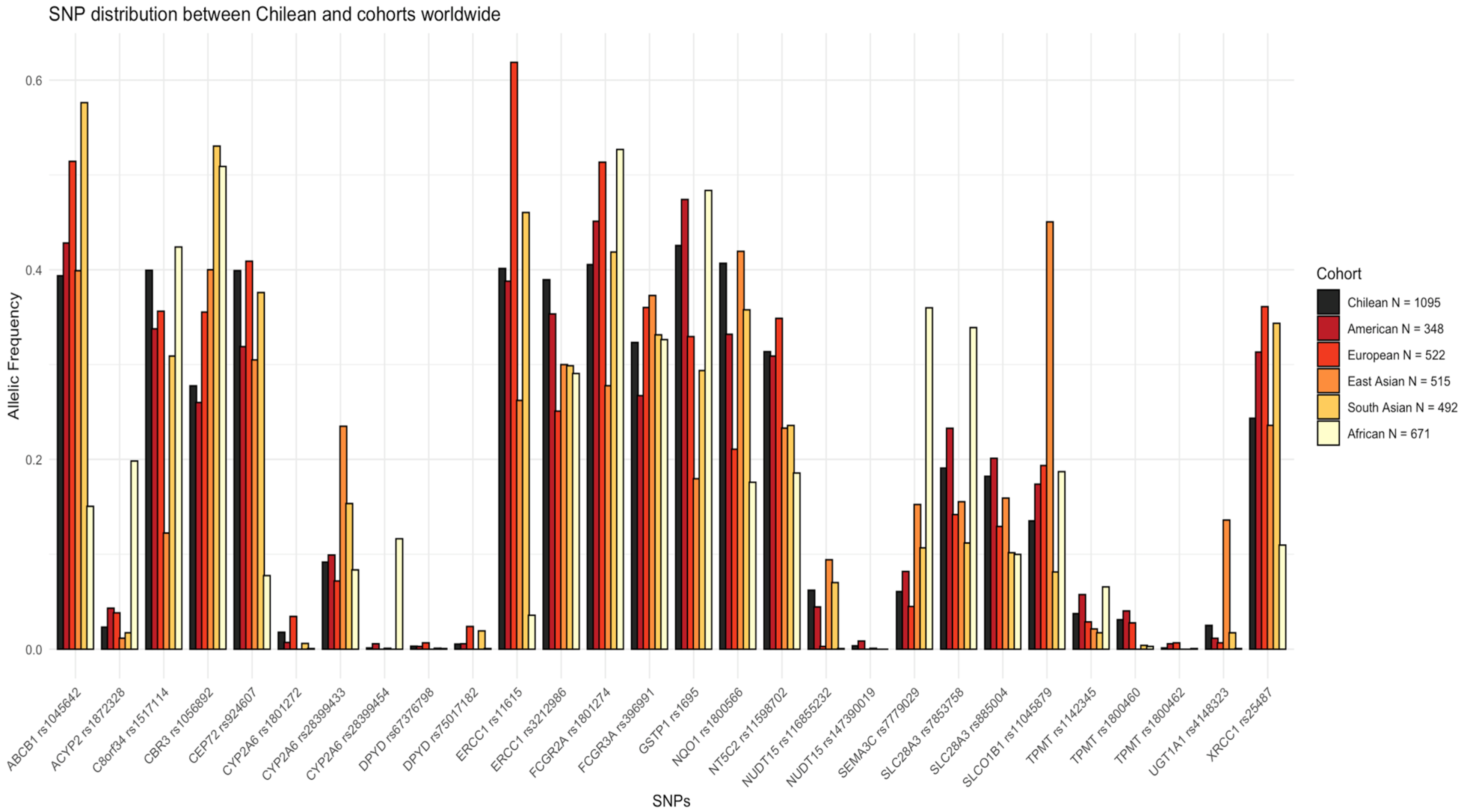
| SNP ID | Gene | MAF CHI | MAF AMR | p | MAF EUR | p | MAF EAS | p | MAF SAS | p | MAF AFR | p | LoE | Clinical Annotation | Related Drugs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs75017182 | DPYD | 0.005 | 0.006 | 1 | 0.024 | 1.08 × 10−5 | 0 | 0.012 | 0.019 | 0.001 | 0.001 | 0.023 | 1A | Toxicity | Fluoropyrimidines |
| rs67376798 | DPYD | 0.003 | 0.003 | 1 | 0.007 | 0.162 | 0 | 0.105 | 0.001 | 0.448 | 0.001 | 0.273 | 1A | Toxicity | Fluoropyrimidines |
| rs1695 | GSTP1 | 0.426 | 0.474 | 0.025 | 0.330 | 1.63 × 10−7 | 0.180 | 3.00 × 10−45 | 0.294 | 1.11 × 10−12 | 0.484 | 0.001 | 2A | Toxicity | Platinum compounds |
| rs116855232 | NUDT15 | 0.062 | 0.045 | 0.093 | 0.003 | 6.24 × 10−20 | 0.094 | 0.001 | 0.070 | 0.391 | 0.001 | 7.83 × 10−28 | 2B | Dosage, Toxicity | Mercaptopurines |
| rs147390019 | NUDT15 | 0.004 | 0.009 | 0.117 | 0.000 | 0.061 | 0.001 | 0.287 | 0 | 0.065 | 0 | 0.028 | 2B | Dosage, Toxicity | Mercaptopurines |
| rs1800462 | TPMT | 0.001 | 0.006 | 0.062 | 0.007 | 0.016 | 0 | 0.556 | 0 | 0.557 | 0.001 | 1 | 1A | Toxicity | Mercaptopurines |
| rs1142345 | TPMT | 0.037 | 0.057 | 0.030 | 0.029 | 0.219 | 0.021 | 0.018 | 0.017 | 0.002 | 0.066 | 2.0 × 10−3 | 1B | Toxicity | Mercaptopurines |
| rs1800460 | TPMT | 0.031 | 0.040 | 0.274 | 0.028 | 0.660 | 0 | 4.42 × 10−12 | 0.004 | 1.87 × 10−7 | 0.003 | 2.55 × 10−10 | 1B | Toxicity | Mercaptopurines |
| rs4148323 | UGT1A1 | 0.025 | 0.011 | 0.036 | 0.007 | 2.0 × 10−3 | 0.136 | 6.92 × 10−32 | 0.017 | 0.198 | 0.001 | 7.66 × 10−11 | 1B | Toxicity | Irinotecan |
| DPYD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Haplotype | rs75017182 | rs2297595 | rs1801265 | CHI | AMR | EUR | EAS | SAS | AFR |
| GTA (H1) | G | T | A | 0.807 | 0.763 | 0.769 | 0.908 | 0.732 | 0.554 |
| GCA (H5) | G | C | A | 0.015 | 0.013 | 0.032 | 0.006 | 0.015 | 0.006 |
| GTG (H2) | G | T | G | 0.148 | 0.173 | 0.110 | 0.072 | 0.205 | 0.414 |
| GCG (H3) | G | C | G | 0.030 | 0.049 | 0.087 | 0.013 | 0.046 | 0.026 |
| ABCB1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Haplotypes | rs1045642 | rs1128503 | CHI | AMR | EUR | EAS | SAS | AFR |
| GG | G | G | 0.435 | 0.507 | 0.443 | 0.335 | 0.327 | 0.803 |
| AG | A | G | 0.089 | 0.088 | 0.143 | 0.039 | 0.083 | 0.060 |
| GA | G | A | 0.171 | 0.065 | 0.042 | 0.265 | 0.096 | 0.047 |
| AA | A | A | 0.305 | 0.340 | 0.371 | 0.359 | 0.492 | 0.091 |
| TPMT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Haplotypes | rs1142345 | rs1800460 | rs1800462 | CHI | AMR | EUR | EAS | SAS | AFR |
| TCC | T | C | C | 0.962 | 0.942 | 0.971 | 0.979 | 0.983 | 0.934 |
| CCC | C | C | C | 0.006 | 0.017 | 0.034 | 0.021 | 0.013 | 0.063 |
| CTC | C | T | C | 0.031 | 0.040 | 0.027 | 0.00 | 0.004 | 0.003 |
| Gene | SNP | p * | MA | LoE | Drug | PharmGKB Annotations |
|---|---|---|---|---|---|---|
| NUDT15 | rs116855232 | 0.00042 | T | 1A | Mercaptopurine | Patients with the rs116855232 CC genotype may have increased dose of mercaptopurine as compared to patients with the CT or TT genotype. |
| NUDT15 | rs869320766 | 0.01043 | AGGAGTC | 1A | Mercaptopurine | The NUDT15*2 allele is assigned as a no function allele by CPIC. Patients with the *2 allele in combination with a normal or no function allele may be at an increased risk of developing leukopenia or neutropenia when treated with mercaptopurine as compared to patients with two normal function alleles. |
| ABCB1 | rs2235047 | 0.00040 | C | 3 | Anthracyclines | Allele C is associated with increased likelihood of cardiotoxicity when exposed to anthracyclines and related substances in children with neoplasms as compared to allele A. |
| BCL2L11 | rs724710 | 0.03475 | T | 3 | Imatinib | Patients with the TT genotype and cancer may have a diminished response when treated with imatinib as compared to patients with the CC genotype. Other genetic and clinical factors may also influence a patient’s response to imatinib. |
| CCAT2 | rs6983267 | 0.01932 | T | 3 | Platinum Compounds | Patients with the TT genotype and lung cancer may have a decreased response to platinum compounds as compared to patients with the GG and GT genotypes. |
| CYP1A1 | rs1048943 | 0.01654 | C | 3 | Capecitabine/Docetaxel | Women with the GG genotype and breast cancer may have increased progression-free survival time when treated with capecitabine and docetaxel as compared to women with the AA genotype. Other genetic and clinical factors may also influence progression-free survival time. |
| DROSHA | rs639174 | 0.00544 | T | 3 | Cyclophosphamide/Cytarabine/Daunorubicin/Mercaptopurine/Methotrexate/Prednisone/ Vincristine | Pediatric patients with precursor cell lymphoblastic leukemia–lymphoma and the TT genotype may have an increased risk of drug toxicity when treated with chemotherapy that includes cyclophosphamide, cytarabine, daunorubicin, mercaptopurine, methotrexate, prednisone and vincristine as compared to patients with the CT and CC genotypes. Other clinical and genetic factors may also influence the risk of drug toxicity in pediatric patients with precursor cell lymphoblastic leukemia-lymphoma who are administered chemotherapy. |
| EPHX1 | rs1051740 | 0.04618 | C | 3 | Cisplatin/Cyclophosphamide | Patients with CT genotype and ovarian cancer who are treated with cisplatin and cyclophosphamide may have an increased risk of grade 1–4 nephrotoxicity as compared to patients with CC or TT genotype. |
| HOTAIR | rs7958904 | 0.03293 | C | 3 | Platinum Compounds | Patients with the CC and CG genotype and lung cancer may have a decreased response to platinum compounds as compared to patients with the GG genotype. |
| KCNQ5 | rs9351963 | 0.00628 | C | 3 | Irinotecan | Patients with the CC genotype and cancer may have an increased risk of diarrhea when treated with irinotecan as compared to patients with the AA or AC genotype. |
| NAT2 | rs1799931 | 0.00280 | A | 3 | Docetaxel/Thalidomide | Patients with AG or GG genotype may have an increased risk of toxicity with docetaxel and thalidomide as compared to patients with the AA genotype. |
| NQO1 | rs1800566 | 0.04034 | A | 3 | Epirubicin/Fluorouracil/Oxaliplatin | Patients with metastatic stomach cancer and the rs1695 AA genotype may have a decreased response to treatment with fluorouracil and oxaliplatin as compared to patients with the GG genotype. Other genetic and clinical factors may also influence response to treatment with fluorouracil and oxaliplatin. |
| NT5C3A | rs3750117 | 0.00308 | A | 3 | Gemcitabine | Patients with AA or AG genotype and cancer may have increased clearance of gemcitabine, and decreased elimination clearance of dFdU (gemcitabine metabolite) as compared to patients with GG genotype. |
| RXRA | rs2234753 | 0.00315 | G | 3 | Docetaxel | Patients with nasopharyngeal cancer and the AG genotype who are treated with docetaxel may have more severe anemia as compared to patients with the AA genotype. |
| SLC31A1 | rs4978536 | 0.01282 | G | 3 | Platinum Compounds | Patients with AG or GG genotype and non-small cell lung cancer who are treated with platinum compounds may have an increased severity of thrombocytopenia as compared to patients with AA genotype. |
| TP53 | rs4968187 | 0.00665 | T | 3 | Cyclophosphamide/Epirubicin/Fluorouracil | Patients with breast cancer and the TT genotype may have a decreased risk of developing neutropenia when treated with cyclophosphamide and fluorouracil as compared to patients with the CC or CT genotypes. Other genetic and clinical factors may also affect a patient’s risk of developing neutropenia. |
| RAD52 | rs11226 | 0.00289 | A | 3 | Cisplatin/Cyclophosphamide | Patients with AA or GG genotype and ovarian cancer who are treated with cisplatin and cyclophosphamide may have an increased risk of grade 3–4 neutropenia as compared to patients with the AG genotype. |
| SLCO1B1 | rs10841753 | 0.02520 | C | 3 | Methotrexate | Patients with precursor cell lymphoblastic leukemia-lymphoma and the CC genotype may have increased concentrations of methotrexate as compared to patients with the CT and TT genotypes. There is no association with risk of mucositis or response to methotrexate. Other clinical and genetic factors may also influence concentrations of methotrexate in patients with precursor cell lymphoblastic leukemia-lymphoma. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owen, G.I.; Cordova-Delgado, M.; Bustos, B.I.; Cerpa, L.C.; Gonzalez, P.; Morales-Pison, S.; Garcia-Bloj, B.; Garrido, M.; Miquel, J.F.; Quiñones, L.A. Assessing the Occurrence and Influence of Cancer Chemotherapy-Related Pharmacogenetic Alleles in the Chilean Population. Pharmaceutics 2024, 16, 561. https://doi.org/10.3390/pharmaceutics16040561
Owen GI, Cordova-Delgado M, Bustos BI, Cerpa LC, Gonzalez P, Morales-Pison S, Garcia-Bloj B, Garrido M, Miquel JF, Quiñones LA. Assessing the Occurrence and Influence of Cancer Chemotherapy-Related Pharmacogenetic Alleles in the Chilean Population. Pharmaceutics. 2024; 16(4):561. https://doi.org/10.3390/pharmaceutics16040561
Chicago/Turabian StyleOwen, Gareth I., Miguel Cordova-Delgado, Bernabé I. Bustos, Leslie C. Cerpa, Pamela Gonzalez, Sebastián Morales-Pison, Benjamín Garcia-Bloj, Marcelo Garrido, Juan Francisco Miquel, and Luis A. Quiñones. 2024. "Assessing the Occurrence and Influence of Cancer Chemotherapy-Related Pharmacogenetic Alleles in the Chilean Population" Pharmaceutics 16, no. 4: 561. https://doi.org/10.3390/pharmaceutics16040561